Chromium Bioaccumulation and Its Impacts on Plants: An Overview
Abstract
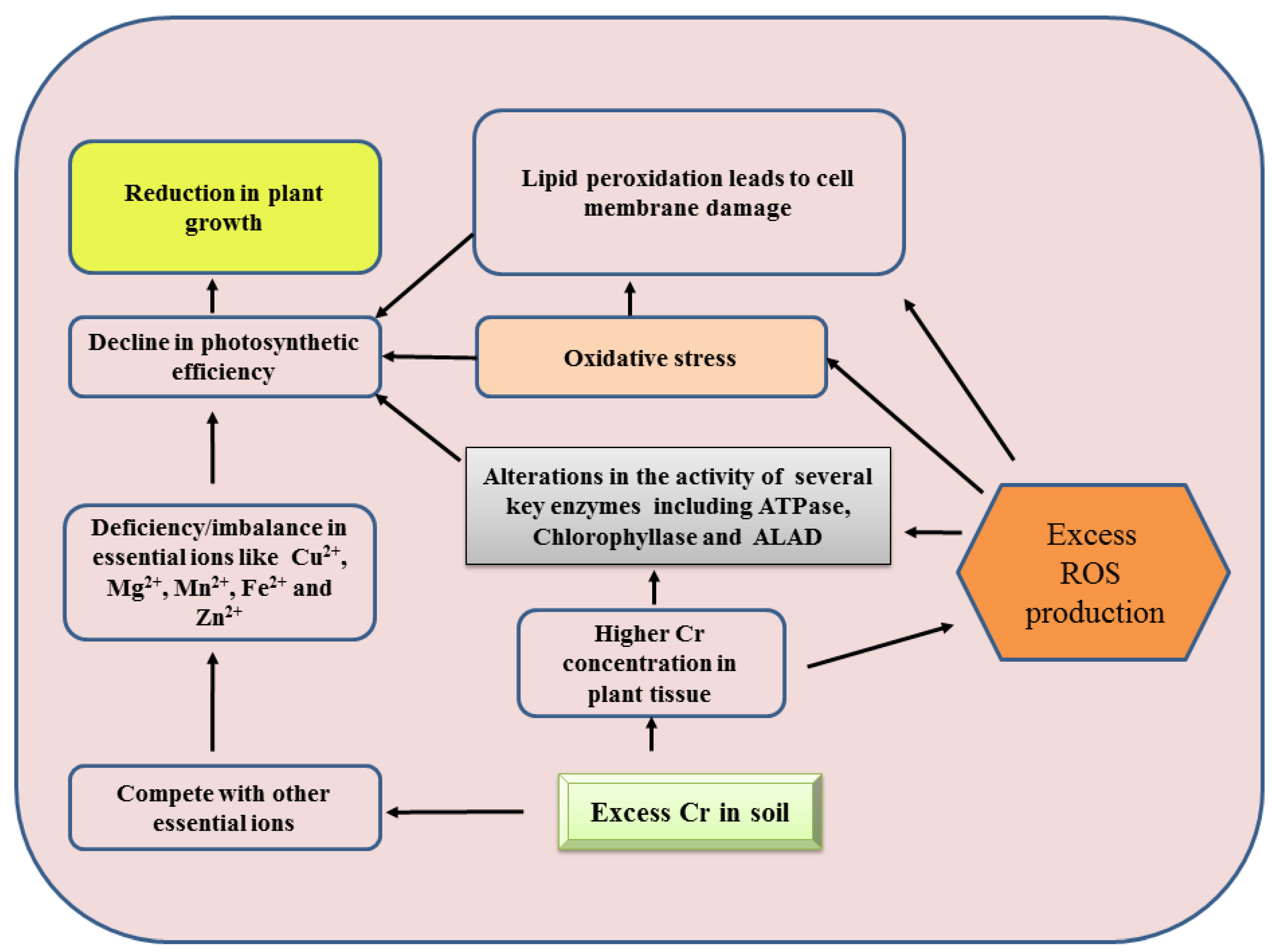
1. Introduction
2. Chromium Uptake, Translocation and Sub-Cellular Distribution
3. Effect of Cr on Nutrient Uptake
4. Effect of Cr on Chlorophyll Molecules and Photosynthetic Performance
5. Reactive Oxygen Species (ROS) and Oxidative Stress
6. Effect of Cr on Enzymatic Antioxidative System
7. Effect of Cr on Non-Enzymatic Antioxidative System
8. Effect of Cr on the Endogenous Levels of Plant Hormones
9. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Nriagu, J.O.; Pacyna, J.; Milford, J.B.; Davidson, C.I. Distribution and characteristic features of chromium in the atmosphere. In Chromium in Natural and Human Environments; Wiley Interscience: New York, NY, USA, 1988; pp. 125–173. [Google Scholar]
- CERCLA Priority List of Hazardous Substances. Agency for Toxic Substances and Disease Registry, USA. 2017. Available online: https://www.atsdr.cdc.gov/spl/ (accessed on 20 September 2019).
