Seedling Characteristics of Three Oily Species before and after Root Pruning and Transplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Environmental Conditions and Substratum
2.3. Germination and Survival
2.4. Aboveground Growth before Transplant
2.5. Leaf Thickness and Weight Measurements
2.6. First Uprooting and Belowground Measurements
2.7. Root Pruning and Transplant
2.8. Aboveground Measurements after Transplanting
2.9. Second Uprooting and Belowground Measurements
2.9.1. Root Distribution Models
2.9.2. Biomass Estimation by Allometric Relationships
2.10. Statistical Analysis
3. Results
3.1. Germination and Survival
3.2. First Phase of Seedling Growth
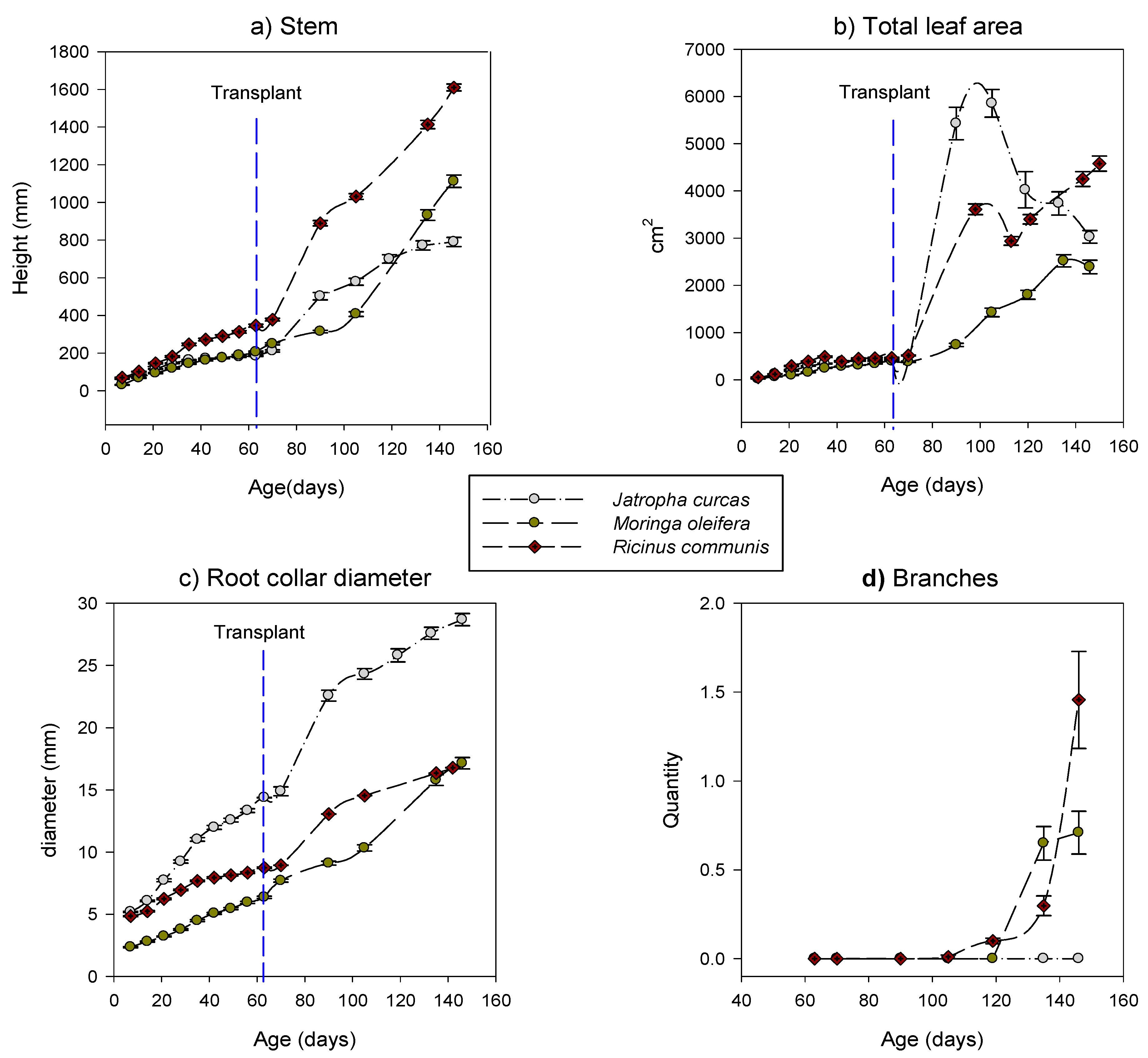
3.2.1. Aboveground Measurements
3.2.2. Leaf Measurements
3.3. Relationships between Above- and Belowground in 63-Day-Old Seedlings
3.4. Second Phase: Plant Performance after Transplanting
3.4.1. Aboveground Measurements
3.4.2. Above- and Belowground Relationships
