What Makes Adventitious Roots?
Abstract
1. What Are Adventitious Roots?
2. What Are the Roles of Adventitious Roots?
3. Where Are Adventitious Roots Formed?
4. When Are the Adventitious Roots Produced?
5. What Resource Availabilities Influence the Formation of Adventitious Roots?
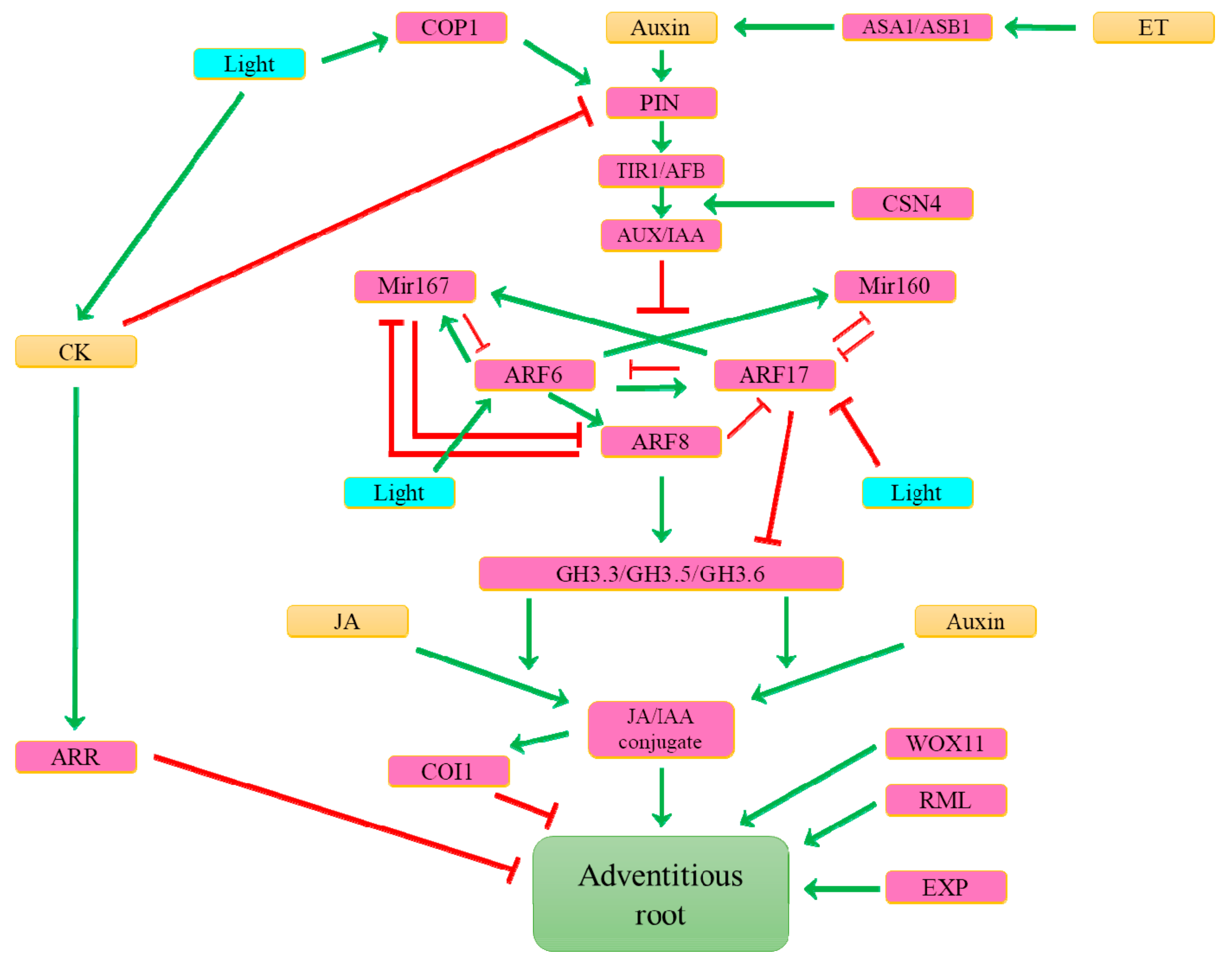
6. What Hormonal Signals Control the Formation of Adventitious Roots?
7. What Are the Genetic Determinants Controlling Constitutive Adventitious Root Formation in Rice?
8. What Are the Main Determinants Involved in Inducible Adventitious Root Formation?
9. What Are the Mechanisms Controlling Adventitious Root Emergence?
10. What Represses Adventitious Root Formation?
11. What Are the Main Perspectives of Research on Adventitious Rooting?
Author Contributions
Funding
Conflicts of Interest
References
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious Roots and Lateral Roots: Similarities and Differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Rebouillat, J.; Dievart, A.; Verdeil, J.L.; Escoute, J.; Giese, G.; Breitler, J.C.; Gantet, P.; Espeout, S.; Guiderdoni, E.; Périn, C. Molecular Genetics of Rice Root Development. Rice 2009, 2, 15–34. [Google Scholar] [CrossRef]
- Coudert, Y.; Le, T.V.A.; Gantet, P. Rice: A Model Plant to Decipher the Hidden Origin of Adventitious Roots. Plant Roots Hidden Half 2013, 4, 157–166. [Google Scholar]
- Coudert, Y.; Périn, C.; Courtois, B.; Khong, N.G.; Gantet, P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Woll, K.; Sauer, M.; Dembinsky, D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann. Bot. 2004, 93, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Bodley, J.H.; Benson, F.C. Stilt-Root Walking by an Iriateoid Palm in the Peruvian Amazon. Biotropica 1980, 12, 67–71. [Google Scholar] [CrossRef]
- Goldsmith, G.R.; Zahawi, R.A. The function of stilt roots in the growth strategy of Socratea exorrhiza (Arecaceae) at two neotropical sites. Rev. Biol. Trop. 2007, 55, 787–793. [Google Scholar] [CrossRef][Green Version]
- Sukumar, P.; Maloney, G.S.; Muday, G.K. Localized Induction of the ATP-Binding Cassette B19 Auxin Transporter Enhances Adventitious Root Formation in Arabidopsis. Plant Physiol. 2013, 162, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Correa, L.; Troleis, J.; Mastroberti, A.A.; Mariath, J.E.A.; Fett-Neto, A.G. Distinct modes of adventitious rooting in Arabidopsis thaliana. Plant Biol. 2012, 14, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl adventitious root organogenesis differs from lateral root development. Front. Plant Sci. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voss, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching Out in Roots: Uncovering Form, Function, and Regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef]
- Bustillo-Avendaño, E.; Ibáñez, S.; Sanz, O.; Sousa Barros, J.A.; Gude, I.; Perianez-Rodriguez, J.; Micol, J.L.; Del Pozo, J.C.; Moreno-Risueno, M.A.; Pérez-Pérez, J.M. Regulation of Hormonal Control, Cell Reprogramming, and Patterning during De Novo Root Organogenesis. Plant Physiol. 2018, 176, 1709–1727. [Google Scholar] [CrossRef]
- Morita, M.T.; Saito, C.; Nakano, A.; Tasaka, M. Endodermal-amyloplast less 1 is a novel allele of Short-Root. Adv. Sp. Res. 2007, 39, 1127–1133. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Tian, H.; Ding, Z. WOX5 is Shining in the Root Stem Cell Niche. Trends Plant Sci. 2015, 20, 601–603. [Google Scholar] [CrossRef]
- Bagchi, R.; Melnyk, C.W.; Christ, G.; Winkler, M.; Kirchsteiner, K.; Salehin, M.; Mergner, J.; Niemeyer, M.; Schwechheimer, C.; Calderón Villalobos, L.I.A.; et al. The Arabidopsis ALF4 protein is a regulator of SCF E3 ligases. EMBO J. 2018, 37, 255–268. [Google Scholar] [CrossRef]