- International Agency for Research on Cancer. Overall evaluations of carcinogenicity: And updating of IARC monographs, vol. 1 to 42. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: Suppl 7. IARC 1987, 7, 1–440. [Google Scholar]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Chocobar Ponce, S.; Pagano, E.; Prado, F.E.; Rosa, M. Differential physiological responses of two Salvinia species to hexavalent chromium at a glance. Aquat. Toxicol. 2016, 175, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Bibi, I.; Niazi, N.K.; Ok, Y.S.; Murtaza, G.; Shahid, M.; Kunhikrishnan, A.; Li, D.; Mahmood, T. Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int. J. Phytoremediation 2017, 19, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Joutey, N.T.; Sayel, H.; Bahafid, W.; El Ghachtouli, N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 233, pp. 45–69. [Google Scholar]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Effluent Limitations Guidelines, Pretreatment Standards, and New Source Performance Standards for the Commercial Hazardous Waste Combustor Subcategory of the Waste Combustors Point Source Category. Fed. Regist. 2000, 00-2019, 4360–4385. [Google Scholar]
- World Health Organization. Air Quality Guidelines for Europe, 2000; WHO Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Reale, L.; Ferranti, F.; Mantilacci, S.; Corboli, M.; Aversa, S.; Landucci, F.; Baldisserotto, C.; Ferroni, L.; Pancaldi, S.; Venanzoni, R. Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 2016, 145, 98–105. [Google Scholar] [CrossRef]
- UdDin, I.; Bano, A.; Masood, S. Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol. Environ. Saf. 2015, 113, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Eqani, S.; Katsoyiannis, A.; Xu, R.; Bibi, S.; Benizri, E.; Chaudhary, H. Phytoextraction of chromium (Cr) and influence of Pseudomonas putida on Eruca sativa growth. J. Geochem. Explor. 2017, 182, 269–274. [Google Scholar] [CrossRef]
- Santos, C.; Rodriguez, E. Review on some emerging endpoints of chromium (VI) and lead phytotoxicity. In Botany; InTech: Rijeka, Croatia, 2012; pp. 61–82. [Google Scholar]
- Eleftheriou, E.; Adamakis, I.-D.; Panteris, E.; Fatsiou, M. Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 15852–15871. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Yadav, A.; Haq, I.; Kumar, S.; Bharagava, R.N.; Singh, S.K.; Raj, A. Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J. Environ. Manag. 2016, 183, 204–211. [Google Scholar] [CrossRef]
- Shanker, A.K.; Djanaguiraman, M.; Venkateswarlu, B. Chromium interactions in plants: Current status and future strategies. Metallomics 2009, 1, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Ibrahim, M.; Gill, R.A.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef]
- Farooq, M.; Ali, S.; Hameed, A.; Bharwana, S.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J. Reduction and efflux of chromate by bacteria. In Molecular Microbiology of Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2007; pp. 407–419. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Khan, I.; Tanveer, M.; Shahid, M.; Shakoor, A.; Wang, L. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 2017, 27, 262–273. [Google Scholar] [CrossRef]
- Tang, M.; Mao, D.; Xu, L.; Li, D.; Song, S.; Chen, C. Integrated analysis of miRNA and mRNA expression profiles in response to Cd exposure in rice seedlings. BMC Genom. 2014, 15, 835. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Bot. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Klejdus, B.I.; Hedbavny, J. Chromium uptake and consequences for metabolism and oxidative stress in chamomile plants. J. Agric. Food Chem. 2013, 61, 7864–7873. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Rout, G.R.; Das, P. Induction, selection and characterization of Cr and Ni-tolerant cell lines of Echinochloa colona (L.) Link in vitro. J. Plant Physiol. 2001, 158, 1281–1290. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M.; Islam, K.S.; Chongling, Y. Effect of Chromium Stress on Antioxidative Enzymes and Malondialdehyde Content Activities in Leaves and Roots of Mangrove Seedlings Kandelia candel (L.) Druce. J. For. Environ. Sci. 2010, 26, 171–179. [Google Scholar]