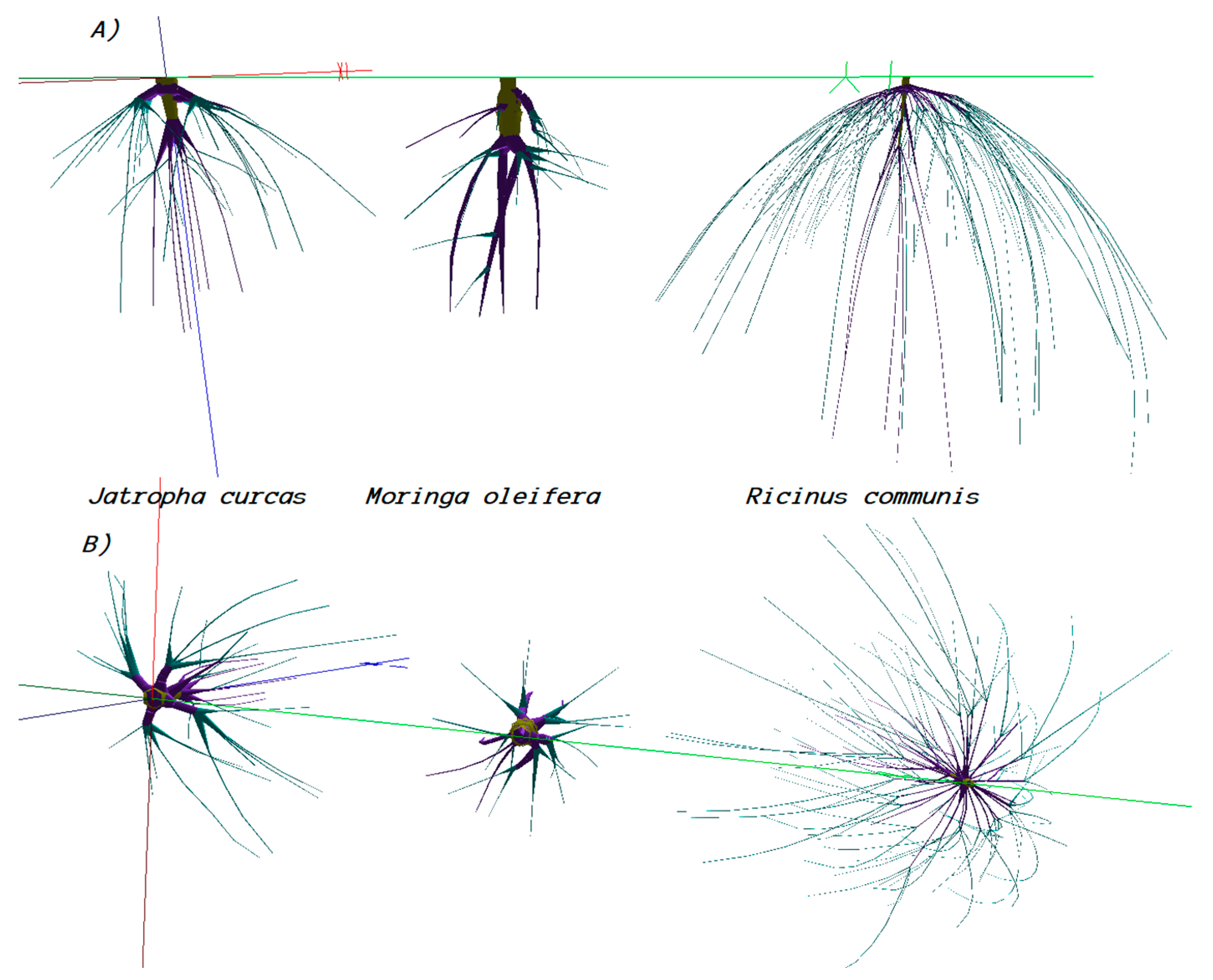
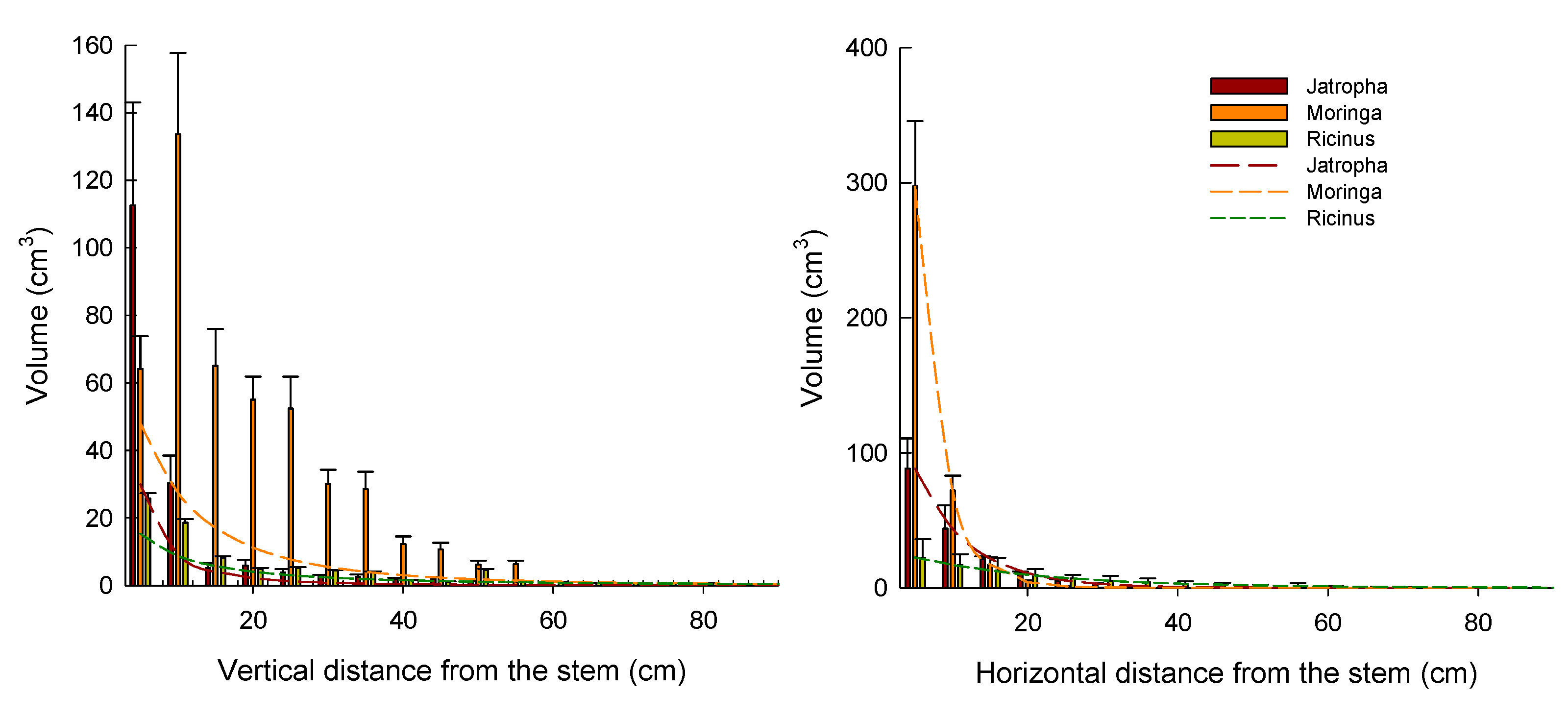
3.4.3. Root Distributions and Their Models
3.5. Plant Fresh and Dry Weight
3.6. Allometric Relationships for Above- and Belowground Biomass Estimation
4. Discussion
4.1. Germination and Survival
4.2. First Phase of Seedling Growth
4.2.1. Stem Growth Rates
4.2.2. Leaf Production
4.2.3. Root Systems and Their Relationships with Aboveground Growth Patterns
4.3. Second Phase: Plant Performance after Transplanting
4.3.1. Survival Rate and Aboveground Growth
4.3.2. Plant Defoliation
4.3.3. Branching and Flowering
4.4. Second Phase: Belowground Development
4.4.1. Root Distribution and Root Distribution Models
4.4.2. Root Trays and Their Implications for Aboveground Development
4.4.3. Above- and Belowground Biomass Estimation by Allometric Data
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velázquez-Zavala, M.; Peón-Escalante, I.E.; Zepeda-Bautista, R.; Jiménez-Arellanes, M.A. Moringa (Moringa oleifera Lam.): Usos potenciales en la agricultura, industria y medicina. Rev. Chapingo Ser. Hortic. 2016, 22, 95–116. [Google Scholar] [CrossRef]
- Clixoo, S. Comprehensive Castor Oil Report; Energy Alternatives India: Gujarat, India, 2016; Available online: http://www.eai.in/ref/reports/castor_oil_comprehensive_report.html (accessed on 10 June 2019).
- Becker, K.; Makkar, H.P.S. Jatropha curcas: A potential source for tomorrow’s oil and biodiesel. Lipid Technol. 2008, 20, 104–107. [Google Scholar] [CrossRef]
- Parrotta, J. Moringa oleifera. In Enzyklopädie der Holzgewächse; Roloff, A., Weisgerber, H., Lang, U., Stimm, B., Eds.; Wiley-Vch: Weinheim, Germany, 2009; pp. 1–8. ISBN 9783527321414. [Google Scholar]
- Solís, B.J.L.; Zamarripa, C.A.; González, A.A.; Rico, P.H.R.; Tapia, V.L.M.; Teniente, O.R.; Zacarías, G.M.; Cruz, R.J.R.; Hernández, M.M. Guía Técnica para la Producción de Higuerilla (Ricinus communis L.) en Chiapas, 1st ed.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Tuxtla, Mexico, 2011.
