Interaction of Huanglongbing and Foliar Applications of Copper on Water Relations of Citrus sinensis cv. Valencia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Treatments
2.2. Foliar Cu Analysis
2.3. Theoretical Model and Measurements to Aid Interpretation of the Impact of HLB and Cu Treatments on Plant Water Relations
2.3.1. Tp (mmoles·s−1)
2.3.2. Stomatal Conductance (mmoles·m−2·s−1)
2.3.3. Tsa (m2)
2.3.4. Uptake Surface Area of the Roots (Usa) (m2)
2.3.5. Feeder Root Lifespan
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
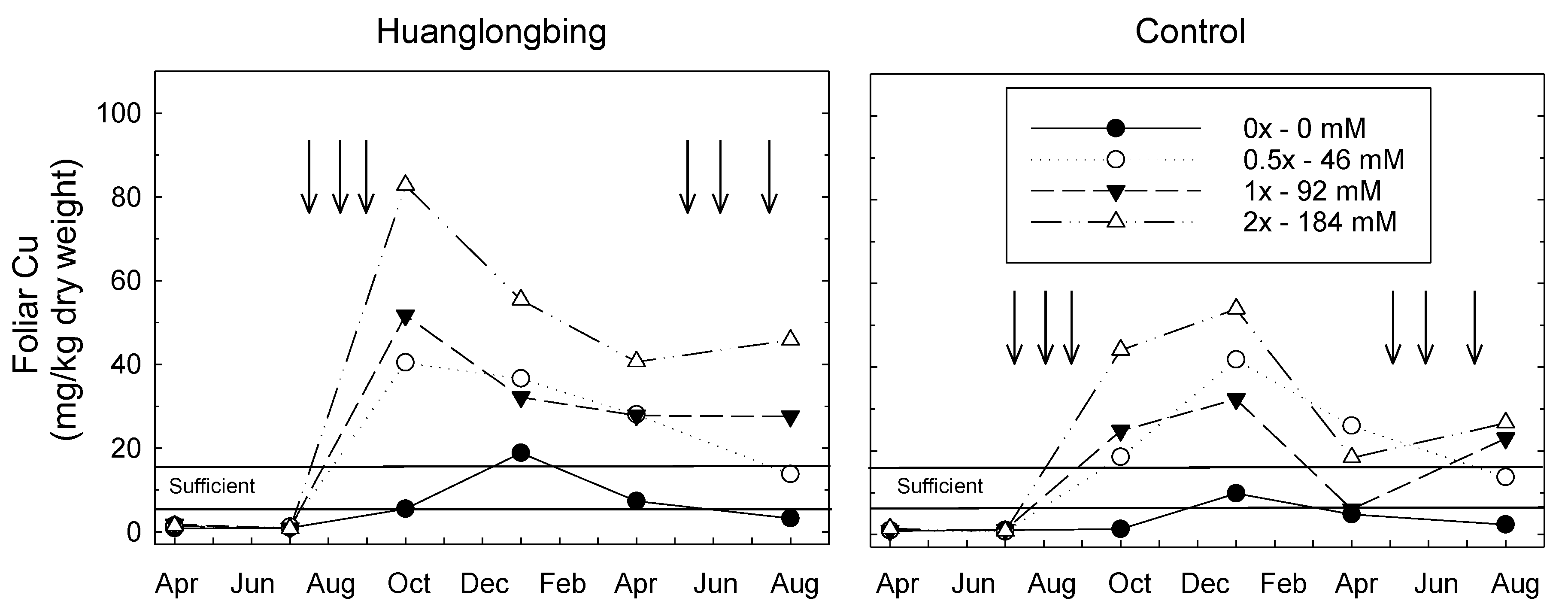
3.1. Foliar Cu Content
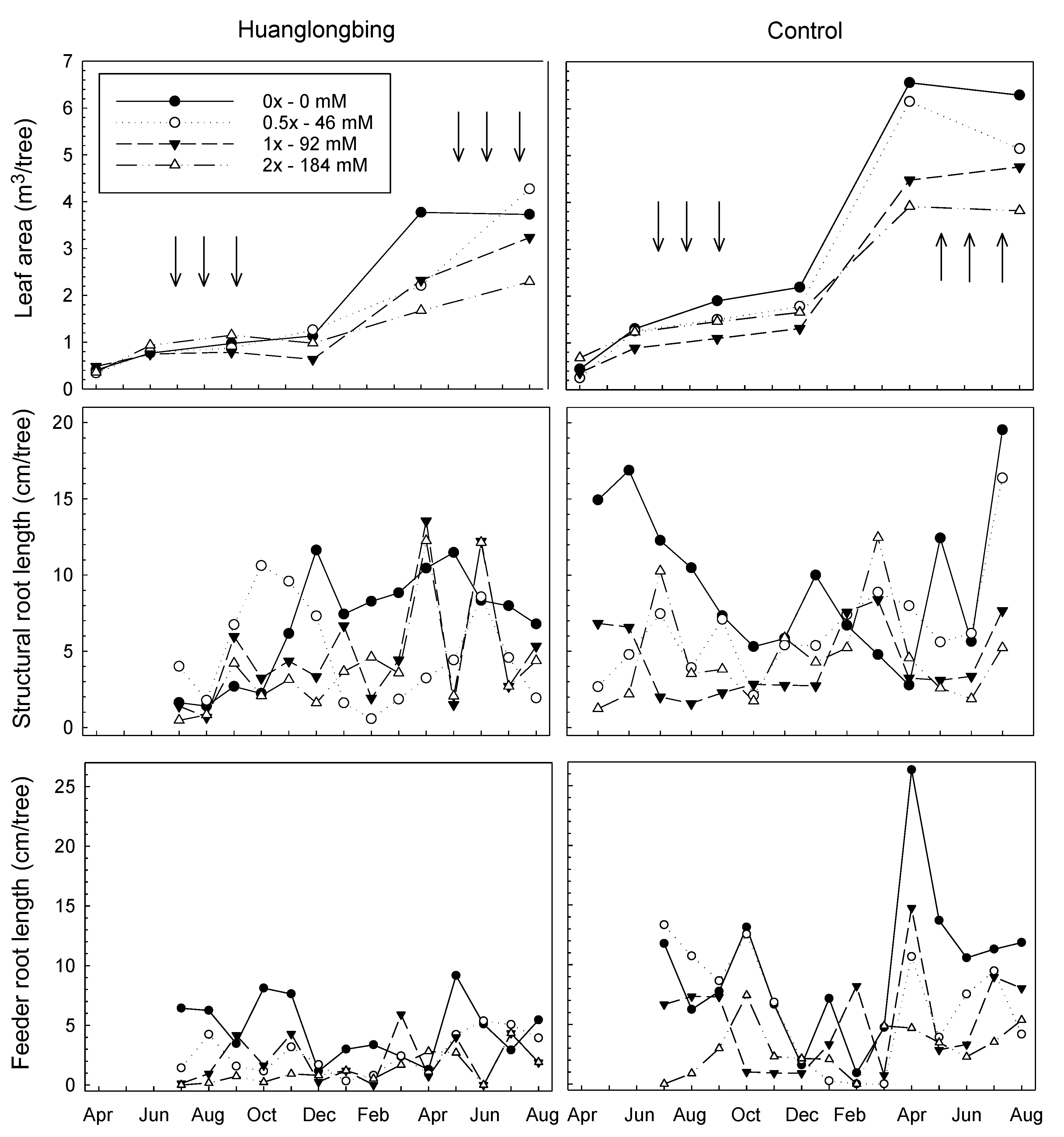