- Rai, V.; Vajpayee, P.; Singh, S.N.; Mehrotra, S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004, 167, 1159–1169. [Google Scholar] [CrossRef]
- Ma, J.; Lv, C.; Xu, M.; Chen, G.; Lv, C.; Gao, Z. Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ. Sci. Pollut. Res. 2016, 23, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.N.; Huang, T.L.; Chi, W.C.; Fu, S.F.; Chen, C.C.; Huang, H.J. Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol. Plant. 2014, 150, 205–224. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xu, X.; Li, T.; Gong, G.; Jia, Y.; Li, Y.; Deng, L. Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J. Hazard. Mater. 2010, 180, 303–308. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Membrane fatty acid composition and saturation levels associated with leaf dehydration tolerance and post-drought rehydration in Kentucky bluegrass. Crop Sci. 2011, 51, 273–281. [Google Scholar] [CrossRef]
- Aldoobie, N.; Beltagi, M. Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. Afr. J. Biotechnol. 2013, 12, 4614–4622. [Google Scholar] [CrossRef]
- Duhan, J.S. Chromium stress on peroxidase, ascorbate peroxidase and acid invertase in pea (Pisum sativum L.) seedling. Int. J. Biotechnol. Mol. Biol. Res. 2012, 3, 15–21. [Google Scholar]
- Paiva, L.B.; Correa, S.F.; Santa Catarina, C.; Floh, E.I.S.; Silva, M.G.D.; Vitória, A.P. Ecophysiological and biochemical parameters for assessing Cr+6 stress conditions in Pterogyne nitens Tul.: New and usual methods for the management and restoration of degraded areas. Environ. Eng. Manag. J. 2014, 13, 3073–3081. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.; Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv CO 4) roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Maiti, S.; Ghosh, N.; Mandal, C.; Das, K.; Dey, N.; Adak, M.K. Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz. J. Plant Physiol. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Yadav, P.; Sharma, A.; Thukral, A.K.; Kumar, V.; Kohli, S.K.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Thukral, A.K.; Sharma, A.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Castasterone and citric acid supplementation alleviates cadmium toxicity by modifying antioxidants and organic acids in Brassica juncea. J. Plant Growth Regul. 2018, 37, 286–299. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, S.; Prakash, S.; Srivastava, M. Fate of trivalent chromium in presence of organic acids: A hydroponic study on the tomato plant. Chem. Speciat. Bioavailab. 1998, 10, 147–150. [Google Scholar] [CrossRef]
- Skeffington, R.; Shewry, P.; Peterson, P. Chromium uptake and transport in barley seedlings (Hordeum vulgare L.). Planta 1976, 132, 209–214. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Luther, A.; Jetschke, G.; Gabrys, H. Modification of chromate toxicity by sulphate in duckweeds (Lemnaceae). Aquat. Toxicol. 2008, 89, 167–171. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Ma, L.Q.; Santos, J.A.; Guilherme, L.R.; Lessl, J.T. Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2014, 184, 187–192. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Gress, J.; De, J.; Rathinasabapathi, B.; Marchi, G.; Chen, Y.; Ma, L.Q. Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere 2016, 147, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sinha, S. Chemical fractionation and heavy metal accumulation in the plant of Sesamum indicum (L.) var. T55 grown on soil amended with tannery sludge: Selection of single extractants. Chemosphere 2006, 64, 161–173. [Google Scholar] [CrossRef]
- Caldelas, C.; Bort, J.; Febrero, A. Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Biol. Toxicol. 2012, 28, 57–68. [Google Scholar] [CrossRef]
