Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals
Abstract
1. Introduction
2. Results
2.1. Effect of Alfalfa Leaf Extracts on Callus Induction
2.2. Effect of Allelopathic Compounds on Callus Growth
2.3. Autotoxic Effect of Alfalfa Leaf Extracts
2.4. Identification of Major Phenolic Compounds and Autotoxic Effects
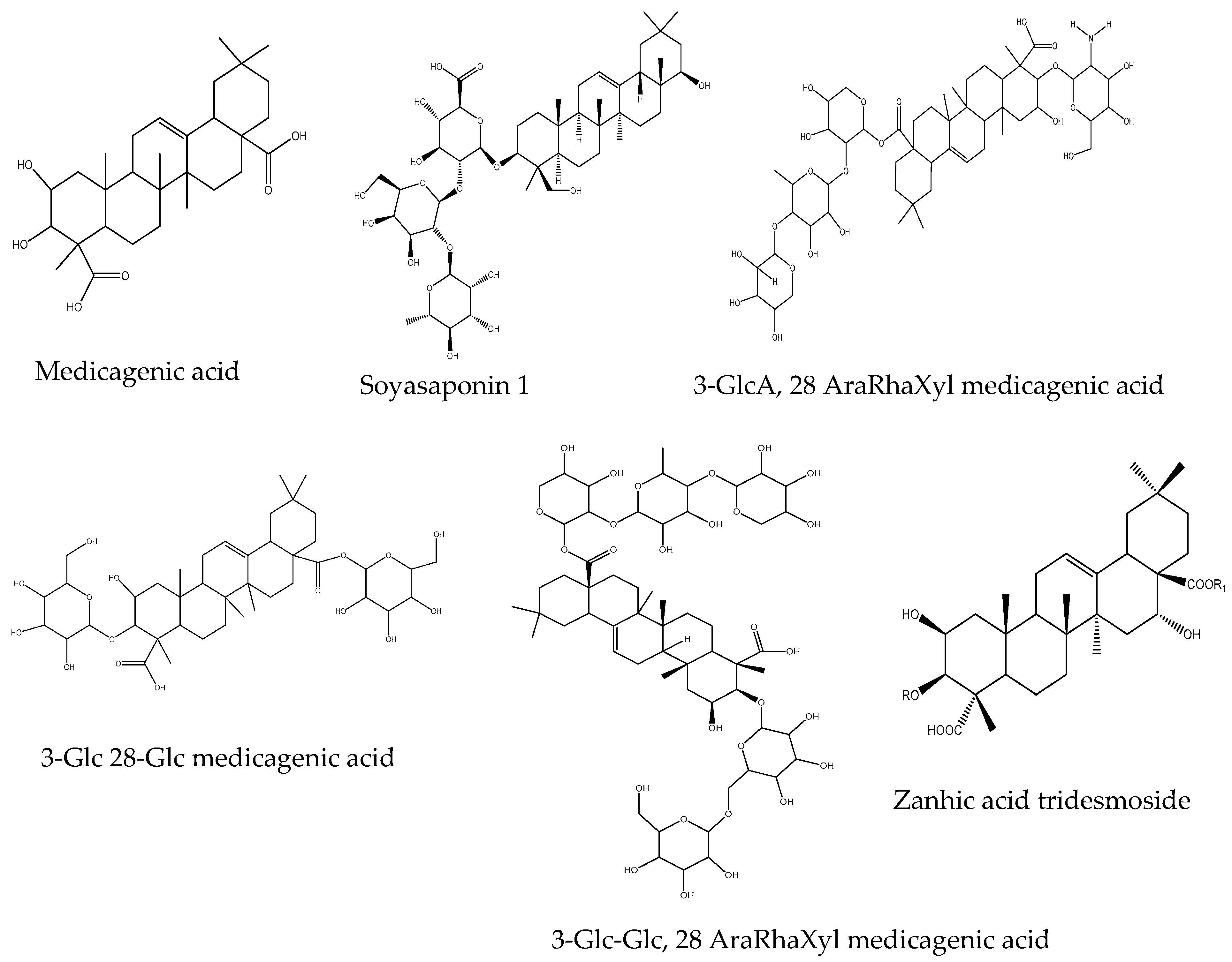
2.5. Identification of Major Saponins and Autotoxic Effects
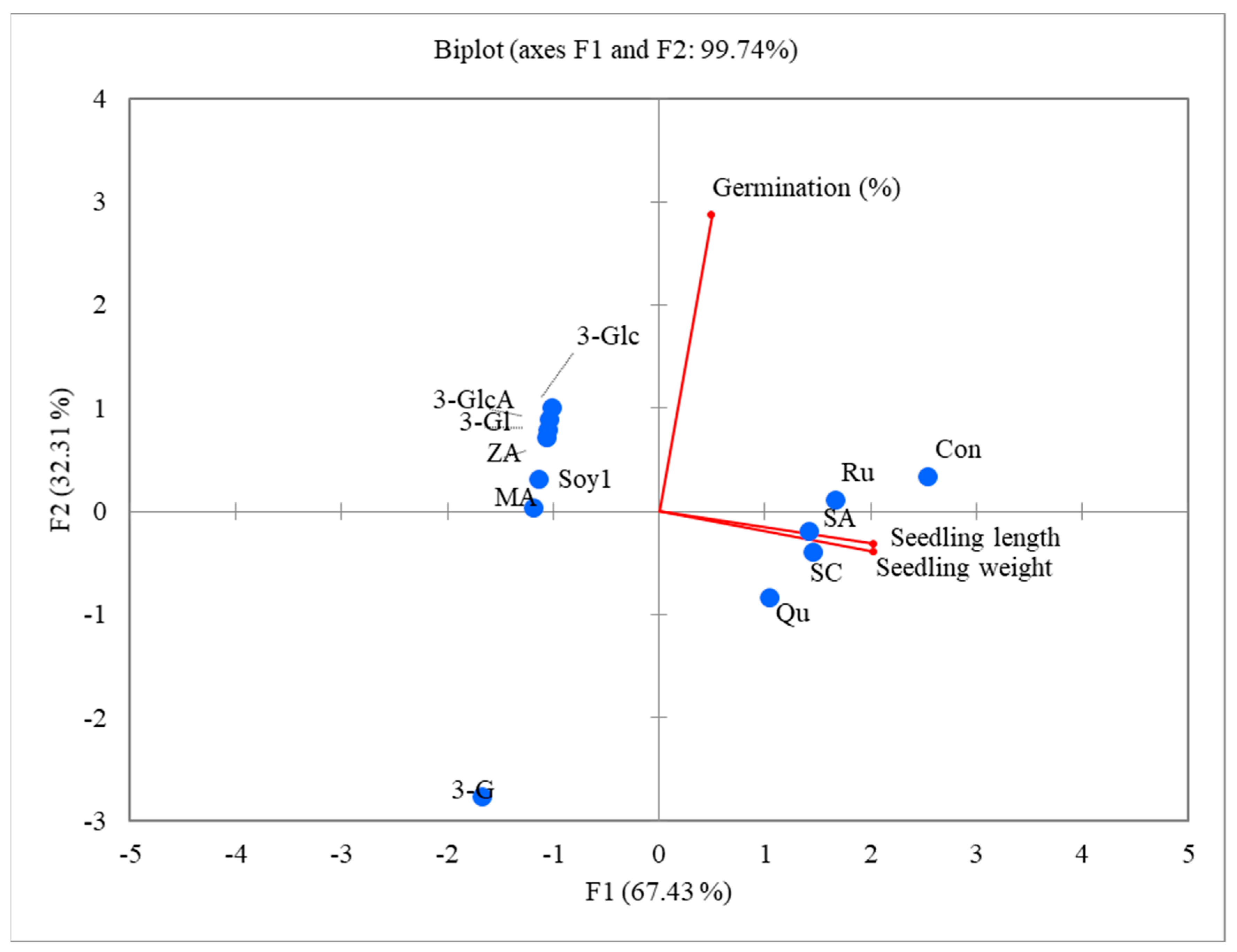
2.6. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Callus Regeneration and Subculture
4.4. Evaluation of Allelopathic Effects of Plant Extracts on Callus Growth
4.5. Effect of Allelopathic Compounds on Callus Growth
4.6. Autotoxic Effect of Alfalfa Leaf Extracts
4.7. Preparation of Plant Extracts
4.8. Phenolic Compound Identification and Quantification Using HPLC
4.9. Identification of Major Saponins in Alfalfa Leaves
4.10. Nuclear Magnetic Resonance (NMR) Spectroscopic Analyses
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griepentrog, H.W.; Dedousis, A.P. Mechanical Weed Control, Soil Engineering; Springer: New York, NY, USA, 2010; pp. 171–179. [Google Scholar]
- Bergin, D. Weed Control Options for Coastal Sand Dunes: A Review; New Zealand Forest Research Institute Limited: Rotorua, New Zealand, 2011; pp. 5–13. [Google Scholar]
- Rueda-Ayala, V.; Rasmussen, J.; Gerhards, R.; Fournaise, N.E. The influence of post-emergence weed harrowing on selectivity, crop recovery and crop yield in different growth stages of winter wheat. Weed Res. 2011, 51, 478–488. [Google Scholar] [CrossRef]
- Zhang, Z.P. Development of chemical weed control and integrated weed management in China. Weed Biol. Manag. 2003, 3, 197–203. [Google Scholar] [CrossRef]
- Bond, W.; Grundy, A. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Carballido, J.; Rodríguez-Lizana, A.; Agüera, J.; Perez-Ruiz, M. Field sprayer for inter and intra-row weed control: Performance and labor savings. Span. J. Agric. Res. 2013, 11, 642–651. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Arno, M.; Costanzo, M.; Malatesta, M.; Séralini, G.E.; Antoniou, M.N. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. J. Environ. Health 2015, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.L.; Leu, A.; Abrahamson, J.; Wallet, B. Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J. Org. Syst. 2014, 9, 6–37. [Google Scholar]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pašti, T.D.; Bondžic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Cattani, D.; Acordi Cesconetto, P.; Kruger Tavares, M.; Parisotto, E.B.; De Oliveira, P.A.; Heinz Rieg, C.E.; Concli Leite, M.; Schröder Prediger, R.D.; Wendt, N.C.; Razzera, G.; et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Fluegge, K.; Fluegge, K. Glyphosate use predicts healthcare utilization for ADHD in the healthcare cost and utilization project net (HCUPnet): A two-way fixed-effects analysis. Pol. J. Environ. Stud. 2016, 25, 1489–1503. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Segatto, M.M.; Hohmann, C.; Miligi, L.; Bakos, L.; Bonamigo, R. Occupational exposure to pesticides with occupational sun exposure increases the risk for cutaneous melanoma. J. Occup. Environ. Med. 2016, 58, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, hard water, and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, J.P. Impact of pesticides application in agricultural industry: An Indian scenario. Int. J. Agric. Food Sci. Technol. 2013, 4, 817–822. [Google Scholar]
- Baziar, M.R.; Farahvash, F.; Mirshekari, B.; Rashidi, V. Allelopathic effect of rye grass (Lolium persicum) and wild mustard (Sinapis arvensis) on barley. Pak. J. Bot. 2014, 46, 2069–2075. [Google Scholar]
- Sitthinoi, P.; Lertmongkol, S.; Chanprasert, W.; Vajrodaya, S. Allelopathic effects of jungle rice (Echinochloa colona (L.) Link) extract on seed germination and seedling growth of rice. Agric. Nat. Resour. 2017, 51, 74–78. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Dorota, S.; Urszula, K. Allelochemicals as bioherbicides—Present and perspectives. Herbic. Curr. Res. Case Stud. Use 2013, 517–542. [Google Scholar] [CrossRef]
- Vyvyan, W.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1632–1646. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Kong, C. The response of allelopathic rice growth and microbial feedback to barnyard grass infestation in a paddy field experiment. Eur. J. Soil Biol. 2013, 56, 26–32. [Google Scholar] [CrossRef]