3.2. Leaf Area (Tsa)
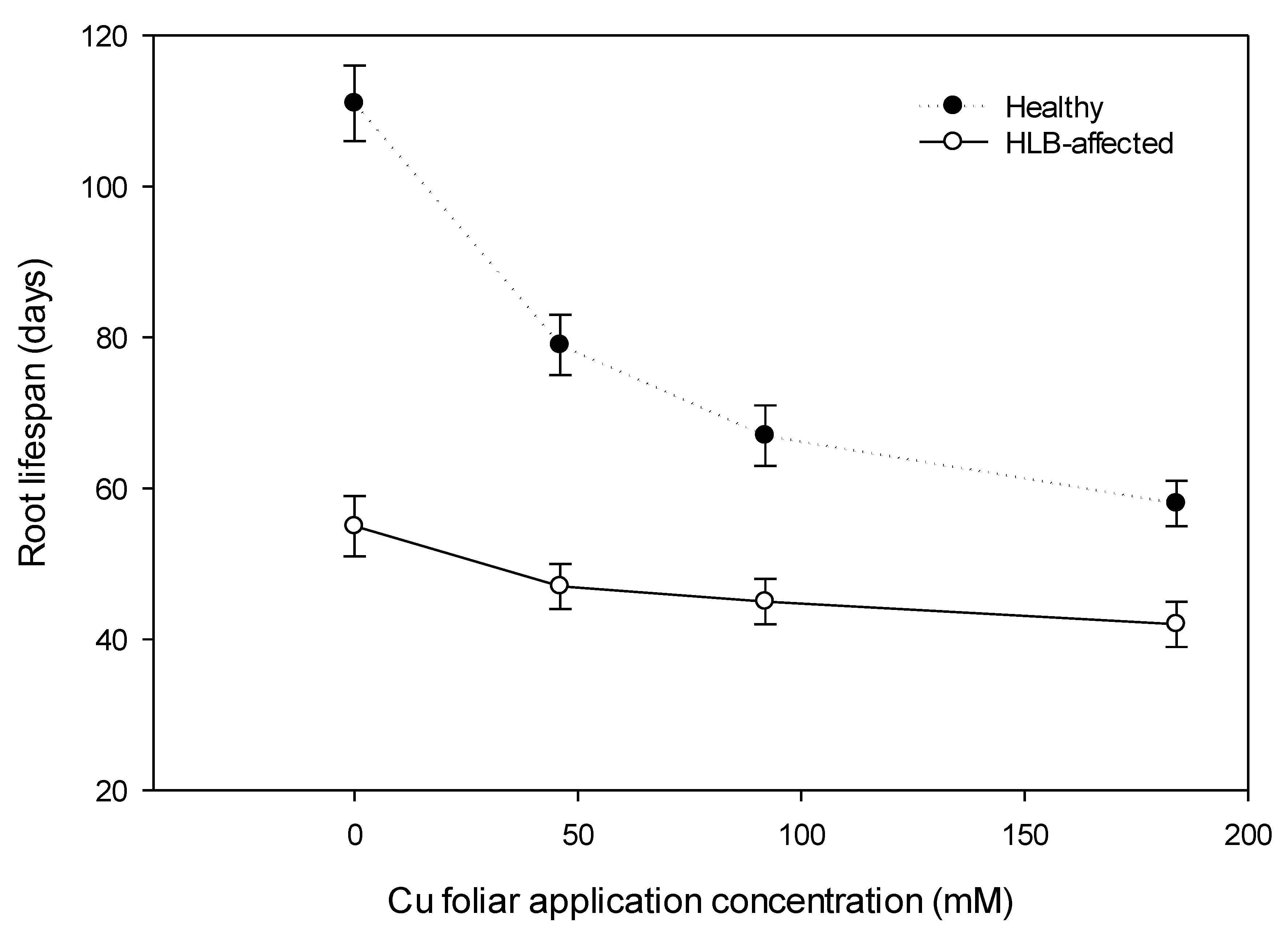
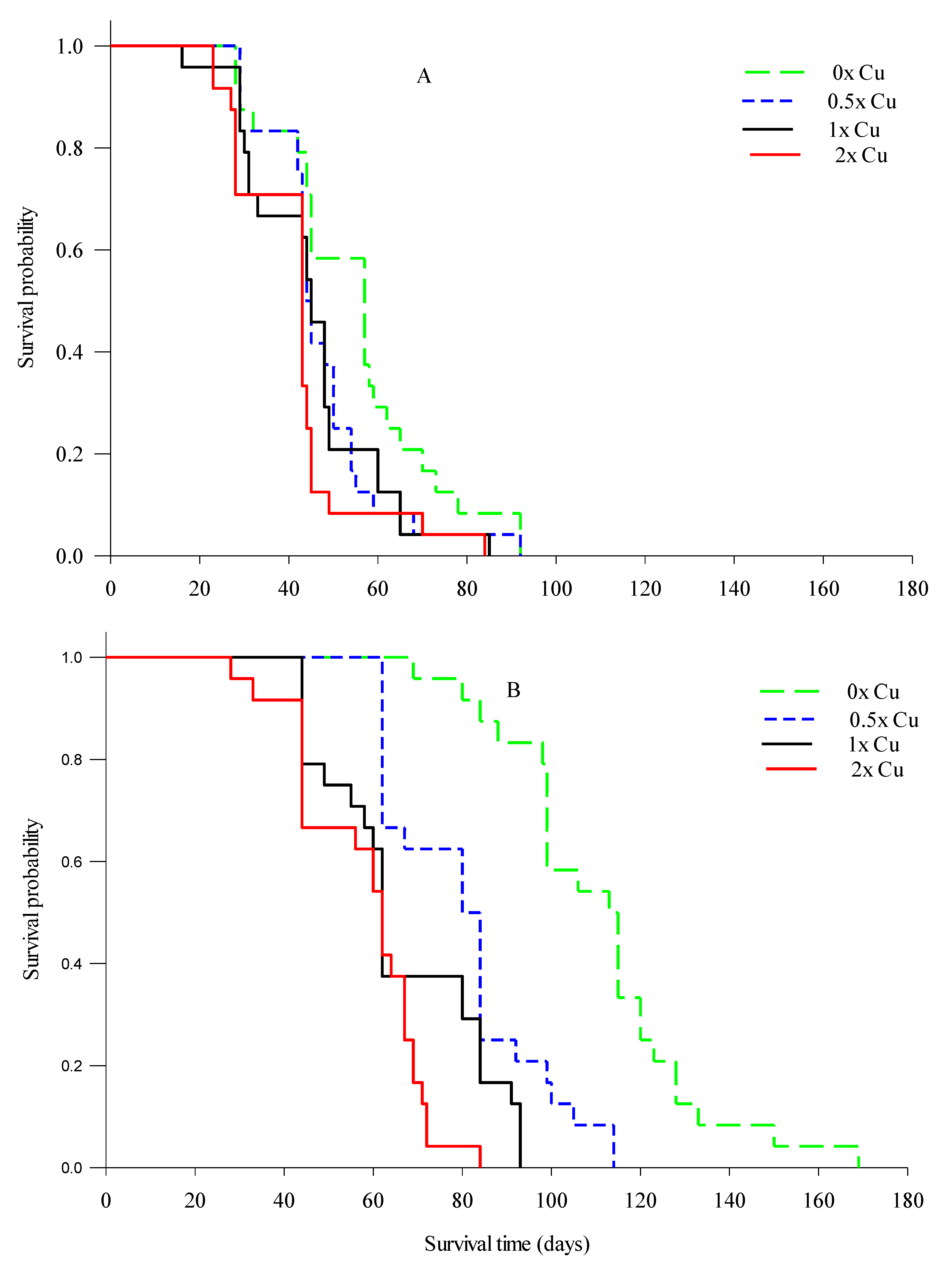
3.3. Total Observable Root Length, Uptake Surface Area (Usa), and Feeder Root Lifespan
3.4. Tsa/Usa (m2·m−2)
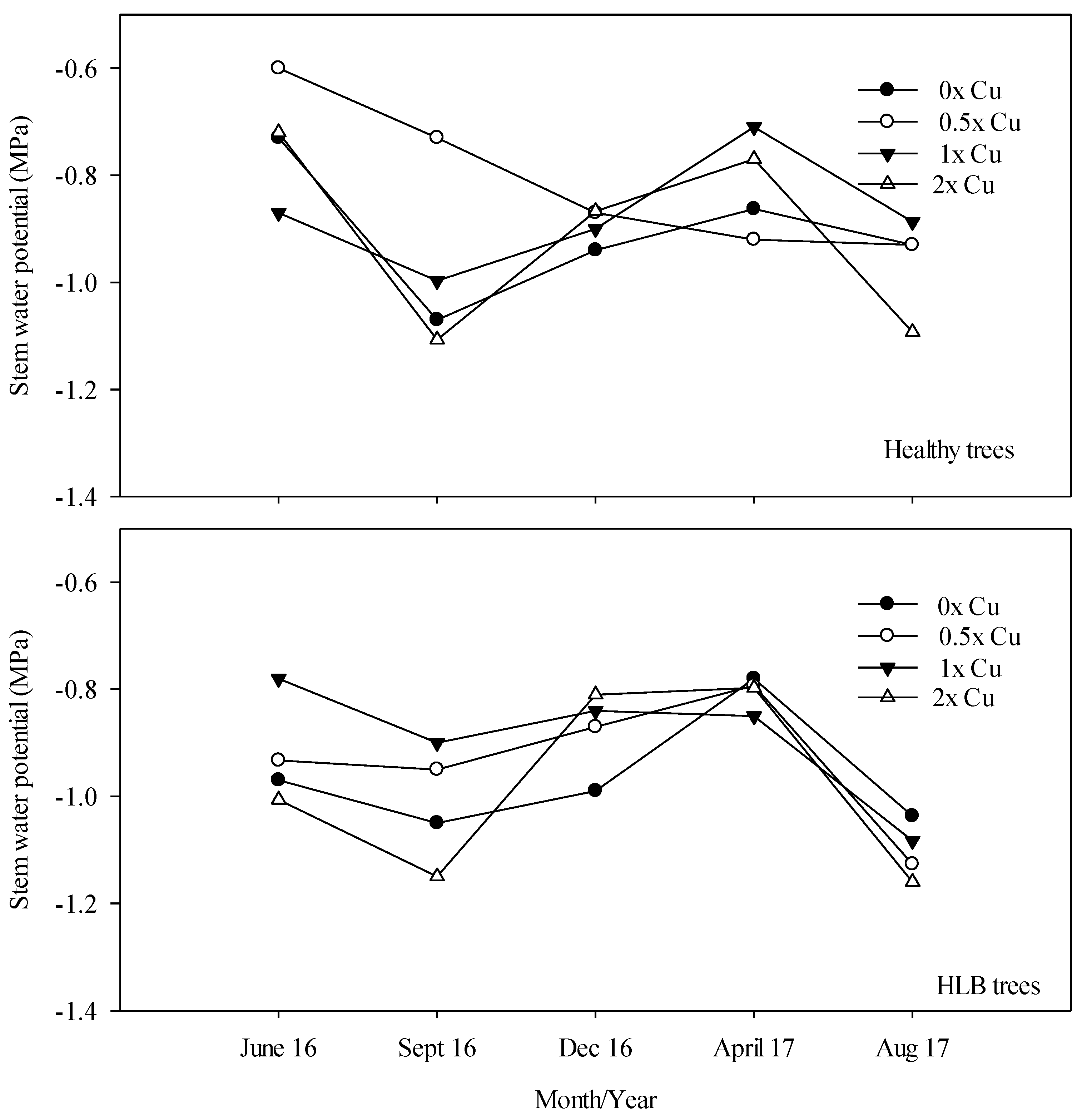
3.5. Ψxylem (MPa)
3.6. Stomatal Conductance (ks)
3.7. Tp (mmoles·s−1)
3.8. Rr+s (MPa·s·mmole−1)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebel, R.C. Huanglongbing: Mechanism of Citrus Decline and Horticulture Management in Florida; Integrated Plant Health Services, LLC: Fort Myers, FL, USA, 2017; ISBN 1532363753. [Google Scholar]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on Huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Kriedemann, P.E.; Barrs, H.D. Citrus orchards. In Water Deficits and Plant Growth; Kozlowsi, T.T., Ed.; Academic Press: London, UK, 1981; pp. 325–417. [Google Scholar]
- Landsberg, J.J.; Jones, H.G. Apple orchards. In Water Deficits and Plant Growth; Kozlowsi, T.T., Ed.; Academic Press: London, UK, 1981; pp. 419–469. [Google Scholar]
- Hamido, S.A.; Morgan, K.T.; Kadyampakeni, D.M. The effect of Huanglongbing on young citrus tree water use. HortTechnology 2017, 27, 659–665. [Google Scholar] [CrossRef]
- Kumar, N.; Kiran, F.; Etxeberria, E. Huanglongbing-induced anatomical changes in citrus fibrous root orders. HortScience 2018, 53, 829–837. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T.; Ebel, R.C.; Kadyampakeni, D.M. Improved irrigation management of sweet orange with Huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Driscoll, P.J. Copper toxicity on Florida citrus—Why did it happen? Proc. Fl. State Horticult. Soc. 2004, 117, 124–127. [Google Scholar]
- USDA-NASS. Citrus Production by Type and Chemical Inventory by State. 2019. Available online: https://quickstats.nass.usda.gov/results/C6A49369-57C7-3F63-BE50-46FAE1A6B601 (accessed on 28 June 2019).