- Kamiya, N.; Nagasaki, H.; Morikami, A.; Sato, Y.; Matsuoka, M. Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J. 2003, 35, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Sakamoto, T.; Ueguchi-tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an Auxin Response Factor in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.I.; Nonomura, K.I.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Wysocka-Diller, J.; Kato, T.; Fujisawa, H.; Benfey, P.N.; Tasaka, M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998, 14, 425–430. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.-J.; van der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Li, S.W.; Xue, L.; Xu, S.; Feng, H.; An, L. Mediators, genes and signaling in adventitious rooting. Bot. Rev. 2009, 75, 230–247. [Google Scholar] [CrossRef]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Greenwood, M.S.; Cui, X.; Xu, F. Response to auxin changes during maturation-related loss of adventitious rooting competence in loblolly pine (Pinus taeda) stem cuttings. Physiol. Plant. 2001, 111, 373–380. [Google Scholar] [CrossRef]
- Naija, S.; Elloumi, N.; Jbir, N.; Ammar, S.; Kevers, C. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured In Vitro. C. R. Biol. 2008, 331, 518–525. [Google Scholar] [CrossRef]
- Agulló-Antón, M.Á.; Sánchez-Bravo, J.; Acosta, M.; Druege, U. Auxins or Sugars: What Makes the Difference in the Adventitious Rooting of Stored Carnation Cuttings? J. Plant Growth Regul. 2011, 30, 100–113. [Google Scholar] [CrossRef]
- Rigal, A.; Yordanov, Y.S.; Perrone, I.; Karlberg, A.; Tisserant, E.; Bellini, C.; Busov, V.B.; Martin, F.; Kohler, A.; Bhalerao, R.; et al. The Aintegumenta Like1 homeotic transcription factor PtAIL1 controls the formation of adventitious root primordia in poplar. Plant Physiol. 2012, 160, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Ghorbani Javid, M.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kareem, A.; Radhakrishnan, D.; Sondhi, Y.; Aiyaz, M.; Roy, M.V.; Sugimoto, K.; Prasad, K. De novo assembly of plant body plan: A step ahead of Deadpool. Regeneration 2016, 3, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Morita, S.; Baba, T. Shoot and Root Development in Rice Related to the Phyllochron. Crop Sci. 1995, 35, 24–29. [Google Scholar] [CrossRef]
- Eysholdt-Derzsó, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019, 21 (Suppl. 1), 103–108. [Google Scholar]
- Tan, X.; Zwiazek, J.J. Stable expression of aquaporins and hypoxia-responsive genes in adventitious roots are linked to maintaining hydraulic conductance in tobacco (Nicotiana tabacum) exposed to root hypoxia. PLoS ONE 2019, 14, e0212059. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef]
- Pernot, C.; Thiffault, N.; DesRochers, A. Contribution of adventitious vs. initial roots to growth and physiology of black spruce seedlings. Physiol. Plant. 2019, 165, 29–38. [Google Scholar] [CrossRef]
- Gaudin, A.C.M.; Mcclymont, S.A.; Holmes, B.M.; Lyons, E.; Raizada, M.N. Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant Cell Environ. 2011, 34, 2122–2137. [Google Scholar] [CrossRef]
- Saengwilai, P.; Tian, X.; Lynch, J.P. Low Crown Root Number Enhances Nitrogen Acquisition from Low-Nitrogen Soils in Maize. Plant Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of Auxin Response Factor Transcripts and MicroRNA Abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Niu, L.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Hu, L.; Dawuda, M.M. The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1047. [Google Scholar] [CrossRef] [PubMed]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Lanteri, M.L.; Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003, 132, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, L.; Lombardo, C.; Pagnussat, G.C.; Lamattina, L. Nitric Oxide Mediates the Indole Acetic Acid Induction Activation of a Mitogen-Activated Protein Kinase Cascade Involved in Adventitious Root Development 1. Society 2004, 135, 279–286. [Google Scholar]