- Zeng, R.S. Allelopathy-the solution is indirect. J. Chem. Ecol. 2014, 40, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Qasem, J.R.; Foy, C.L. Weed allelopathy, its ecological impacts and future prospects: A review. J. Crop. Prod. 2001, 4, 43–92. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Alias, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity against germination and seedling emergence in Cistus ladanifer L. Plant Soil. 2006, 282, 327–332. [Google Scholar] [CrossRef]
- Duke, S.O. Proving Allelopathy in Crop-Weed Interactions. Weed Sci. Spec. Issue 2015, 63, 121–132. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, C.; Baskar, P. Allelopathy in weed management: A critical review. Afr. J. Agric. Res. 2015, 10, 1004–1015. [Google Scholar]
- Cseke, L.J.; Kaufman, P.B. Regulation of metabolite synthesis in plants. In Natural Products from Plants; Cseke, L.J., Kirakosyan, A., Kaufman, P.B., Warber, S., Duke, J.A., Brielmann, H., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 101–141. [Google Scholar]
- Einhellig, F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 88, 886–893. [Google Scholar] [CrossRef]
- Pramanik, M.H.R.; Nagal, M.; Asao, M.; Matsui, Y. Effect of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Weston, L.A.I. Root exudation: An overview. In Root Ecology. Ecological Studies (Analysis and Synthesis); de Kroon, H., Visser, E.J.W., Eds.; Springer: Heidelberg, Germany, 2003; Volume 168, pp. 235–255. [Google Scholar]
- Xuan, T.D.; Tsuzuki, E.; Terao, H.; Matsuo, M.; Khanh, T.D. Identification of potential allelochemicals in kava (Piper methysticum L.) root. Allelopath. J. 2003, 12, 197–204. [Google Scholar]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Blum, U.; Shafer, S.R.; Lehman, M.E. Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: Concepts vs. an experimental model. Crit. Rev. Plant Sci. 1999, 18, 673–693. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions-an update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Tabaglio, V.; Gavazzi, C.; Schulz, M.; Marocco, A. Alternative weed control using the allelopathic effect of natural benzoxazinoids from rye mulch. Agron. Sustain. Dev. 2008, 28, 397–401. [Google Scholar] [CrossRef]