- Bakshi, S.; He, Z.L.; Harris, W.G. Particulate copper in soils and surface runoff from contaminated sandy soils under citrus production. Environ. Sci. Pollut. Res. 2013, 20, 8801–8812. [Google Scholar] [CrossRef] [PubMed]
- Behlau, F.; Belasque, J., Jr.; Graham, J.H.; Leite, R.P., Jr. Effect of frequency of copper applications on control of citrus canker and the yield of young bearing sweet orange trees. Crop Protect. 2010, 29, 300–305. [Google Scholar] [CrossRef]
- Fan, J.; He, Z.; Ma, L.Q.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soils Sediments 2011, 11, 639–648. [Google Scholar] [CrossRef]
- Ebel, R.C.; Hamido, S.; Morgan, K.T. Interaction of Huanglongbing and foliar applications of copper on growth and nutrient acquisition of Citrus sinensis cv. Valencia. HortScience 2019, 54, 297–302. [Google Scholar] [CrossRef]
- Handique, U.; Ebel, R.C.; Morgan, K.T. Influence of soil-applied fertilizer on greening development in new growth flushes of sweet orange. Proc. Fl. State Horticult. Soc. 2012, 125, 36–40. [Google Scholar]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Obreza, T.A.; Morgan, K.T. Nutrition of Florida Citrus Trees, Cooperative Extension Service; Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2008. [Google Scholar]
- Hanlon, E.A.; Gonzalez, J.S.; Bartos, J.M. Institute of Food and Agricultural Sciences (IFAS) Extension Soil Testing Laboratory (ESTL) and Analytical Research Laboratory (ARL) Chemical Procedures and Training Manual; Circule 812; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Jones, J.B.J.; Case, V.W. Sample, Handling, and Analyzing Plant Tissue Samples; Westerman, R.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Plank, C.O. Plant analysis reference procedures for the southern region of the United States. South Coop. Ser. Bull. 1992, 368. Available online: http://www.ncagr.gov/agronomi/pdffiles/sera368.pdf (accessed on 10 January 2019).
- Anderson, D.L.; Henderson, L.J. Comparing sealed chamber digestion with other digestion methods used for plant-tissue analysis. Agron. J. 1988, 80, 549–552. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Obreza, T.A.; Rouse, R.E.; Sherrod, J.B. Economics of controlled-release fertilizer use on young citrus trees. J. Prod. Agric. 1999, 12, 69–73. [Google Scholar] [CrossRef]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.L.; Mathesius, U. Root-to-shoot signaling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2016, 43, 87–104. [Google Scholar] [CrossRef]
- Cohen, Y. Determination of orchard water requirement by a combined trunk sap flow and meteorological approach. Irrig. Sci. 1991, 12, 93–98. [Google Scholar] [CrossRef]
- Barkataky, S.; Morgan, K.T.; Ebel, R.C. Water use of ‘Hamlin’ sweet orange during cold acclimation. Irrig. Sci. 2013, 31, 431–443. [Google Scholar] [CrossRef]
- Ebel, R.C.; Proebsting, E.L.; Evans, R.G. Apple tree and fruit responses to early termination of irrigation in a semi-arid environment. HortScience 2001, 36, 1197–1201. [Google Scholar] [CrossRef]
- Milliron, L.K.; Olivos, A.; Saa, S.; Sanden, B.L.; Shackel, K.A. Dormant stem water potential responds to laboratory manipulation of hydration as well as contrasting rainfall field conditions in deciduous tree crops. Biosyst. Eng. 2018, 165, 2–9. [Google Scholar] [CrossRef]
- Garnier, E.; Berger, A. Testing water potential in peach trees as an indicator of water stress. J. Horticult. Sci. 1985, 60, 47–56. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.L.; Epron, D.; Badot, P.M. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004, 166, 1213–1218. [Google Scholar] [CrossRef]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Cook, C.M.; Kostidou, A.; Vardaka, E.; Lanaras, T. Effects of copper on the growth, photosynthesis and nutrient concentrations of Phaseolus plants. Photosynthetica 1997, 34, 179–193. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Burchett, M.D. Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar. Environ. Res. 2002, 54, 65–84. [Google Scholar] [CrossRef]