- Achten, W.M.J.; Nielsen, L.R.; Aerts, R.; Lengkeek, A.G.; Kjaer, E.D.; Trabucco, A.; Hansen, J.K.; Maes, W.H.; Graudal, L.; Akinnifesi, F.K.; et al. Towards domestication of Jatropha curcas. Biofuels 2010, 1, 91–107. [Google Scholar] [CrossRef]
- Valdés Rodríguez, O.A.; Pérez Vázquez, A.; García Pérez, E.; Inurreta Aguirre, H.D. Condiciones agroecológicas de procedencias nativas de Jatropha curcas L. In Energía Alterna y Biocombustibles; Perez Vazquez, A., Garcia Perez, E., Eds.; Colegio de Postgraduados: Montecillo, Mexico, 2013; pp. 143–152. ISBN 9786077151043. [Google Scholar]
- SDA, (Secretaría de Desarrollo Agropecuario). Manual de Reforestación, 1st ed.; SDA, (Secretaría de Desarrollo Agropecuario), Ed.; Gobierno del Estado de México: Toluca, Mexico, 2006; ISBN 8495458233.
- Struve, D.K. Tree establishment: A review of some of the factors affecting transplant survival and establishment. Arboric. Urban. 2009, 35, 10–13. [Google Scholar]
- Gao, K.; Zhu, T.; Wang, L.; Gao, Y. Effects of root pruning radius and time on yield of tuberous roots and resource allocation in a crop of Helianthus tuberosus L. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.R.; Morgenroth, J.; Koeser, A.K. The effects of root pruning on growth and physiology of two Acer species in New Zealand. Urban For. Urban Green. 2018, 38, 64–73. [Google Scholar] [CrossRef]
- Bailey, P.H.J.; Currey, J.D.; Fitter, A.H. The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. J. Exp. Bot. 2002. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Bouma, T.J.; Eissenstat, D.M. Linking root traits to potential growth rate in six temperate tree species. Oecologia 2002, 132, 34–43. [Google Scholar] [CrossRef]
- Valdés-Rodríguez, O.A.; Giadrossich, F.; Pérez-Vázquez, A.; Moreno-Seceña, J.C. Above- and below-ground biomass and allometry of Moringa oleifera and Ricinus communis grown in a compacted clayey soil. Flora 2018, 241, 35–45. [Google Scholar] [CrossRef]
- Smith, G.D.; Jangawad, L.; Grivastava, K. Castor roots in a vertic inceptisol. In Development in Agricultural and Management Forest Ecology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 24, pp. 533–543. [Google Scholar]
- Valdés-Rodríguez, O.A.; Sánchez-Sánchez, O.; Pérez-Vázquez, A.; Caplan, J.S.; Danjon, F. Jatropha curcas L. root structure and growth in diverse soils. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bayala, R.; Hernandez, R.R.; Diédhiou, I.; Mbengue, A.A.; Diallo, D.; Sène, A.; Diédhiou, P.M.; Diémé, R. Allometric equations and carbon stocks in tree biomass of Jatropha curcas L. in Senegal’s Peanut Basin. Glob. Ecol. Conserv. 2016, 9, 61–69. [Google Scholar]
- Muhl, Q.E. Seed Germination, Tree Growth and Flowering Responses of Moringa oleifera Lam. (Horseradish tree) to Temperature. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2009. [Google Scholar]
- Ghestem, M.; Veylon, G.; Bernard, A.; Vanel, Q.; Stokes, A. Influence of plant root system morphology and architectural traits on soil shear resistance. Plant Soil 2014, 377, 43–61. [Google Scholar] [CrossRef]
- Reubens, B.; Achten, W.M.J.; Maes, W.H.; Danjon, F.; Aerts, R.; Poesen, J.; Muys, B. More than biofuel? Jatropha curcas root system symmetry and potential for soil erosion control. J. Arid Environ. 2011, 75, 201–205. [Google Scholar]
- Liv Soares, S.; Silva Do Vale, L.; De Macedo Beltrao, N.E. A simple Method for Measurement of Jatropha curcas Leaf Area. Rev. Bras. Oleag. Fibr. 2007, 11, 9–14. [Google Scholar]
- Jain, T.C.; Misra, D.K. Leaf area estimation by linear measurements in Ricinus communis. Nature 1966, 212, 741–742. [Google Scholar] [CrossRef]
- GFORGE-INRIA. Openalea-OpenAlea. Available online: http://openalea.gforge.inria.fr/dokuwiki/doku.php?id=openalea (accessed on 2 March 2016).