- Rasmussen, J.A.; Einhellig, F.A. Synergistic inhibitory effects of p-coumaric and ferulic acids on germination and growth of grain sorghum. J. Chem. Ecol. 1977, 3, 197–205. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Gaudreau, A.; Lecouturier, D.; Kapel, R.; Lutin, F.; Vezina, L.-P.; Bazinet, L. Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 2009, 329, 60–67. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT–Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Ullah, F.; Ahmad, S.; Wahab, S.; Zeb, A.; Khattak, M.K.; Khan, S.; Kang, M. Quality Evaluation of Biscuits Supplemented with Alfalfa Seed Flour. Foods 2016, 5, 68. [Google Scholar] [CrossRef]
- Hawkins, C.; Yu, L.X. Recent progress in alfalfa (Medicago sativa L.) genomics and genomic selection. Crop. J. 2018, 6, 565–575. [Google Scholar] [CrossRef]
- Kechang, L.; Ping, Z.; Cash, D. Biology and management of major alfalfa diseases and pests. In Alfalfa Management Guide for Ningxia; Cash, D., Yuegao, H., Kechang, L., Suqin, W., Ping, Z., Rong, G., Eds.; China Agricultural Press: Beijing, China, 2009; pp. 37–62. [Google Scholar]
- Mölgaard, J.; von Schenck, H.; Olsson, A.G. Alfalfa seeds lower low density lipoprotein cholesterol and apolipoprotein B concentrations in patients with type II hyperlipoproteinemia. Atherosclerosis 1987, 65, 173–179. [Google Scholar] [CrossRef]
- Dornbos, D.L., Jr.; Spencer, G.F. Natural products phytotoxicity. A bioassay suitable for small quantities of slightly water soluble compounds. J. Chem. Ecol. 1990, 16, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Márton, M.; Mándoki, Z.S.; Csapó, J. Evaluation of biological value of sprouts I. F at content, fatty acid composition. Acta Univ. Sapientiae Aliment. 2010, 3, 53–65. [Google Scholar]
- Plaza, L.; de Ancos, B.; Cano, M.P. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum.L) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Compton, M.E.; Preece, J.E. Response of tobacco callus to shoot tip exudation from five species. HortScience 1988, 23, 208–210. [Google Scholar]