- Lanteri, M.L.; Pagnussat, G.C.; Lamattina, L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J. Exp. Bot. 2006, 57, 1341–1351. [Google Scholar] [CrossRef]
- Lanteri, M.L.; Laxalt, A.M.; Lamattina, L. Nitric Oxide Triggers Phosphatidic Acid Accumulation via Phospholipase D during Auxin-Induced Adventitious Root Formation in Cucumber. Plant Physiol. 2008, 147, 188–198. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, C.; Ma, X.; Li, G.; Gan, L.; Ng, D.; Xia, K. Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung bean seedlings. PLoS ONE 2013, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hilo, A.; Shahinnia, F.; Druege, U.; Franken, P.; Melzer, M.; Rutten, T.; von Wirén, N.; Hajirezaei, M.-R. A specific role of iron in promoting meristematic cell division during adventitious root formation. J. Exp. Bot. 2017, 68, 4233–4247. [Google Scholar] [CrossRef] [PubMed]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, Y.; Yang, A.; Zhang, W.-H. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Jeník, J. Clonal growth in woody plants: A review. Folia Geobot. Phytotaxon. 1994, 29, 291–306. [Google Scholar] [CrossRef]
- Vacek, S.; Hejcman, M. Natural layering, foliation, fertility and plant species composition of a Fagus sylvatica stand above the alpine timberline in the Giant (Krkonoše) Mts., Czech Republic. Eur. J. For. Res. 2012, 131, 799–810. [Google Scholar] [CrossRef]
- Dawood, T.; Rieu, I.; Wolters-Arts, M.; Derksen, E.B.; Mariani, C.; Visser, E.J.W. Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiao, F.; Wu, Z.; Li, Y.; Wang, X.; He, X.; Zhong, W.; Wu, P. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 2008, 146, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Falasca, G.; Zaghi, D.; Possenti, M.; Altamura, M.M. Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep. 2004, 23, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef]
- Walk, T.C.; Jaramillo, R.; Lynch, J.P. Architectural Tradeoffs between Adventitious and Basal Roots for Phosphorus Acquisition. Plant Soil 2006, 279, 347–366. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Cervera, M.T.; Delarue, M.; Beeckman, T.; Dewitte, W.; Bellini, C.; Caboche, M.; Van Onckelen, H.; Van Montagu, M.; Inzé, D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 1995, 7, 1405–1419. [Google Scholar]
- Lin, C.; Sauter, M. Polar Auxin Transport Determines Adventitious Root Emergence and Growth in Rice. Front. Plant Sci. 2019, 10, 444. [Google Scholar] [CrossRef]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. 2006, 63, 2738–2754. [Google Scholar] [CrossRef]
- Agulló-Antón, M.Á.; Ferrández-Ayela, A.; Fernández-García, N.; Nicolás, C.; Albacete, A.; Pérez-Alfocea, F.; Sánchez-Bravo, J.; Pérez-Pérez, J.M.; Acosta, M. Early steps of adventitious rooting: Morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol. Plant. 2014, 150, 446–462. [Google Scholar] [CrossRef]