- Ali, A.; Xu, M.S.; Zhao, Y.T.; Zhang, Q.Q.; Zhou, L.L.; Yang, X.D.; Yan, E.R. Allometric biomass equations for shrub and small tree species in subtropical China. Silva Fenn. 2015, 49, 1–10. [Google Scholar] [CrossRef]
- Achten, W.M.J.M.J.; Maes, W.H.H.; Reubens, B.; Mathijs, E.; Singh, V.P.P.; Verchot, L.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bioenergy 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Sinacore, K.; Hall, J.S.; Potvin, C.; Royo, A.A.; Ducey, M.J.; Ashton, M.S. Unearthing the hidden world of roots: Root biomass and architecture differ among species within the same guild. PLoS ONE 2017, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Valdés-rodríguez, O.A.; Pérez-vázquez, A.; Muñoz-Gamboa, C. Efecto de peso y talla de semilla sobre plántulas de Moringa y Ricinus. Rev. Mex. Cienc. Agrícolas 2018, 9, 1411–1422. [Google Scholar] [CrossRef]
- Valdés Rodríguez, O.A.; Palacios Wassenaar, O.M.; Ruíz, R.; Pérez Vázquez, A. Potencial de la asociación Moringa y Ricinus en el subtrópico veracruzano. Rev. Mex. Cienc. Agrícolas 2014, 9, 1673–1686. [Google Scholar] [CrossRef]
- Valdés-Rodríguez, O.A.; Sánchez-Sánchez, O.; Pérez-Vázquez, A. Effects of soil texture on germination and survival of non-toxic Jatropha curcas seeds. Biomass Bioenergy 2013, 48, 167–170. [Google Scholar] [CrossRef]
- Valdés Rodríguez, O.A. Estudio de Jatropha curcas L. no tóxica: Semillas, Plántulas y Primeros estadíos del Sistema de raíces. Ph.D. Thesis, Universidad Veracruzana, Veracruz, Mexico, 2012. [Google Scholar]
- Ribeiro, P.R.; Fernandez, L.G.; De Castro, R.D.; Ligterink, W.; Hilhorst, H.W.M. Physiological and biochemical responses of Ricinus communis seedlings to different temperatures: A metabolomics approach. BMC Plant Biol. 2014, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Fotouo-M, H.; Du Toit, E.S.; Robbertse, P.J. Germination and ultrastructural studies of seeds produced by a fast-growing, drought-resistant tree: Implications for its domestication and seed storage. AoB Plants 2015, 7, 1–12. [Google Scholar]
- Díaz-Chuquizuta, P.; Valdés-Rodríguez, O.A.; Tello-Salas, C. Germination responses in physic nut (Jatropha curcas L.) seeds to pregerminative treatments Respuestas de germinación en semillas de piñón (Jatropha curcas L.) a tratamientos pregerminativos. Rev. Chapingo Ser. Hortic. 2017, 23, 89–95. [Google Scholar] [CrossRef]
- Van Gelder, H.A.; Poorter, L.; Sterck, F.J. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phytol. 2004, 171, 367–378. [Google Scholar] [CrossRef]
- Pérez-Vázquez, A.; Hernández-Salinas, G.; Ávila-Reséndiz, C.; Valdés-Rodríguez, O.A.; Gallardo-López, F.; García-Pérez, E.; Ruiz-Rosado, O. Effect of the soil water content on Jatropha seedlings in a tropical climate. Int. Agrophys. 2013, 27, 351–357. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W.; Xu, W.; Wei, Y.; Wang, R. Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves. Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Azcón-Bieto, J.; Talón, M. Fundamentos de Fisiología Vegetal, 2nd ed.; McGraw-Hill Interamericana de España: Barcelona, Spain, 2013; Volume 1, ISBN 9788448192938. [Google Scholar]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Kader, J.C., Michel, D., Eds.; Academic Press: London, UK, 2010; Volume 55, pp. 179–225. ISBN 9780123808684. [Google Scholar]
- Izumi, Y.; Iijima, M. Fractal and Multifractal Analysis of Cassava Root System Grown by the Root-Box Method. Plant Prod. Sci. 2002, 5, 146–151. [Google Scholar] [CrossRef] [Green Version]
- González-gonzález, C.E.; Crespo-lópez, G.J. Response of Moringa oleifera Lam to fertilization strategies on lixiviated Ferralitic Red soil. Pastos y Forrajes 2016, 39, 173–177. [Google Scholar]