- Braga, K.Q.; Coimbra, M.C.; Castro, A.H.F. In vitro germination, callus induction and phenolic compounds contents from Pyrostegia venusta (Ker Gawl.) Miers. Acta Sci. Biol. Sci. 2015, 37, 151–158. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The effect of phenols on respiratory enzymes in seed germination respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stansley, P.D.; Holmes, E. Application on NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Sorgona, A.; Albano, S.; Cacco, G. Coumarin differentially affects the morphology of different toot types of maize seedlings. J. Chem. Ecol. 2004, 30, 1871–1883. [Google Scholar] [CrossRef]
- Testa, B. The Metabolism of Drugs and Other Xenobiotics; Academic Press: New York, NY, USA, 1995; p. 475. [Google Scholar]
- Hammondkosak, K.E.; Jones, J.D.G. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar]
- Kekec, G.; Mutlu, S.; Alpsoy, L.; Sakcali, M.S.; Atici, O. Genotoxic effects of catmint (Nepeta meyeri Benth.) essential oils on some weed and crop plants. Toxicol. Ind. Health 2013, 29, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Sunar, S.; Yildirim, N.; Aksakal, O.; Agar, G. Determination of the genotoxic effects of Convolvulus arvensis extracts on corn (Zea mays L.) seeds. Toxicol. Ind. Health 2013, 29, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Meazza, G.; Scheffler, B.E.; Tellez, M.R.; Rimando, A.M.; Romagni, J.G.; Li, Y.Z.; Liang, W.J.; Zhang, X.K.; Liu, F.M.; Zhu, X.H. Allelopathic activity of root saponins of alfalfa on wheat, corn and barnyard grass. Allelopath. J. 2005, 15, 119–123. [Google Scholar]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Wu, F.Z.; Pan, K.; Ma, F.M.; Wang, X.D. Effects of cinnamic acid on photosynthesis and cell ultrastructure of cucumber seedlings. Acta Hortic. Sin. 2004, 31, 183–188. [Google Scholar]
- Harun, M.A.Y.A.; Robinson, R.W.; Johnson, J.; Uddin, M.N. Allelopathic potential of Chrysanthemoides monilifera subsp. monilifera (bone seed): A novel weapon in the invasion processes. S. Afr. J. Bot. 2014, 93, 157–166. [Google Scholar] [CrossRef]
- Sunmonu, T.O.; Van Staden, J. Phytotoxicity evaluation of six fast- growing tree species in South Africa. S. Afr. J. Bot. 2014, 90, 101–106. [Google Scholar] [CrossRef][Green Version]
- Nonaka, M. Variable sensitivity of Trichoderma viride to Medicago sativa saponins. Phytochemistry 1986, 25, 73–75. [Google Scholar] [CrossRef]
- Oleszek, W.; Price, K.R.; Colquhoun, I.J.; Jurzysta, M.; Ploszynski, M.; Fenwick, G.R. Isolation and identification of alfalfa (Medicago sativa L.) root saponins: Their activity in relation to a fungal bioassay. J. Agric. Food Chem. 1990, 38, 1810–1817. [Google Scholar] [CrossRef]