- Klerk, G.J.D.; Keppel, M.; Brugge, J.T.; Meekes, H. Timing of the phases in adventitous root formation in apple microcuttings. J. Exp. Bot. 1995, 46, 965–972. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins Determine Arabidopsis Root-Meristem Size by Controlling Cell Differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hidemi, K.; Inukai, Y. Molecular mechanism of crown root initiation and the different mechanisms between crown root and radicle in rice. Plant Signal. Behav. 2011, 6, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. Cytokinin Oxidase/Dehydrogenase4 Integrates Cytokinin and Auxin Signaling to Control Rice Crown Root Formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.-R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010, 61, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Negi, S.; Sukumar, P.; Muday, G.K. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 2011, 138, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Veloccia, A.; Fattorini, L.; Della Rovere, F.; Sofo, A.; D’Angeli, S.; Betti, C.; Falasca, G.; Altamura, M.M. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6445–6458. [Google Scholar] [CrossRef]
- Rasmussen, A.; Hu, Y.; Depaepe, T.; Vandenbussche, F.; Boyer, F.-D.; Van Der Straeten, D.; Geelen, D. Ethylene Controls Adventitious Root Initiation Sites in Arabidopsis Hypocotyls Independently of Strigolactones. J. Plant Growth Regul. 2017, 36, 897–911. [Google Scholar] [CrossRef]
- Lin, C.; Sauter, M. Control of Adventitious Root Architecture in Rice by Darkness, Light, and Gravity. Plant Physiol. 2018, 176, 1352–1364. [Google Scholar] [CrossRef]
- Jackson, M.B. Waterlogging and Submergence. Annu. Rev. Phytopathol. 1985, 36, 145–174. [Google Scholar]
- Eysholdt-Derzsó, E.; Sauter, M. Root Bending Is Antagonistically Affected by Hypoxia and ERF-Mediated Transcription via Auxin Signaling. Plant Physiol. 2017, 175, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Lombardi, L.; Iacopino, S.; Pencik, A.; Novak, O.; Perata, P.; Giuntoli, B.; Licausi, F. Endogenous Hypoxia in Lateral Root Primordia Controls Root Architecture by Antagonizing Auxin Signaling in Arabidopsis. Mol. Plant 2019, 12, 538–551. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Hanecakova, J. Ethylene and rooting of mung bean cuttings. The role of auxin induced ethylene synthesis and phase-dependent effects. Plant Growth Regul. 2008, 56, 203–209. [Google Scholar] [CrossRef]
- Lombardi-Crestana, S.; da Silva Azevedo, M.; de Silva, G.F.F.; Pino, L.E.; Appezzato-da-Glória, B.; Figueira, A.; Nogueira, F.T.S.; Peres, L.E.P. The Tomato (Solanum Lycopersicum cv. Micro-Tom) Natural Genetic Variation Rg1 and the DELLA Mutant Procera Control the Competence Necessary to Form Adventitious Roots and Shoots. J. Exp. Bot. 2012, 63, 5689–5703. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, J.I.; Kotula, L.; Santa-María, G.E.; Colmer, T.D. Root phenotypes of dwarf and “overgrowth” SLN1 barley mutants, and implications for hypoxic stress tolerance. J. Plant Physiol. 2019, 234–235, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Westfall, C.S.; Hicks, L.M.; Wang, S.; Jez, J.M. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J. Biol. Chem. 2010, 285, 29780–29786. [Google Scholar] [CrossRef]
- Westfall, C.S.; Herrmann, J.; Chen, Q.; Wang, S.; Jez, J.M. Modulating plant hormones by enzyme action. Plant Signal. Behav. 2010, 5, 1607–1612. [Google Scholar] [CrossRef]
- Wang, S.; Bai, Y.; Shen, C.; Wu, Y.; Zhang, S.; Jiang, D.; Guilfoyle, T.J.; Chen, M.; Qi, Y. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G.; et al. Auxin Controls Arabidopsis Adventitious Root Initiation by Regulating Jasmonic Acid Homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef]
- Fattorini, L.; Della Rovere, F.; Andreini, E.; Ronzan, M.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid induces ectopic formation of metaxylem in the hypocotyl of arabidopsis thaliana without conversion into indole-3-acetic acid and with a positive interaction with ethylene. Int. J. Mol. Sci. 2017, 18, 2474. [Google Scholar] [CrossRef] [PubMed]