- Reddy, K.R.; Matcha, S.K. Quantifying nitrogen effects on castor bean (Ricinus communis L.) development, growth, and photosynthesis. Ind. Crops Prod. 2010, 31, 185–191. [Google Scholar] [CrossRef]
- Valdés-Rodríguez, O.A.; Sánchez-Sánchez, O.; Pérez-Vázquez, A.; Ruiz-Bello, R. Soil texture effects on the development of Jatropha seedlings-Mexican variety “piñón manso”. Biomass Bioenergy 2011, 35, 3529–3536. [Google Scholar] [CrossRef]
- Schurr, U.; Heckenberger, U.; Herdel, K.; Walter, A.; Feil, R. Leaf development in Ricinus communis during drought stress: Dynamics of growth processes, of cellular structure and of sink-source transition. J. Exp. Bot. 2000, 51, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.H.; Achten, W.M.J.; Reubens, B.; Raes, D.; Samson, R.; Muys, B. Plant-water relationships and growth strategies of Jatropha curcas L. seedlings under different levels of drought stress. J. Arid Environ. 2009, 73, 877–884. [Google Scholar] [CrossRef]
- Noda-Leyva, Y.; Pérez-Vázquez, A.; Valdés-Rodríguez, O.A. Establecimiento de tres especies de oleaginosas bajo asociación. Agron. Mesoam. 2015, 26, 323–332. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Zaman-Allah, M.; Marimuthu, S.; Wani, S.P.; Kesava Rao, A.V.R. Root growth in Jatropha and its implications for drought adaptation. Biomass Bioenergy 2012, 39, 247–252. [Google Scholar] [CrossRef]


| Species | Moringa | Ricinus | Jatropha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Exp1 | Exp2 | This experiment | Exp1 | Exp2 | This experiment | Exp3 | Exp3 | This experiment |
| Germination percentage | 95.0 | 60.0 | 86.0 | 96.0 | 80.0 | 100.0 | 77.0 | 25.0 | 97.0 |
| Mean germination time (days) | 5.9 | 13.0 | 14.1 | 4.7 | 10.0 | 7.5 | 6.2 | 10.8 | 9.1 |
| Substratum | Sand | Clay | S-C (5:1) † | Sand | Clay | S-C (5:1) † | Sand | Clay | S-C (5:1) † |
| Average Temperature °C) | 27.0 | 25.5 | 23.2 | 28.7 | 25.5 | 22.2 | 27.4 | 27.4 | 23.2 |
| Humidity (%) | 88.7 | 78.7 | 73.9 | 88.5 | 78.7 | 71.3 | 73.8 | 73.8 | 73.3 |
| Variable | Moringa | Ricinus | Jatropha | |||
|---|---|---|---|---|---|---|
| Exp2 | This experiment | Exp2 | This experiment | Exp1 | This experiment | |
| Plant age (days) | 45 | 63 | 45 | 63 | 45 | 63 |
| Height | 4.66 ± 0.09 | 3.30 ± 0.07 | 5.08 ± 0.09 | 5.49 ± 0.12 | 4.22 ± 0.12 | 2.93 ± 0.05 |
| Root collar diameter | 0.09 ± 0.00 | 0.10 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.00 | 0.19 ± 0.02 | 0.23 ± 0.00 |
| Leaf production | 0.11 ± 0.00 | 0.16 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.11 ± 0.01 |
| Substratum | Sand | S-C (5:1) † | Sand | S-C (5:1) | Sand | S-C (5:1) |
| Temperature (°C) | 26.01 ± 1.17 | 24.69 ± 2.27 | 26.01 ± 1.17 | 24.69 ± 2.27 | 24.69 ± 1.45 | 24.69 ± 2.27 |
| Humidity (%) | 88.54 ±3.03 | 75.00 ± 5.90 | 88.54 ± 3.03 | 75.00 ± 5.90 | 75.00 ± 6.61 | 75.00 ± 5.90 |
| Parameter (n = 100) | Moringa | Ricinus | Jatropha |
|---|---|---|---|
| Tap root length (mm) | 173.59 ± 4.20 c | 411.14 ± 5.92 a | 280.40 ± 6.14 b |
| Main lateral roots length (mm) | 196.25 ± 4.79 c | 351.60 ± 4.17 a | 289.00 ± 5.24 b |
| Maximum tap root diameter (mm) | 18.5 ± 0.35 a | 6.09 ± 0.12 c | 9.71 ± 0.29 b |
| Main lateral roots diameter (mm) | 1.85 ± 0.13 b | 1.05 ± 0.02 c | 3.97 ± 0.17 a |
| Number of lateral roots | 1.09 ± 0.03 c | 25.18 ± 0.48 a | 5.15 ± 0.28 b |
| Tap root + lateral roots volume (cm3) | 23.82 ± 1.69 a | 11.11 ± 0.29 b | 23.33 ± 1.52 a |
| Tap root diameter/Main lateral roots diameter | 12.53 ± 0.54 a | 5.90 ± 0.13 b | 2.5 ± 0.10 c |
| Tap root volume/laterals root volume | 19.88 ± 1.14 a | 1.29 ± 0.07 b | 1.40 ± 0.14 b |