- Mangabeira, P.A.; Ferreira, A.S.; de Almeida, A.-A.F.; Fernandes, V.F.; Lucena, E.; Souza, V.L.; dos Santos Júnior, A.J.; Oliveira, A.H.; Grenier-Loustalot, M.F.; Barbier, F. Compartmentalization and ultrastructural alterations induced by chromium in aquatic macrophytes. Biometals 2011, 24, 1017–1026. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Assuncao, A.G.; Herrero, E.; Lin, Y.F.; Huettel, B.; Talukdar, S.; Smaczniak, C.; Immink, R.G.; van Eldik, M.; Fiers, M.; Schat, H.; et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 10296–10301. [Google Scholar] [CrossRef]
- Nishida, S.; Mizuno, T.; Obata, H. Involvement of histidine-rich domain of ZIP family transporter TjZNT1 in metal ion specificity. Plant Physiol. Biochem. 2008, 46, 601–606. [Google Scholar] [CrossRef]
- Gustin, J.L.; Loureiro, M.E.; Kim, D.; Na, G.; Tikhonova, M.; Salt, D.E. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. Cell Mol. Biol. 2009, 57, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Kobae, Y.; Sekino, T.; Yoshioka, H.; Nakagawa, T.; Martinoia, E.; Maeshima, M. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol. 2006, 47, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. Cell Mol. Biol. 2007, 50, 207–218. [Google Scholar] [CrossRef]
- Zayed, A.; Lytle, C.M.; Qian, J.-H.; Terry, N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 1998, 206, 293–299. [Google Scholar] [CrossRef]
- Cary, E.E.; Allaway, W.H.; Olson, O.E. Control of chromium concentrations in food plants. 1. Absorption and translocation of chromium by plants. J. Agric. Food Chem. 1977, 25, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sinam, G.; Kumar Mishra, R.; Sinha, S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L. Ecotoxicol. Environ. Saf. 2010, 73, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Chigonum, W.J.; Ikenna, O.C.; John, C.U. Dynamic Impact of Chromium on Nutrient Uptake from Soil by Fluted Pumpkin (Telfairia occidentalis). Am. J. Biosci. Bioeng. 2019, 7, 1–9. [Google Scholar]
- Osu Charles, I.; Onyema, M.O. Vanadium inhibition capacity on nutrients and heavy metal uptake by Cucumis Sativus. J. Am. Sci. 2016, 12. [Google Scholar] [CrossRef]
- Dube, B.; Tewari, K.; Chatterjee, J.; Chatterjee, C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 2003, 53, 1147–1153. [Google Scholar] [CrossRef]
- Turner, M.; Rust, R. Effects of Chromium on Growth and Mineral Nutrition of Soybeans 1. Soil Sci. Soc. Am. J. 1971, 35, 755–758. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. C. R. Biol. 2010, 333, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K.; Kirkby, E. Principles of Plant Nutrition; International Potash Institute: Worblaufen, Switzerland, 1987; pp. 687–695. [Google Scholar]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2019, 38, 1–23. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Chandra, P.; Kulshreshtha, K. Chromium accumulation and toxicity in aquatic vascular plants. Bot. Rev. 2004, 70, 313–327. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: An overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef]
- Rajendran, M.; An, W.-H.; Li, W.-C.; Perumal, V.; Wu, C.; Sahi, S.V.; Sarkar, S.K. Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides (L.) Roberty). J. Cent. South Univ. 2019, 26, 489–500. [Google Scholar] [CrossRef]
- Sinha, S.; Saxena, R.; Singh, S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes. Chemosphere 2005, 58, 595–604. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Vincent, C.; Zotarelli, L.; Liu, G.; Mattson, N.S.; Rathinasabapathi, B.; Martínez-Nicolas, J.J.; Garcia-Sanchez, F. Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one. Environ. Exp. Bot. 2017, 140, 8–18. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yu, K.; Zhang, Z.; Jiang, W.; Liu, D. Antioxidant response system and chlorophyll fluorescence in chromium (VI)-treated Zea mays L. seedlings. Acta Biol. Crac. Ser. Bot. 2009, 51, 23–33. [Google Scholar]