- Terrile, M.C.; París, R.; Calderõn-Villalobos, L.I.A.; Iglesias, M.J.; Lamattina, L.; Estelle, M.; Casalongué, C.A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis Transport Inhibitor Response 1 auxin receptor. Plant J. 2012, 70, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Tao, L.; Zhu, C. Does nitric oxide play a pivotal role downstream of auxin in promoting crown root primordia initiation in monocots? Plant Signal. Behav. 2009, 4, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z.-M. (Max) Physiological and Molecular Regulation of Adventitious Root Formation. CRC Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Niu, L.; Yu, J.; Liao, W.; Yu, J.; Zhang, M.; Dawuda, M.M. Calcium and Calmodulin Are Involved in Nitric Oxide-Induced Adventitious Rooting of Cucumber under Simulated Osmotic Stress. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Li, Y.; Leng, Y.; Zeng, X.-Y.; Ma, Y.-H. Nitric oxide donor improves adventitious rooting in mung bean hypocotyl cuttings exposed to cadmium and osmotic stresses. Environ. Exp. Bot. 2019, 164, 114–123. [Google Scholar] [CrossRef]
- Li, S.-W.; Leng, Y.; Shi, R.-F. Transcriptomic profiling provides molecular insights into hydrogen peroxide-induced adventitious rooting in mung bean seedlings. BMC Genom. 2017, 18, 188. [Google Scholar] [CrossRef]
- Syros, T.; Yupsanis, T.; Zafiriadis, H.; Economou, A. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 2004, 161, 69–77. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Yu, X.; Yu, J.; He, X.; Zhang, S.; Shou, H.; Wu, P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005, 43, 47–56. [Google Scholar] [CrossRef]
- Taramino, G.; Sauer, M.; Stauffer, J.L.; Multani, D.; Niu, X.; Sakai, H.; Hochholdinger, F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007, 50, 649–659. [Google Scholar] [CrossRef]
- Orman-Ligeza, B.; Parizot, B.; Gantet, P.P.; Beeckman, T.; Bennett, M.J.; Draye, X. Post-embryonic root organogenesis in cereals: Branching out from model plants. Trends Plant Sci. 2013, 18, 459–467. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Xing, J.; Yan, L.; Wang, R.; Zhao, Y. The YUCCA-Auxin-WOX11 Module Controls Crown Root Development in Rice. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Mai, C.D.; Phung, N.T.; To, H.T.; Gonin, M.; Hoang, G.T.; Nguyen, K.L.; Do, V.N.; Courtois, B.; Gantet, P. Genes controlling root development in rice. Rice 2014, 7, 30. [Google Scholar] [CrossRef]
- Kitomi, Y.; Ogawa, A.; Kitano, H.; Inukai, Y. CRL4 regulates crown root formation through auxin transport in rice. Plant Root 2008, 2, 19–28. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Wang, L.; Wang, X.; Xue, Y.; Wu, P.; Shou, H. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009, 19, 1110–1119. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Coudert, Y.; Dievart, A.; Droc, G.; Gantet, P. ASL/LBD Phylogeny Suggests that Genetic Mechanisms of Root Initiation Downstream of Auxin Are Distinct in Lycophytes and Euphyllophytes. Mol. Biol. Evol. 2013, 30, 569–572. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Gan, T.; Liu, L.; Long, W.; Wang, Y.; Niu, M.; Li, X.; Zheng, M.; Jiang, L.; et al. CRL6, a member of the CHD protein family, is required for crown root development in rice. Plant Physiol. Biochem. 2016, 105, 185–194. [Google Scholar] [CrossRef]
- Coudert, Y.; Le, V.A.T.; Adam, H.; Bès, M.; Vignols, F.; Jouannic, S.; Guiderdoni, E.; Gantet, P. Identification of Crown Rootless1-regulated genes in rice reveals specific and conserved elements of postembryonic root formation. N. Phytol. 2015, 206, 243–254. [Google Scholar] [CrossRef]