| Species (n = 100) | Parameter | Height | RCD | Number of Leaves | Leaf Area of the Youngest Fully Mature Leaf |
|---|---|---|---|---|---|
| Jatropha | Tap root length | 0.48 ** | 0.51 ** | 0.34 * | 0.22 |
| Laterals length | 0.49 ** | 0.52 ** | 0.24 | 0.23 | |
| Tap root diameter | 0.44 ** | 0.54 ** | 0.10 | 0.26 | |
| Lateral roots diameter | 0.40 ** | 0.57 ** | 0.18 | 0.12 | |
| Number of Laterals | 0.16 | 0.15 | −0.11 | −0.03 | |
| Root volume | 0.62 ** | 0.64 ** | 0.17 | 0.21 | |
| Moringa | Tap root length | 0.18 | 0.01 | −0.06 | 0.16 |
| Lateral roots length | 0.31 ** | 0.09 | −0.11 | 0.31 ** | |
| Tap root diameter | 0.29 ** | 0.44 ** | 0.02 | 0.14 | |
| Lateral roots diameter | 0.33 ** | 0.40 ** | 0.03 | 0.35 ** | |
| Number of lateral roots | 0.10 | 0.07 | −0.06 | 0.13 | |
| Root volume | 0.66 ** | 0.43 ** | −0.06 | 0.59 ** | |
| Ricinus | Tap root length | 0.30 * | 0.40 ** | 0.06 | 0.08 |
| Lateral roots length | 0.23 * | 0.39 ** | 0.16 | 0.11 | |
| Tap root diameter | 0.29 ** | 0.42 ** | −0.07 | 0.05 | |
| Lateral roots diameter | −0.09 | −0.11 | −0.17 | 0.20* | |
| Number of lateral roots | 0.26 ** | 0.08 | 0.07 | −0.16 | |
| Root volume | 0.37 ** | 0.40 ** | −0.07 | 0.70 ** |
| Parameter | Moringa1 | Ricinus1 | Jatropha2 |
|---|---|---|---|
| Plant height (mm) | 321.4 ± 158.0 | 450.0 ± 249.7 | 209.4 ± 26.6 |
| RCD | 9.7 ± 4.1 | 12.27 ± 7.0 | 12.1 ± 1.6 |
| Number of leaves | NA | NA | 0.5 ± 0.7 |
| Area of average leaf | NA | NA | 4.84 ± 0.21 |
| Number of branches | NA | NA | 0.0 ± 0.0 |
| Plant age (days) | 103 | 103 | 110 |
| Soil type | Clay | Clay | Sandy |
| Growth conditions | Open field | Open field | Plastic bag (25 × 60 cm) |
| Temperature °C | 24.2 ± 1.5 | 24.2 ± 1.5 | 24.3 ± 2.7 |
| Humidity (%) | 78.4 ± 5.9 | 78.4 ± 5.9 | 74.3 ± 12.6 |
| Dimension (n = 20) | Moringa | Ricinus | Jatropha |
|---|---|---|---|
| Tap root length at trimmed tip | 89.69 ± 3.42 b | 123.84 ± 10.63 a | 64.38 ± 2.67 c |
| Tap root total length | 409.25 ± 12.82 b | 831.9 ± 32.36 a | 478.50 ± 14.10 b |
| Tap root diameter | 58.74 ± 1.03 a | 10.01 ± 0.42 c | 28.15 ± 0.76 b |
| Tap root diameter at trimmed tip | 41.25 ± 1.96 a | 5.35 ± 0.34 c | 21.61 ± 1.10 b |
| Number of roots from tap root | 3.44 ± 0.21 c | 4.60 ± 0.40 b | 6.10 ± 0.32 a |
| Tap root inclination (θ roots from tap) | −60.20 ± 12.19 b | −81.00 ± 1.81 a | −83.71 ± 1.09 a |
| Number of main lateral roots | 2.00 ± 0.24 c | 33.37 ± 2.56 a | 5.60 ± 0.15 b |
| Main lateral roots total length | 395.19 ± 12.53 b | 758.58 ± 33.49 a | 484.55 ± 22.13 b |
| Main lateral roots diameter | 22.40 ± 1.16 a | 2.45 ± 0.12 c | 16.74 ± 0.78 b |
| Main lateral roots diameter at trimmed tip | 16.78 ± 1.05 a | 1.03 ± 0.04 c | 10.64 ± 0.53 b |
| Lateral root inclination (θ laterals) | −53.33 ± 4.90 a | −24.25 ± 2.99 b | −31.50 ± 5.34 b |
| Tap root length/stem length | 0.32 ± 0.01 b | 0.66 ± 0.02 a | 0.60 ± 0.01 a |
| Total tap root length/total lateral lengths | 1.04 ± 0.02 a | 1.11 ± 0.04 a | 1.00 ± 0.03 a |
| Root collar diameter/tap root diameter | 0.66 ± 0.02 b | 1.63 ± 0.06 a | 1.41 ± 0.03 a |
| Tap root diameter/main lateral roots diameter | 2.84 ± 0.13 a | 4.28 ± 0.27 a | 1.72 ± 0.05 b |
| Tap root + main lateral roots volume (cm3) | 275.69 ± 19.22 a | 76.89 ± 5.97 c | 218.24 ± 27.50 b |
| Species (n = 20) | Parameter | Height | RCD | Number of Leaves | Leaf Area | Number of Branches |
|---|---|---|---|---|---|---|
| Jatropha | Tap root length | 0.61 ** | 0.58 ** | 0.48 * | 0.32 * | -- |
| Lateral roots length | 0.42 * | 0.65 ** | 0.44 * | 0.50 ** | -- | |
| Tap root diameter | 0.70 ** | 0.81 ** | 0.73 ** | 0.12 | -- | |