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am. J. Plant Sci. 2013, 4, 720–726. [Google Scholar] [CrossRef]
- Tang, J.; Xu, J.; Wu, Y.; Li, Y.; Tang, Q. Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis. L.). Afr. J. Biotechnol. 2012, 11, 2248–2255. [Google Scholar]
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Analysis of growth response and tolerance index of Glycine max (L.) Merr. under hexavalent chromium stress. Adv. Life Sci. 2014, 1, 231–241. [Google Scholar]
- Muslu, A.; Ergün, N. Effects of copper and chromium and high temperature on growth, proline and protein content in wheat seedlings. Bangladesh J. Bot. 2013, 42, 105–112. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Vajpayee, P.; Rai, U.; Ali, M.; Tripathi, R.; Yadav, V.; Sinha, S.; Singh, S. Chromium-induced physiologic changes in Vallisneria spiralis L. and its role in phytoremediation of tannery effluent. Bull. Environ. Contam. Toxicol. 2001, 67, 246–256. [Google Scholar] [CrossRef]
- Zlobin, I.; Kholodova, V.; Rakhmankulova, Z.; Kuznetsov, V.V. Brassica napus responses to short-term excessive copper treatment with decrease of photosynthetic pigments, differential expression of heavy metal homeostasis genes including activation of gene NRAMP4 involved in photosystem II stabilization. Photosynth. Res. 2015, 125, 141–150. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Iqbal, M.; Ashraf, M.Y.; Ashraf, M.A.; Ali, S. Organic chelants-mediated enhanced lead (Pb) uptake and accumulation is associated with higher activity of enzymatic antioxidants in spinach (Spinacea oleracea L.). J. Hazard. Mater. 2016, 317, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, C. Chromium uptake and toxicity effects on growth and metabolic activities in wheat, Triticum aestivum L. cv. UP 2003. Indian J. Exp. Biol. 1996, 34, 689–691. [Google Scholar] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Hameed, A.; Farid, M.; Hussain, S.; Yasmeen, T.; Najeeb, U.; Bharwana, S.A.; Abbasi, G.H. Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol. Environ. Saf. 2014, 109, 38–47. [Google Scholar] [CrossRef]
- Davies, F., Jr.; Puryear, J.; Newton, R.; Egilla, J.; Saraiva Grossi, J. Mycorrhizal fungi increase chromium uptake by sunflower plants: Influence on tissue mineral concentration, growth, and gas exchange. J. Plant Nutr. 2002, 25, 2389–2407. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Turkan, I.; Uzilday, B.; Dietz, K.J.; Brautigam, A.; Ozgur, R. Reactive oxygen species and redox regulation in mesophyll and bundle sheath cells of C4 plants. J. Exp. Bot. 2018, 69, 3321–3331. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.; Khan, I.; Samad, R.A.; Tung, S.A.; Anam, M.; Shahzad, B. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean–Soil Air Water 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Grover, P.; Rekhadevi, P.; Danadevi, K.; Vuyyuri, S.; Mahboob, M.; Rahman, M. Genotoxicity evaluation in workers occupationally exposed to lead. Int. J. Hyg. Environ. Health 2010, 213, 99–106. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Shahzad, B.; Mughal, M.N.; Tanveer, M.; Gupta, D.; Abbas, G. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. Res. 2017, 24, 103–115. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Morina, F.; Jovanovic, L.; Mojovic, M.; Vidovic, M.; Pankovic, D.; Veljovic Jovanovic, S. Zinc-induced oxidative stress in Verbascum thapsus is caused by an accumulation of reactive oxygen species and quinhydrone in the cell wall. Physiol. Plant. 2010, 140, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010, 178, 307–311. [Google Scholar] [CrossRef]
- Duquesnoy, I.; Champeau, G.M.; Evray, G.; Ledoigt, G.; Piquet-Pissaloux, A. Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. C. R. Biol. 2010, 333, 814–824. [Google Scholar] [CrossRef]
- Márquez-García, B.; Pérez-López, R.; Ruíz-Chancho, M.J.; López-Sánchez, J.F.; Rubio, R.; Abreu, M.M.; Nieto, J.M.; Córdoba, F. Arsenic speciation in soils and Erica andevalensis Cabezudo & Rivera and Erica australis L. from São Domingos Mine area, Portugal. J. Geochem. Explor. 2012, 119, 51–59. [Google Scholar]