- Kitomi, Y.; Ito, H.; Hobo, T.; Aya, K.; Kitano, H.; Inukai, Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011, 67, 472–484. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.-X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, S.; Song, Y.; Huang, Y.; Zhou, S.; Liu, X.; Zhou, D.-X. The Interaction between Rice ERF3 and WOX11 Promotes Crown Root Development by Regulating Gene Expression Involved in Cytokinin Signaling. Plant Cell 2015, 27, 2469–2483. [Google Scholar] [CrossRef]
- Lohmann, J.U.; Hong, R.L.; Hobe, M.; Busch, M.A.; Parcy, F.; Simon, R.; Weigel, D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 2001, 105, 793–803. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, S.; Zhang, Q.; Song, H.; Zhou, D.X.; Zhao, Y. Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J. Exp. Bot. 2017, 68, 2787–2798. [Google Scholar] [CrossRef]
- Liu, W.; Yu, J.; Ge, Y.; Qin, P.; Xu, L. Pivotal role of LBD16 in root and root-like organ initiation. Cell. Mol. Life Sci. 2018, 75, 3329–3338. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, D.; Ren, Y.; Zhang, X.; Zhao, J. Characteristic and Expression Analysis of a Metallothionein Gene, OsMT2b, Down-Regulated by Cytokinin Suggests Functions in Root Development and Seed Embryo Germination of Rice. Plant Physiol. 2008, 146, 1637–1650. [Google Scholar] [CrossRef]
- Shang, X.L.; Xie, R.R.; Tian, H.; Wang, Q.L.; Guo, F.Q. Putative zeatin O-glucosyltransferase OscZOG1 regulates root and shoot development and formation of agronomic traits in rice. J. Integr. Plant Biol. 2016, 58, 627–641. [Google Scholar] [CrossRef]
- Negishi, N.; Oishi, M.; Kawaoka, A. Chemical screening for promotion of adventitious root formation in Eucalyptus globulus. BMC Proc. 2011, 5, 139. [Google Scholar] [CrossRef]
- Stevens, M.E.; Woeste, K.E.; Pijut, P.M. Localized gene expression changes during adventitious root formation in black walnut (Juglans nigra L.). Tree Physiol. 2018, 38, 877–894. [Google Scholar] [CrossRef]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1-and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Pacurar, M.L.; Lakehal, A.; Pacurar, A.M.; Ranjan, A.; Bellini, C. The Arabidopsis Cop9 signalosome subunit 4 (CNS4) is involved in adventitious root formation. Sci. Rep. 2017, 7, 628. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Hoyt, M.J.; Hamilton, A.A.; Alonso, J.M. A Link between Ethylene and Auxin Uncovered by the Characterization of Two Root-Specific Ethylene-Insensitive Mutants in Arabidopsis. Plant Cell 2005, 17, 2230–2242. [Google Scholar] [CrossRef]
- Fett-Neto, A.G.; Fett, J.P.; Goulart, L.W.V.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464. [Google Scholar] [CrossRef]
- Ibáñez, S.; Ruiz-Cano, H.; Fernández, M.Á.; Sánchez-García, A.B.; Villanova, J.; Micol, J.L.; Pérez-Pérez, J.M. A Network-Guided Genetic Approach to Identify Novel Regulators of Adventitious Root Formation in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 461. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Lv, Y.; Cen, X.; Ding, X.; Wu, H.; Li, X. Genetic control of the root system in rice under normal and drought stress conditions by genome-wide association study. PLoS Genet. 2017, 13, e1006889. [Google Scholar] [CrossRef]
- Hutchison, K.W.; Singer, P.B.; McInnis, S.; Diaz-Sala, C.; Greenwood, M.S. Expansins are conserved in conifers and expressed in hypocotyls in response to exogenous auxin. Plant Physiol. 1999, 120, 827–832. [Google Scholar] [CrossRef]
- Xu, M.; Xie, W.; Huang, M. Two Wuschel-related Homeobox genes, PeWOX11a and PeWOX11b, are involved in adventitious root formation of poplar. Physiol. Plant. 2015, 155, 446–456. [Google Scholar] [CrossRef]