| Lateral roots diameter | 0.50 * | 0.63 ** | 0.61 ** | 0.05 | -- | |
| Number of Laterals | 0.46 * | 0.44 * | 0.35 * | 0.37 * | -- | |
| Root volume | 0.67 ** | 0.84 | 0.69 ** | 0.25 | -- | |
| Moringa | Tap root length | 0.55 ** | 0.66 ** | 0.44 * | 0.29 | 0.18 |
| Lateral roots length | 0.40 * | 0.53 ** | 0.20 | 0.26 | 0.05 | |
| Tap root diameter | 0.76 ** | 0.80 ** | 0.55 ** | 0.42 * | 0.01 | |
| Lateral roots diameter | 0.13 | 0.15 | 0.27 | −0.21 | 0.21 | |
| Number of Laterals | 0.66 ** | 0.76 ** | 0.58 ** | 0.21 | 0.20 | |
| Root volume | 0.76 ** | 0.76 ** | 0.59 ** | 0.32 | 0.12 | |
| Ricinus | Tap root length | 0.57 ** | 0.23 | 0.25 | −0.20 | 0.09 |
| Lateral roots length | 0.30 * | 0.22 | 0.30 * | −0.13 | 0.12 | |
| Tap root diameter | 0.03 | 0.51 ** | 0.16 | 0.00 | 0.26* | |
| Lateral roots diameter | 0.34 ** | 0.21 | 0.12 | 0.06 | −0.07 | |
| Number of lateral roots | 0.06 | −0.12 | −0.08 | 0.17 | −0.02 | |
| Root volume | 0.43 ** | 0.42 ** | 0.24 | 0.15 | 0.10 |
| Parameters and Model | Jatropha | Moringa | Ricinus | |
|---|---|---|---|---|
| Vertical distribution | Vertical root volume (cm3) at a distance D | |||
| SS | 37.64 | 3195.32 | 26.40 | |
| r2 | 0.99 | 0.85 | 0.97 | |
| Horizontal distribution | Horizontal root volume (cm3) at a distance D | |||
| SS | 710.21 | 1.38 | 26.35 | |
| r2 | 0.93 | 0.99 | 0.96 | |
| Parameter (n = 20) | Moringa | Ricinus | Jatropha |
|---|---|---|---|
| Leaf fresh weight | 63.72 ± 7.70 b | 74.83 ± 3.41 a | 75.10 ± 1.85 a |
| Stem fresh weight | 127.19 ± 12.76 b | 136.00 ± 5.06 b | 187.91 ± 5.78 a |
| Root fresh weight | 136.78 ± 11.85 a | 75.16 ± 3.87 b | 59.03 ± 1.83 c |
| Above/below ground fresh weight | 1.42 ± 0.07 c | 2.92 ± 0.37 b | 4.84 ± 0.21 a |
| Leaf dry weight | 12.07 ± 1.40 b | 9.37 ± 0.41 c | 14.10 ± 0.12 a |
| Stem dry weight | 26.48 ± 2.12 b | 20.17 ± 0.77 b | 81.67 ± 3.29 a |
| Root dry weight | 19.69 ± 1.89 a | 7.54 ± 0.37 c | 12.54 ± 0.40 b |
| Above/below ground dry weight | 2.14 ± 0.15 c | 4.01 ± 0.15 b | 7.65 ± 0.18 a |
| Biomass (mg) | Moringa | Ricinus | Jatropha |
|---|---|---|---|
| L = Leaf | |||
| Variables | HNA = H N A | HNA = H N A | NA = N A |
| Model | L = −1.8 + 10.3ln (HNA) | L = 15.3(HNA)0.6 | L = 17.7 + 3.8ln (NA) |
| Type | Logarithm | Power | Logarithm |
| r2 | 0.83 | 0.67 | 0.57 |
| SS | 106.6 | 47.8 | 9.8 |
| S = Stem | |||
| Variables | HNA = H N A | DH = D H | D |
| Model | S = 5.4 + 15.8ln (HNA) | S = 540(DH)0.84 | S = 267.1 + 59.6ln(D) |
| Type | Logarithm | Power | Logarithm |
| r2 | 0.87 | 0.70 | 0.54 |
| SS | 187.5 | 138.9 | 1733.2 |
| R = Root | |||
| Variables | HNA = H N A | DH = D H | DNA = D N A |
| Model | R = 2.9 + 12.7ln(HNA) | R = 182.6(DH)0.81 | R = 67.1DNA0.40 |
| Type | Logarithm | Exponential | Power |
| r2 | 0.71 | 0.41 | 0.57 |
| SS | 328.6 | 61.9 | 36.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Rodríguez, O.A.; Pérez-Vázquez, A. Seedling Characteristics of Three Oily Species before and after Root Pruning and Transplant. Plants 2019, 8, 258. https://doi.org/10.3390/plants8080258
Valdés-Rodríguez OA, Pérez-Vázquez A. Seedling Characteristics of Three Oily Species before and after Root Pruning and Transplant. Plants. 2019; 8(8):258. https://doi.org/10.3390/plants8080258
Chicago/Turabian StyleValdés-Rodríguez, Ofelia Andrea, and Arturo Pérez-Vázquez. 2019. "Seedling Characteristics of Three Oily Species before and after Root Pruning and Transplant" Plants 8, no. 8: 258. https://doi.org/10.3390/plants8080258
APA StyleValdés-Rodríguez, O. A., & Pérez-Vázquez, A. (2019). Seedling Characteristics of Three Oily Species before and after Root Pruning and Transplant. Plants, 8(8), 258. https://doi.org/10.3390/plants8080258