- Körpe, D.A.; Aras, S. Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2011, 719, 29–34. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Gupta, D.; Nicoloso, F.; Schetinger, M.; Rossato, L.; Pereira, L.; Castro, G.; Srivastava, S.; Tripathi, R. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Gupta, D.; Huang, H.; Yang, X.; Razafindrabe, B.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Lee, S.; Moon, J.S.; Ko, T.-S.; Petros, D.; Goldsbrough, P.B.; Korban, S.S. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 2003, 131, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Dupuy, C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat. Res./Rev. Mutat. Res. 2012, 751, 77–81. [Google Scholar] [CrossRef]
- Potocký, M.; Pejchar, P.; Gutkowska, M.; Jiménez-Quesada, M.J.; Potocká, A.; de Dios Alché, J.; Kost, B.; Žárský, V. NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant Physiol. 2012, 169, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 121–147. [Google Scholar]
- Pourrut, B.; Perchet, G.; Silvestre, J.; Cecchi, M.; Guiresse, M.; Pinelli, E. Potential role of NADPH-oxidase in early steps of lead-induced oxidative burst in Vicia faba roots. J. Plant Physiol. 2008, 165, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Ullah, A.; Shahzad, B.; Tanveer, M.; Nadeem, F.; Sharma, A.; Lee, D.J.; Rehman, A. Abiotic Stress Tolerance in Plants Through Pre-sowing Seed Treatments with Mineral Elements and Growth Regulators. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 427–445. [Google Scholar] [CrossRef]
- Fahad, S.; Rehman, A.; Shahzad, B.; Tanveer, M.; Saud, S.; Kamran, M.; Ihtisham, M.; Khan, S.U.; Turan, V.; Ur Rahman, M.H. Chapter 14—Rice Responses and Tolerance to Metal/Metalloid Toxicity. Advances in Rice Research for Abiotic Stress Tolerance 2019, 299–312. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Iqbal, M.; Bharwana, S.A.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Panda, S.; Choudhury, S. Chromium stress in plants. Braz. J. Plant Physiol. 2005, 17, 95–102. [Google Scholar] [CrossRef]
- Mhamdi, A.; Hager, J.; Chaouch, S.; Queval, G.; Han, Y.; Taconnat, L.; Saindrenan, P.; Gouia, H.; Issakidis-Bourguet, E.; Renou, J.-P. Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010, 153, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, C.; Tripathi, R. Phytotoxic lesions of chromium in maize. Chemosphere 2003, 51, 63–68. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Meyer, A.J.; Hell, R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth. Res. 2005, 86, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Galant, A.; Preuss, M.L.; Cameron, J.C.; Jez, J.M. Plant glutathione biosynthesis: Diversity in biochemical regulation and reaction products. Front. Plant Sci. 2011, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Simultaneous measurement of foliar glutathione, γ-glutamylcysteine, and amino acids by high-performance liquid chromatography: Comparison with two other assay methods for glutathione. Anal. Biochem. 1998, 264, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Di Toppi, L.S.; Fossati, F.; Musetti, R.; Mikerezi, I.; Favali, M.A. Effects of Hexavalent Chromium on Maize, Tomato, and Cauliflower Plants. J. Plant Nutr. 2002, 25, 701–717. [Google Scholar] [CrossRef]
- Shanker, A.K.; Pathmanabhan, G. Speciation dependant antioxidative response in roots and leaves of sorghum (Sorghum bicolor (L.) Moench cv CO 27) under Cr (III) and Cr (VI) stress. Plant Soil 2004, 265, 141–151. [Google Scholar] [CrossRef]
- McAuley, A.; Olatunji, M.A. Metal-ion oxidations in solution. Part XIX. Redox pathways in the oxidation of penicillamine and glutathione by chromium (VI). Can. J. Chem. 1977, 55, 3335–3340. [Google Scholar] [CrossRef]