- Wang, X.-F.; He, F.-F.; Ma, X.-X.; Mao, C.-Z.; Hodgman, C.; Lu, C.-G.; Wu, P. OsCAND1 is required for crown root emergence in rice. Mol. Plant 2011, 4, 289–299. [Google Scholar] [CrossRef]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The Root Meristemless1/Cadmium Sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–110. [Google Scholar] [CrossRef]
- Kornet, N.; Scheres, B. Members of the GCN5 histone acetyltransferase complex regulate Plethora-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell 2009, 21, 1070–1079. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D.-X. Rice Homeodomain Protein WOX11 Recruits a Histone Acetyltransferase Complex to Establish Programs of Cell Proliferation of Crown Root Meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef]
- Jin, J.; Chen, H.; Cai, W. Transcriptome Analysis of Oryza sativa Calli Under Microgravity. Microgravity Sci. Technol. 2015, 27, 437–453. [Google Scholar] [CrossRef]
- Won, S.K.; Choi, S.B.; Kumari, S.; Cho, M.; Lee, S.H.; Cho, H.T. Root hair-specific expansin B genes have been selected for graminaceae root hairs. Mol. Cells 2010, 30, 369–376. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, B.; He, X.; Lv, S.; Bai, Y.; Ding, W.; Chen, M.; Cho, H.T.; Wu, P. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011, 66, 725–734. [Google Scholar]
- Ma, N.; Wang, Y.; Qiu, S.; Kang, Z.; Che, S.; Wang, G.; Huang, J. Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 2013, 8, e75997. [Google Scholar] [CrossRef]
- Debi, B.R.; Taketa, S.; Ichii, M. Cytokinin inhibits lateral root initiation but stimulates lateral root elongation in rice (Oryza sativa). J. Plant Physiol. 2005, 162, 507–515. [Google Scholar] [CrossRef]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; et al. Cytokinins Act Directly on Lateral Root Founder Cells to Inhibit Root Initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar] [CrossRef]
- Ramirez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 759–771. [Google Scholar] [CrossRef]
- Kuroha, T.; Satoh, S. Involvement of cytokinins in adventitious and lateral root formation. Plant Root 2007, 1, 27–33. [Google Scholar] [CrossRef]
- Meng, W.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-B Arabidopsis Response Regulators is Critical to the Specification of Shoot Stem Cell Niche by Dual Regulation of Wuschel. Plant Cell 2017, 29, 00640. [Google Scholar] [CrossRef]
- Da Rocha Correˆa, L.; Paim, D.C.; Schwambach, J.; Fett-Neto, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regul. 2005, 45, 63–73. [Google Scholar] [CrossRef]
- Thompson, A.J.; Thorne, E.T.; Burbidge, A.; Jackson, A.C.; Sharp, R.E.; Taylor, I.B. Complementation of notabilis, an abscisic acid-deficient mutant of tomato: Importance of sequence context and utility of partial complementation. Plant Cell Environ. 2004, 27, 459–471. [Google Scholar] [CrossRef]
- Busov, V.; Meilan, R.; Pearce, D.W.; Rood, S.B.; Ma, C.; Tschaplinski, T.J.; Strauss, S.H. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 2006, 224, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.D.; Chen, L.-J.; Yu, S.-M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Chou Guan, J.; Koch, K.E.; Suzuki, M.; Wu, S.; Latshaw, S.; Petruff, T.; Goulet, C.; Klee, H.J.; McCarty, D.R. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol. 2012, 160, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Beveridge, C.A.; Geelen, D. Inhibition of strigolactones promotes adventitious root formation. Plant Signal. Behav. 2012, 7, 694–697. [Google Scholar] [CrossRef]
- Sun, H.; Tao, J.; Hou, M.; Huang, S.; Chen, S.; Liang, Z.; Xie, T.; Wei, Y.; Xie, X.; Yoneyama, K.; et al. A strigolactone signal is required for adventitious root formation in rice. Ann. Bot. 2015, 115, 1155–1162. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Baurens, F.-C.; Nicolleau, J.; Legavre, T.; Verdeil, J.-L.; Monteuuis, O. Genomic DNA methylation of juvenile and mature Acacia mangium micropropagated in vitro with reference to leaf morphology as a phase change marker. Tree Physiol. 2004, 24, 401–407. [Google Scholar] [CrossRef]