- Bevington, K.B.; Castle, W.S. Annual root growth pattern of young citrus trees in relation to shoot growth, soil temperature, and soil water content. J. Am. Soc. Hortric. Sci. 1985, 110, 840–845. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press, Inc.: San Diego, CA, USA, 1995; Volume 30–41, pp. 128–130. ISBN 0-12-473543-6. [Google Scholar]
- Gao, Y.; Duan, A.; Qiu, X.; Liu, Z.; Sun, J.; Zhang, J.; Wang, H. Distribution of roots and root length density in a maize/soybean strip intercropping system. Agric. Water Manag. 2010, 98, 199–212. [Google Scholar] [CrossRef]
- Nickel, S.; Crookston, R.K.; Russelle, M.P. Root growth and distribution are affected by corn–soybean cropping sequence. Agron. J. 1995, 87, 895–902. [Google Scholar] [CrossRef]
- Abrisqueta, J.M.; Mounzer, O.; Álvarez, S.; Conejero, W.; García-Orellana, Y.; Tapia, L.M.; Veraa, J.; Abrisquetaa, I.; Ruiz-Sánchezab, M.C. Root dynamics of peach trees submitted to partial rootzone drying continuous deficit irrigation. Agric. Water Manag. 2008, 95, 959–967. [Google Scholar] [CrossRef]
- Bernier, P.Y.; Robitaille, G. A plane intersect methods for estimating fine root productivity of trees from minirhizotrons images. Plant Soil 2004, 265, 165–173. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M.; Lakso, A.N. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 2000, 147, 171–178. [Google Scholar] [CrossRef]
- Crocker, T.L.; Hendrick, R.L.; Ruess, R.W.; Pregitzer, K.S.; Burton, A.J.; Allen, M.F.; Shan, J.; Morris, L.A. Substituting root numbers for length: Improving the use of minirhizotrons to study fine root dynamics. Appl. Soil Ecol. 2003, 23, 127–135. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Plana, V.; Ortuño, M.F.; Tapia, L.M.; Abrisqueta, J.M. Spatial root distribution of apricot trees in different soil tillage practices. Plant Soil 2005, 272, 211–221. [Google Scholar] [CrossRef]
- Wells, C.E.; Glenn, D.M.; Eissenstat, D.M. Changes in the risk of fine-root mortality with age: A case study in peach Prunus persica (Rosaceae). Am. J. Bot. 2002, 89, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Barney, C.W. Effects of soil temperature and light intensity on root growth of loblolly pine seedlings. Plant Physiol. 1951, 26, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.F. Root and Shoot Growth of Shortleaf and Loblolly Pines in Relation to Certain Environmental Conditions; BoD: Norderstedt, Germany, 1939; Volume 4, p. 52. [Google Scholar]
- Wilcox, H.E. Growth studies of the root of incense cedar, Librocedrus decurrens II. Morphological features of the growth system and root behaviour. Am. J. Bot. 1962, 49, 237–245. [Google Scholar] [CrossRef]
- Zambrosi, F.C.B.; Mesquita, G.L.; Tanaka, F.A.O.; Quaggio, J.A.; Mattos, D., Jr. Phosphorus availability and rootstock affect copper-induced damage to the root ultrastructure of Citrus. Env. Exp. Bot. 2013, 95, 25–33. [Google Scholar] [CrossRef]
- Wutscher, H.K.; Smith, P.F. Citrus. In Nutrient Deficiencies & Toxicities of Crop Plants; Bennet, W.F., Ed.; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Gasque, M.; Martí, P.; Granero, B.; González-Altozano, P. Effects of long-term summer deficit irrigation on ‘Navelina’ citrus trees. Agric. Water Manag. 2016, 169, 140–147. [Google Scholar] [CrossRef]