- Wyman-Simpson, C.L.; Waller, G.R.; Jurzysta, M.; McPherson, J.K.; Young, C.C. Biological activity and chemical isolation of root saponins of six cultivars of alfalfa (Medicago sativa L.). Plant Soil 1991, 135, 83–94. [Google Scholar] [CrossRef]
- Oleszek, W. Allelopathic potentials of alfalfa (Medicago sativa) saponins: Their relation to antifungal and hemolytic activities. J. Chem. Ecol. 1993, 19, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Waller, G.R.; Yang, C.F.; Chen, L.F.; Liou, R.M.; Wu, S.C.; Young, C.C.; Lee, M.R.; Lee, J.S.; Cheng, C.C.; Chou, C.H.; et al. Saponins produced during the life cycle of mungbeans and their role as allelochemicals. Stud. Plant Sci. 1999, 6, 105–130. [Google Scholar]
- Waller, G.R.; Yang, C.F.; Chen, L.F.; Su, C.H.; Liou, R.M.; Wu, S.C.; Young, C.C.; Lee, M.R.; Lee, J.S.; Waller, G.R.; et al. Allelopathic activity of root saponins from alfalfa (Medicago sativa L) on weeds and wheat. Bot. Bull. Acad. Sin. 1993, 34, 1–11. [Google Scholar]
- Scognamiglio, M.; D’Abrosca, B.; Fiumano, V.; Chambery, A.; Severino, V.; Tsafantakis, N.; Pacifico, S.; Esposito, A.; Fiorentino, A. Oleanane saponins from Bellis sylvestris Cyr. and evaluation of their phytotoxicity on Aegilops geniculata Roth. Phytochemistry 2012, 84, 125–134. [Google Scholar] [CrossRef]
- Ohana, P.; Delmer, D.P.; Carlson, R.W.; Glushka, J.; Azadi, P.; Bacic, T.; Benziman, M. Identification of a novel triterpenoid saponin from Pisum sativum as a specific inhibitor of the diguanylate cyclase of Acetobacter xylinum. Plant Cell Physiol. 1998, 39, 144–152. [Google Scholar] [CrossRef][Green Version]
- Macias, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2004, 48, 2512–2521. [Google Scholar] [CrossRef]
- Takahashi, L.; Sert, M.A.; Kelmer-Bracht, A.M.; Bracht, A.; Ishii-Iwamoto, E.L. Effects of rutin and quercetin on mitochondrial metabolism and on ATP levels in germinating tissues of Glycirte Max. Plant Physiol. Biochem. 1998, 36, 495–501. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Plant secondary metabolite rutin affects the photosynthesis and excitation energy flux responses in Arabidopsis thaliana. Allelopath. J. 2016, 38, 215–228. [Google Scholar]