- Monteuuis, O.; Doulbeau, S.; Verdeil, J.L. DNA methylation in different origin clonal offspring from a mature Sequoiadendron giganteum genotype. Trees-Struct. Funct. 2008, 22, 779–784. [Google Scholar] [CrossRef]
- Nilsson, O.; Olsson, O. Getting to the root: The role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol. Plant. 1997, 100, 463–473. [Google Scholar] [CrossRef]
- Sedira, M.; Holefors, A.; Welander, M. Protocol for transformation of the apple rootstock Jork 9 with the rolB gene and its influence on rooting. Plant Cell Rep. 2001, 20, 517–524. [Google Scholar] [CrossRef]
- Zhu, L.H.; Li, X.Y.; Ahlman, A.; Welander, M. The rooting ability of the dwarfing pear rootstock BP10030 (Pyrus communis) was significantly increased by introduction of the rolB gene. Plant Sci. 2003, 165, 829–835. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Jahnke, S.; Menzel, M.I.; Van Dusschoten, D.; Roeb, G.W.; Bühler, J.; Minwuyelet, S.; Blümler, P.; Temperton, V.M.; Hombach, T.; Streun, M.; et al. Combined MRI-PET dissects dynamic changes in plant structures and functions. Plant J. 2009, 59, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.R.; Roberts, J.A.; Black, C.R.; McNeill, A.; Davidson, R.; Mooney, S.J. The X-factor: Visualizing undisturbed root architecture in soils using X-ray computed tomography. J. Exp. Bot. 2010, 61, 311–313. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, Y.S.; Kwon, C.-W.; Park, H.K.; Jeong, J.S.; Kim, J.-K. Overexpression of the Transcription Factor AP37 in Rice Improves Grain Yield under Drought Conditions. Plant Physiol. 2009, 150, 1368–1379. [Google Scholar] [CrossRef]
- Kenrick, P. The Origin of Roots. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–13. ISBN 978-0-8247-0631-9. [Google Scholar]

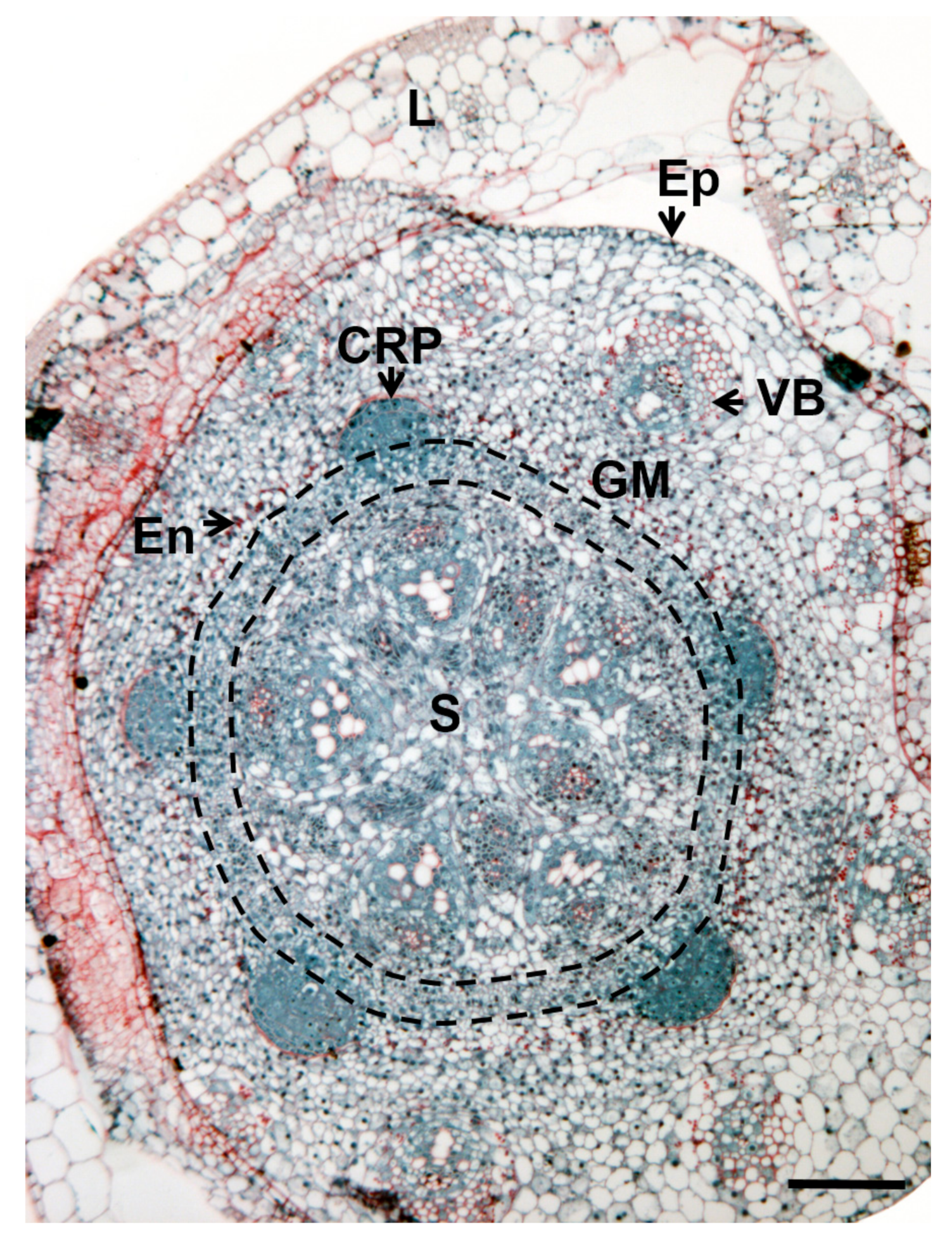
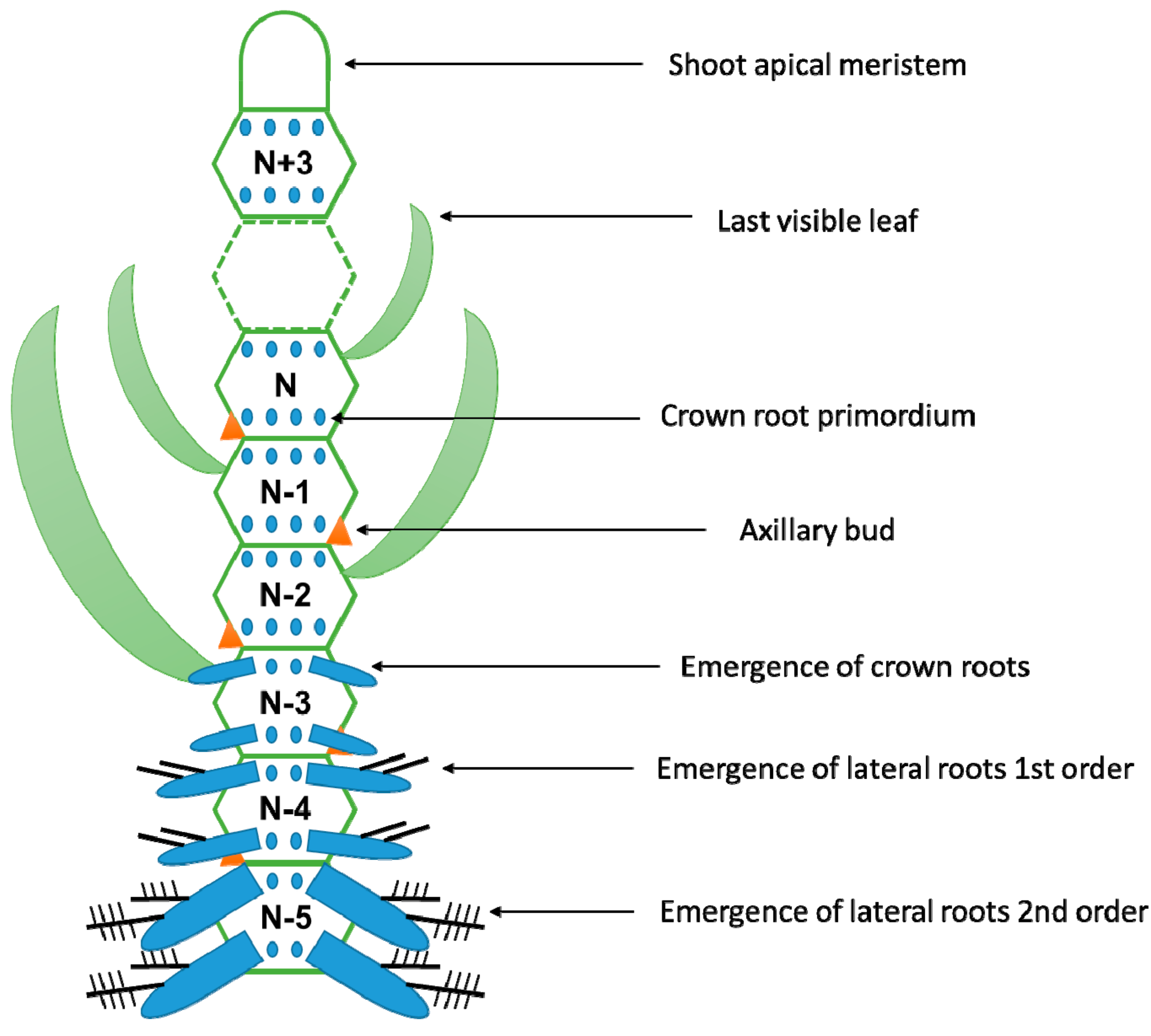
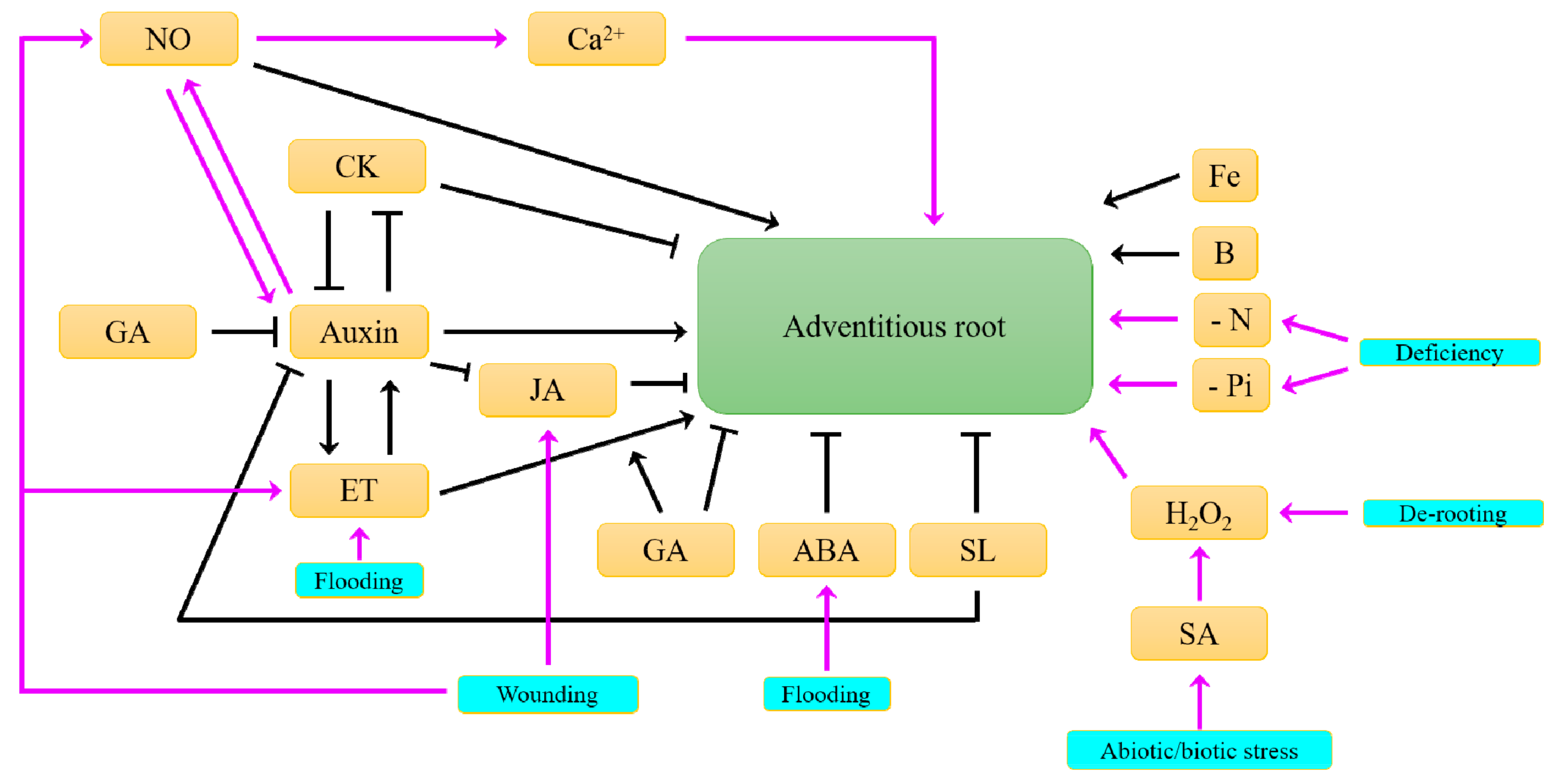
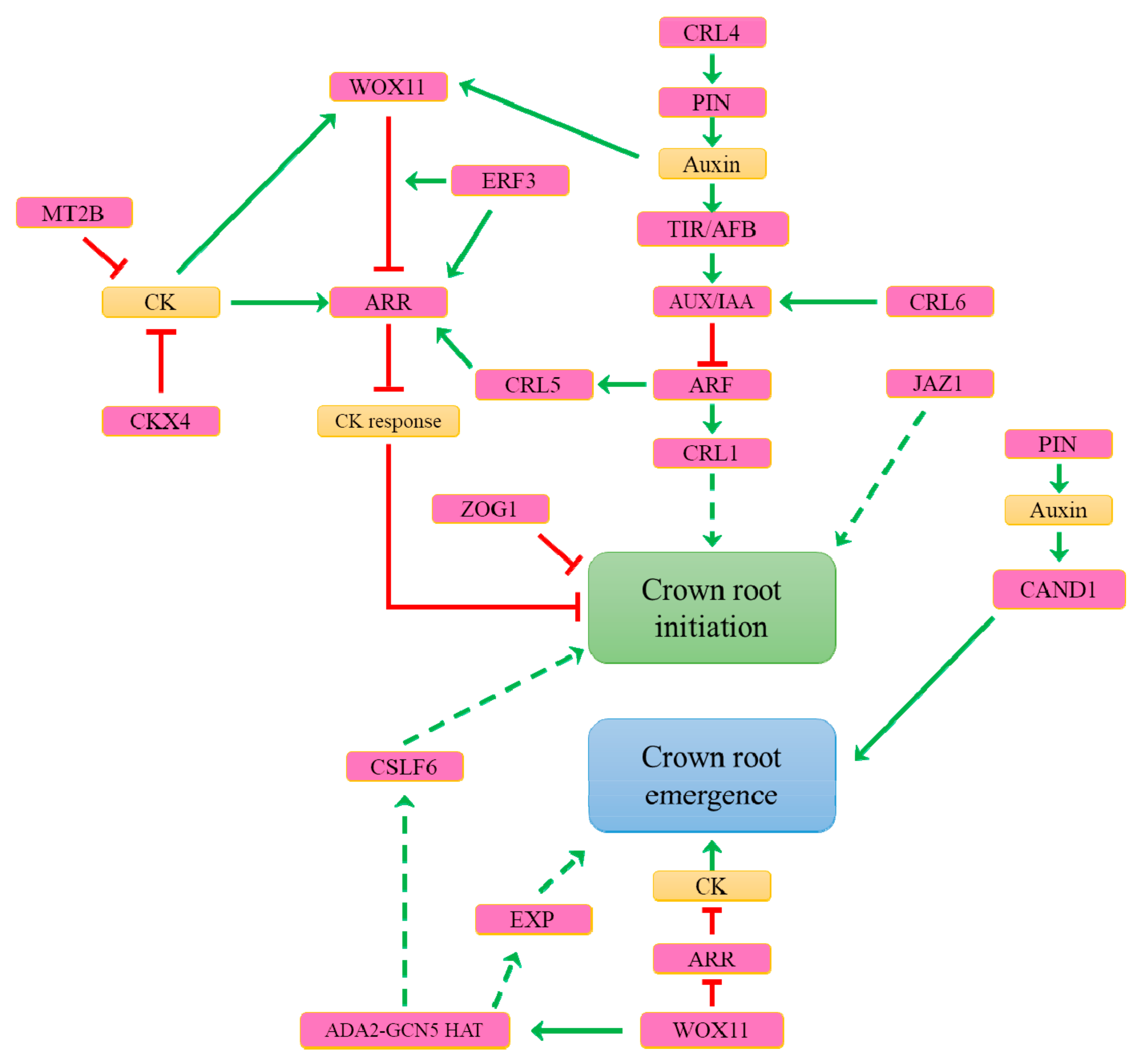
| Species | Constitutive | Inducible | |
|---|---|---|---|
| Dicots | Arabidopsis | no | mechanical damage [12] dark/light [11,43,44] flooding [26,36] in vitro culture [16,18] |
| Cucumber | n.d. | salt [45] mechanical damage [46,47,48,49,50] | |
| Epiphyte | yes [3] | n.d. | |
| Ficus | yes [3] | n.d. | |
| Mung bean | n.d. | mechanical damage [36,51] biotic stress [51] | |
| Petunia | n.d. | mechanical damage [6,7,32,52] | |
| Sunflower | n.d. | mechanical damage [26] | |
| Tobacco | n.d. | flooding [37] | |
| Tomato | n.d. | flooding [53] | |
| Monocots | Barley | yes [1] | flooding [54] |
| Maize | yes [1,5] | n.d. | |
| Rice | yes [2,3] | flooding [38,55,56], Pi starvation [57] | |
| Socratea exorrhiza | yes | mechanical damage [9] | |
| Woody species | yes | mechanical damage [26,27,28] in vitro culture [26,27,28] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What Makes Adventitious Roots? Plants 2019, 8, 240. https://doi.org/10.3390/plants8070240
Gonin M, Bergougnoux V, Nguyen TD, Gantet P, Champion A. What Makes Adventitious Roots? Plants. 2019; 8(7):240. https://doi.org/10.3390/plants8070240
Chicago/Turabian StyleGonin, Mathieu, Véronique Bergougnoux, Thu D. Nguyen, Pascal Gantet, and Antony Champion. 2019. "What Makes Adventitious Roots?" Plants 8, no. 7: 240. https://doi.org/10.3390/plants8070240
APA StyleGonin, M., Bergougnoux, V., Nguyen, T. D., Gantet, P., & Champion, A. (2019). What Makes Adventitious Roots? Plants, 8(7), 240. https://doi.org/10.3390/plants8070240