- Gomes, M.D.M.D.A.; Lagôa, A.M.M.A.; Medina, C.L.; Machado, E.C.; Machado, M.A. Interactions between water potential, stomatal conductance and abscisic acid content of orange trees submitted to drought stress. Braz. J. Plant Physiol. 2004, 16, 155–161. [Google Scholar] [CrossRef]
- Syvertsen, J.P. Partial shoot removal increases net CO2 assimilation and alters water relations of Citrus seedlings. Tree Physiol. 1994, 14, 497–508. [Google Scholar] [CrossRef]
- Davies, F.S.; Bower, J. Water stress, gas exchange and fruit set of ‘Olinda’ Valencia orange trees in Eastern Transvaal area of South Africa. Acta. Horticult. 1994, 365, 121–127. [Google Scholar] [CrossRef]
- Machado, E.C.; Medina, C.L.; Gomes, M.D.M.D.A.; Habermann, G. Seasonal variation of photosynthetic rates, stomatal conductance and leaf water potential in ‘Valencia’ orange trees. Sci. Agricola 2002, 59, 53–58. [Google Scholar] [CrossRef]
- Syvertsen, J.P. Minimum leaf water potential and stomatal closure in citrus leaves of different ages. Ann. Bot. 1982, 47, 827–834. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T. Harvesting method affects water dynamics and yield of sweet orange with Huanglongbing. Agriculture 2018, 8, 38. [Google Scholar] [CrossRef]
- Espadafor, M.; Orgaz, F.; Testi, L.; Lorite, I.J.; González-Dugo, V.; Fereres, E. Responses of transpiration and transpiration efficiency of almond trees to moderate water deficits. Sci. Horticult. 2017, 225, 6–14. [Google Scholar] [CrossRef]
- Do Vale Gomes, A.M.S.; de Oliveira Reis, F.; de Lemos, R.N.S.; Mondego, J.M.; Braun, H.; Araujo, J.R.G. Physiological characteristics of citrus plants infested with citrus blackfly. Rev. Bras. Entomol. 2019, 63, 119–123. [Google Scholar] [CrossRef]
- Wilcox, D.A.; Davies, F.S.; Buchanan, D.W. Root temperature, water relations, and cold hardiness in two citrus rootstocks. J. Am. Horticult. Sci. 1983, 108, 318–321. [Google Scholar]
| Model Variables and Main Effects Means 2 | Foliar Cu (mg·kg−1 Dry Weight) | Tsa (m2) | Total Observable Root Length (cm) | Tsa/EUsa (×104 m2·m−2) | Ψxylem (MPa) | ks (mmoles·m−2·s−1) | Tp (mmoles·s−1) | Rr+s (×10−4 MPa·s·mmole−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Structural | Feeder | ||||||||
| HLB | 0.01 | 0.01 | 0.31 | <0.01 | 0.74 | <0.01 | 0.82 | <0.01 | 0.13 |
| Cu | <0.01 | 0.30 | 0.03 | <0.01 | 0.48 | 0.93 | 0.10 | <0.01 | 0.75 |
| HLB*Cu | <0.01 | 0.36 | <0.01 | 0.02 | 0.50 | 0.94 | 0.83 | 0.01 | 0.71 |
| MAFT | <0.01 | 0.09 | 0.15 | 0.47 | 0.20 | <0.01 | <0.01 | <0.01 | 0.61 |
| HLB*MAFT | <0.01 | 0.29 | 0.01 | 0.96 | 0.54 | --- | --- | 0.35 | --- |
| Cu*MAFT | <0.01 | 0.09 | 0.03 | 0.03 | 0.47 | --- | --- | 0.02 | --- |
| HLB*Cu*MAFT | <0.01 | 0.03 | <0.01 | <0.01 | 0.43 | --- | --- | 0.01 | --- |
| HLB treatment | |||||||||
| HLB | 17.1a | 2.1b | 4.0 | 2.5b | 8.3 | −1.07a | 180 | 2.5b | 1.6 |
| Control | 12.0b | 3.4a | 7.7 | 5.3a | 11.9 | −0.95b | 183 | 3.0a | 2.9 |
| Cu treatment (mM) | |||||||||
| 0 | 4.9c | 3.4a | 9.5a | 7.1a | 3.9 | −1.03 | 176 | 3.8a | 2.1 |
| 46 | 13.6b | 2.9ab | 6.2b | 4.5b | 18.0 | −0.98 | 194 | 3.1b | 2.9 |
| 92 | 15.8b | 2.5bc | 4.3c | 3.7bc | 10.0 | −1.01 | 182 | 2.4bc | 1.8 |
| 184 | 23.7a | 2.2c | 3.4c | 2.1c | 9.0 | −1.01 | 173 | 2.1c | 2.5 |
| Time of Event-Day | Number of Roots Died | Live Roots at the Start of the Day (n) | Survival Probability | Standard Error | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu Rates–HLB Affected Roots | |||||||||||||||||||
| 0× | 0.5× | 1× | 2× | 0× | 0.5× | 1× | 2× | 0× | 0.5× | 1× | 2× | 0× | 0.5× | 1× | 2× | 0× | 0.5× | 1× | 2× |
| 28 | 29 | 16 | 23 | 2 | 4 | 1 | 2 | 24 | 24 | 24 | 24 | 0.917 | 0.833 | 0.958 | 0.917 | 0.056 | 0.076 | 0.041 | 0.056 |
| 29 | 42 | 29 | 27 | 1 | 2 | 3 | 1 | 22 | 20 | 23 | 22 | 0.875 | 0.750 | 0.833 | 0.875 | 0.068 | 0.088 | 0.076 | 0.068 |