- Marwa, E.M.; Meharg, A.A.; Rice, C.M. Risk assessment of potentially toxic elements in agricultural soils and maize tissues from selected districts in Tanzania. Sci. Total. Environ. 2012, 416, 180–186. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant. 2009, 31, 261–269. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Baskaran, L.; Chidambaram, A.; Sundaramoorthy, P. Influence of chromium stress on proline accumulation in soybean (Glycine max L. Merr.) genotypes. Glob. J. Environ. Res. 2009, 3, 106–108. [Google Scholar]
- Bitrián, M.; Zarza, X.; Altabella, T.; Tiburcio, A.F.; Alcázar, R. Polyamines under abiotic stress: Metabolic crossroads and hormonal crosstalks in plants. Metabolites 2012, 2, 516–528. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Souto, C.; Reigosa, M. Ecophysiological responses of three native herbs to phytotoxic potential of invasive Acacia melanoxylon R. Br. Agrofor. Syst. 2011, 83, 149–166. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Nguyen, Q.T.; Fu, S.F.; Lin, C.Y.; Chen, Y.C.; Huang, H.J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, L.; Liu, L.; Wang, J.; Li, C.; Wang, Q. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternata f. sp. lycopersici. J. Exp. Bot. 2013, 64, 637–650. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential antioxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar]
- Matilla-Vazquez, M.; Matilla, A. Ethylene: Role in plants under environmental stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–222. [Google Scholar]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Sponsel, V.; Hedden, P. Gibberellin biosynthesis and inactivation. In Plant Hormones; Davis, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 63–94. [Google Scholar] [CrossRef]
- Atici, Ö.; Ağar, G.; Battal, P. Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol. Plant. 2005, 49, 215–222. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
| Plant Species | Physiological Response | Reference |
|---|---|---|
| Camellia sinensis | Increased SOD and CAT activities | Tang et al. [26] |
| Capsicum annuum | Increased carotenoid content | Oliveira [27] |
| Chamomilla recutita | Increased MDA level | Kováčik et al. [28] |
| Echinochloa colona | Increased CAT and POD activities | Samantaray et al. [29] |
| Kandelia candel | Increased MDA content, and activities of CAT and SOD | Rahman et al. [30] |
| Ocimum tenuiflorum | Increased proline level | Rai et al. [31] |
| Oryza sativa | Increased POD activity | Ma et al. [32] |
| Oryza sativa | Increased ethylene synthesis | Trinh et al. [33] |
| Oryza sativa | Increased CAT and SOD activities | Zhang et al. [34] |
| Oryza sativa | Increased POD activity | Xu et al. [35] |
| Phaseolus vulgaris | Decreased carotenoids | Aldoobie and Beltagi [36] |
| Pisum sativum | Decreased APX activity | Duhan [37] |
| Pterogyne nitens | Increased spermidine level | Paiva et al. [38] |
| Raphanus sativus | Increased glycine-betaine content | Choudhary et al. [39] |
| Triticum aestivum | Increased MDA contents | Ali et al. [22] |
| Triticum aestivum | Increased lipid peroxidation | Zhang et al. [34] |
| Vigna radiata | Decreased glutathione level | Shanker et al. [40] |
| Zea mays | Increased SOD and GPX activities | Maiti et al. [41] |
| Zea mays | Increased lipid peroxidation and H2O2 content | Maiti et al. [41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. https://doi.org/10.3390/plants9010100
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants. 2020; 9(1):100. https://doi.org/10.3390/plants9010100
Chicago/Turabian StyleSharma, Anket, Dhriti Kapoor, Junfeng Wang, Babar Shahzad, Vinod Kumar, Aditi Shreeya Bali, Shivam Jasrotia, Bingsong Zheng, Huwei Yuan, and Daoliang Yan. 2020. "Chromium Bioaccumulation and Its Impacts on Plants: An Overview" Plants 9, no. 1: 100. https://doi.org/10.3390/plants9010100
APA StyleSharma, A., Kapoor, D., Wang, J., Shahzad, B., Kumar, V., Bali, A. S., Jasrotia, S., Zheng, B., Yuan, H., & Yan, D. (2020). Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants, 9(1), 100. https://doi.org/10.3390/plants9010100