| 32 | 43 | 30 | 28 | 1 | 5 | 1 | 4 | 21 | 18 | 20 | 21 | 0.833 | 0.542 | 0.792 | 0.708 | 0.076 | 0.102 | 0.083 | 0.093 |
| 42 | 44 | 31 | 43 | 1 | 1 | 2 | 9 | 20 | 13 | 19 | 17 | 0.792 | 0.542 | 0.708 | 0.333 | 0.083 | 0.102 | 0.093 | 0.096 |
| 44 | 45 | 33 | 44 | 2 | 2 | 1 | 2 | 19 | 12 | 17 | 8 | 0.708 | 0.542 | 0.667 | 0.250 | 0.093 | 0.101 | 0.096 | 0.088 |
| 45 | 48 | 43 | 45 | 3 | 1 | 1 | 3 | 17 | 10 | 16 | 6 | 0.583 | 0.542 | 0.625 | 0.125 | 0.101 | 0.099 | 0.099 | 0.068 |
| 57 | 50 | 44 | 49 | 5 | 3 | 2 | 1 | 14 | 9 | 15 | 3 | 0.375 | 0.542 | 0.542 | 0.083 | 0.099 | 0.088 | 0.102 | 0.056 |
| 58 | 54 | 45 | 70 | 1 | 2 | 2 | 1 | 9 | 6 | 13 | 2 | 0.333 | 0.542 | 0.458 | 0.042 | 0.096 | 0.076 | 0.102 | 0.041 |
| 59 | 55 | 48 | 84 | 1 | 1 | 4 | 1 | 8 | 4 | 11 | 1 | 0.292 | 0.542 | 0.292 | 0.000 | 0.093 | 0.068 | 0.093 | 0.000 |
| 62 | 59 | 49 | 1 | 1 | 2 | 7 | 3 | 7 | 0.250 | 0.542 | 0.208 | 0.088 | 0.056 | 0.083 | |||||
| 65 | 68 | 60 | 1 | 1 | 2 | 6 | 2 | 5 | 0.208 | 0.542 | 0.125 | 0.083 | 0.041 | 0.068 | |||||
| 70 | 92 | 65 | 1 | 1 | 2 | 5 | 1 | 3 | 0.167 | 0.542 | 0.042 | 0.076 | 0.000 | 0.041 | |||||
| 73 | 85 | 1 | 1 | 4 | 1 | 0.125 | 0.000 | 0.068 | 0.000 | ||||||||||
| 78 | 1 | 3 | 0.083 | 0.056 | |||||||||||||||
| 92 | 2 | 2 | 0.000 | 0.000 | |||||||||||||||
| Healthy Roots | |||||||||||||||||||
| 69 | 62 | 44 | 28 | 1 | 8 | 5 | 1 | 24 | 24 | 24 | 24 | 0.958 | 0.667 | 0.792 | 0.958 | 0.041 | 0.096 | 0.083 | 0.041 |
| 80 | 67 | 49 | 33 | 1 | 1 | 1 | 1 | 23 | 16 | 19 | 23 | 0.917 | 0.625 | 0.750 | 0.917 | 0.056 | 0.099 | 0.088 | 0.056 |
| 84 | 80 | 55 | 44 | 1 | 3 | 1 | 6 | 22 | 15 | 18 | 22 | 0.875 | 0.500 | 0.708 | 0.667 | 0.068 | 0.102 | 0.093 | 0.096 |
| 88 | 84 | 58 | 56 | 1 | 6 | 1 | 1 | 21 | 12 | 17 | 16 | 0.833 | 0.250 | 0.667 | 0.625 | 0.076 | 0.088 | 0.096 | 0.099 |
| 98 | 92 | 60 | 60 | 1 | 1 | 1 | 2 | 20 | 6 | 16 | 15 | 0.792 | 0.208 | 0.625 | 0.542 | 0.083 | 0.083 | 0.099 | 0.102 |
| 99 | 99 | 62 | 62 | 5 | 1 | 6 | 3 | 19 | 5 | 15 | 13 | 0.583 | 0.167 | 0.375 | 0.417 | 0.101 | 0.076 | 0.099 | 0.101 |
| 106 | 100 | 80 | 64 | 1 | 1 | 2 | 1 | 14 | 4 | 9 | 10 | 0.542 | 0.125 | 0.292 | 0.375 | 0.102 | 0.068 | 0.093 | 0.099 |
| 113 | 105 | 84 | 67 | 1 | 1 | 3 | 3 | 13 | 3 | 7 | 9 | 0.500 | 0.083 | 0.167 | 0.250 | 0.102 | 0.056 | 0.076 | 0.088 |
| 115 | 114 | 91 | 69 | 4 | 2 | 1 | 2 | 12 | 2 | 4 | 6 | 0.333 | 0.000 | 0.125 | 0.167 | 0.096 | 0.000 | 0.068 | 0.076 |
| 120 | 93 | 71 | 2 | 3 | 1 | 8 | 3 | 4 | 0.250 | 0.000 | 0.125 | 0.088 | 0.000 | 0.068 | |||||
| 123 | 72 | 1 | 2 | 6 | 3 | 0.208 | 0.042 | 0.083 | 0.041 | ||||||||||
| 128 | 84 | 2 | 1 | 5 | 1 | 0.125 | 0.000 | 0.068 | 0.000 | ||||||||||
| 133 | 1 | 3 | 0.083 | 0.056 | |||||||||||||||
| 150 | 1 | 2 | 0.042 | 0.041 | |||||||||||||||
| 169 | 1 | 1 | 0.000 | 0.000 | |||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamido, S.A.; Ebel, R.C.; Morgan, K.T. Interaction of Huanglongbing and Foliar Applications of Copper on Water Relations of Citrus sinensis cv. Valencia. Plants 2019, 8, 298. https://doi.org/10.3390/plants8090298
Hamido SA, Ebel RC, Morgan KT. Interaction of Huanglongbing and Foliar Applications of Copper on Water Relations of Citrus sinensis cv. Valencia. Plants. 2019; 8(9):298. https://doi.org/10.3390/plants8090298
Chicago/Turabian StyleHamido, Said A., Robert C. Ebel, and Kelly T. Morgan. 2019. "Interaction of Huanglongbing and Foliar Applications of Copper on Water Relations of Citrus sinensis cv. Valencia" Plants 8, no. 9: 298. https://doi.org/10.3390/plants8090298
APA StyleHamido, S. A., Ebel, R. C., & Morgan, K. T. (2019). Interaction of Huanglongbing and Foliar Applications of Copper on Water Relations of Citrus sinensis cv. Valencia. Plants, 8(9), 298. https://doi.org/10.3390/plants8090298