- Ohana, P.; Delmer, D.P.; Volman, G.; Benziman, M. Glycosylated triterpenoid saponin: A specific inhibitor of diguanylate cyclase from Acetobacter xylinum. Biological activity and distribution. Plant Cell Physiol. 1998, 39, 153–159. [Google Scholar] [CrossRef]
- Adel, M.M.; Sehnal, F.; Jurzysta, M. Effects of alfalfa saponins on the moth Spodoptera littoralis. J. Chem. Ecol. 2000, 26, 1065–1078. [Google Scholar] [CrossRef]
- Golawska, S. Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J. Chem. Ecol. 2007, 33, 1598–1606. [Google Scholar] [CrossRef]
- Sen, S.; Makkar, H.P.S.; Becker, K. Alfalfa saponins and their implication in animal nutrition. J. Agric. Food Chem. 1998, 46, 131–140. [Google Scholar] [CrossRef]
- Tava, A.; Odoardi, M. Saponins from Medicago sp.: Chemical characterization and biological activity against insects. In Saponins Used in Food and Agriculture; Waller, G.R., Yamasaki, K., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 97–109. [Google Scholar]
- Agrell, J.; Oleszek, W.; Stochmal, A.; Olsen, M.; Anderson, P. Herbivore-induced responses in alfalfa (Medicago sativa). J. Chem. Ecol. 2003, 29, 303–320. [Google Scholar] [CrossRef]
- De Geyter, E.; Geelen, D.; Smagghe, G. First results on the insecticidal action of saponins. Commun. Agric. Appl. Biol. Sci. 2007, 72, 645–648. [Google Scholar]
- Gorski, P.M.; Miersch, J.; Ploszynski, M. Production and biological activity of saponins and canavanine in alfalfa seedlings. J. Chem Ecol. 1991, 17, 1135–1143. [Google Scholar] [CrossRef]
- Golawska, S.; Lukasik, I.; Leszczynski, B. Effect of alfalfa saponins and flavonoids on pea aphid. Entomol. Exp. Appl. 2008, 128, 147–153. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yu, C.Y.; Chung, I.M. Assessment of the phenolic profile, antimicrobial activity and oxidative stability of transgenic Perilla frutescens L. overexpressing tocopherol methyltransferase (g-tmt) gene. Plant Physiol. Biochem. 2017, 118, 77–87. [Google Scholar] [CrossRef]
- Tava, A.; Avato, P. Chemical and biological activity of triterpene saponins from Medicago species. Nat. Prod. Commun. 2006, 1, 1159–1180. [Google Scholar] [CrossRef]
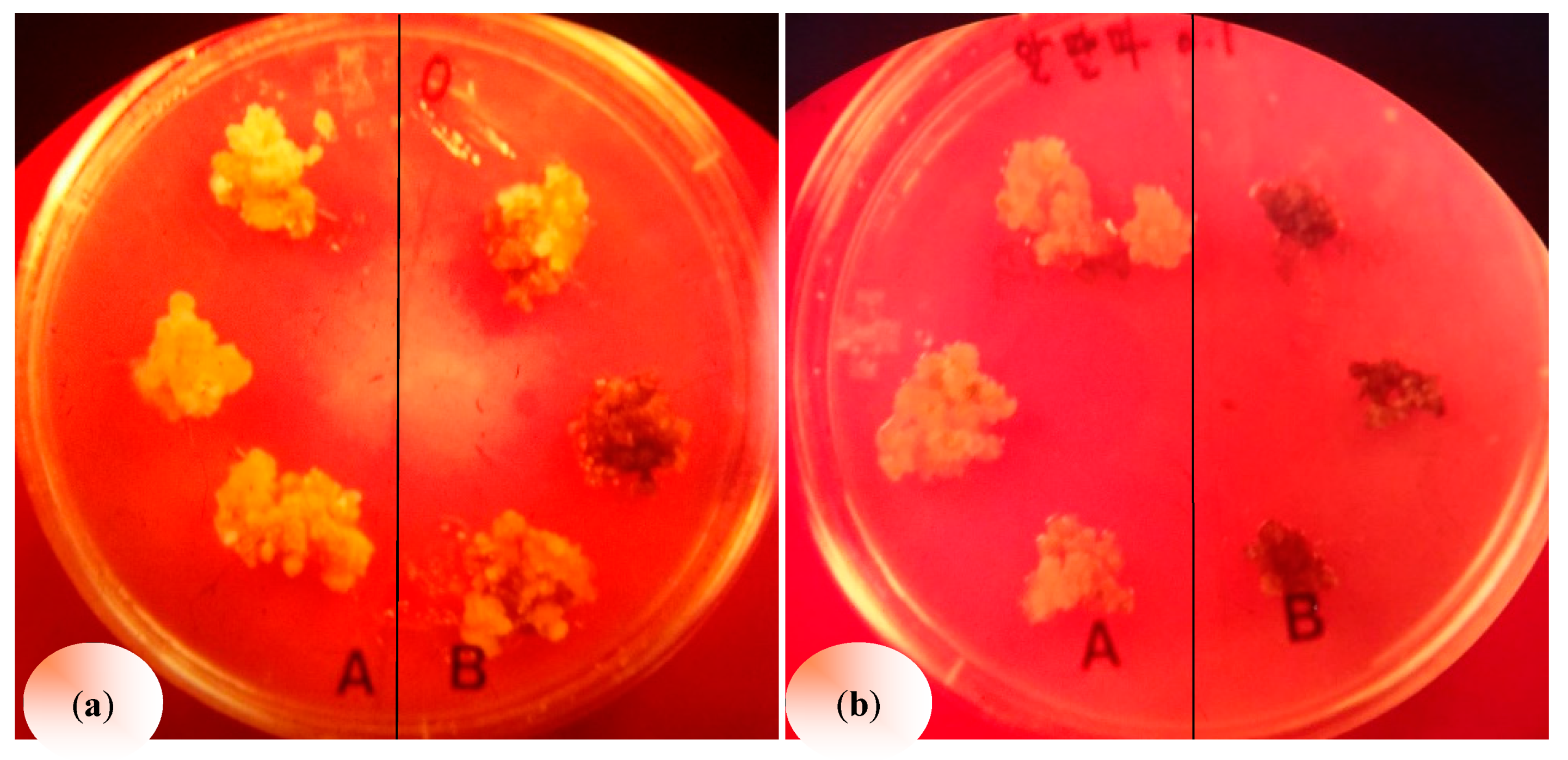
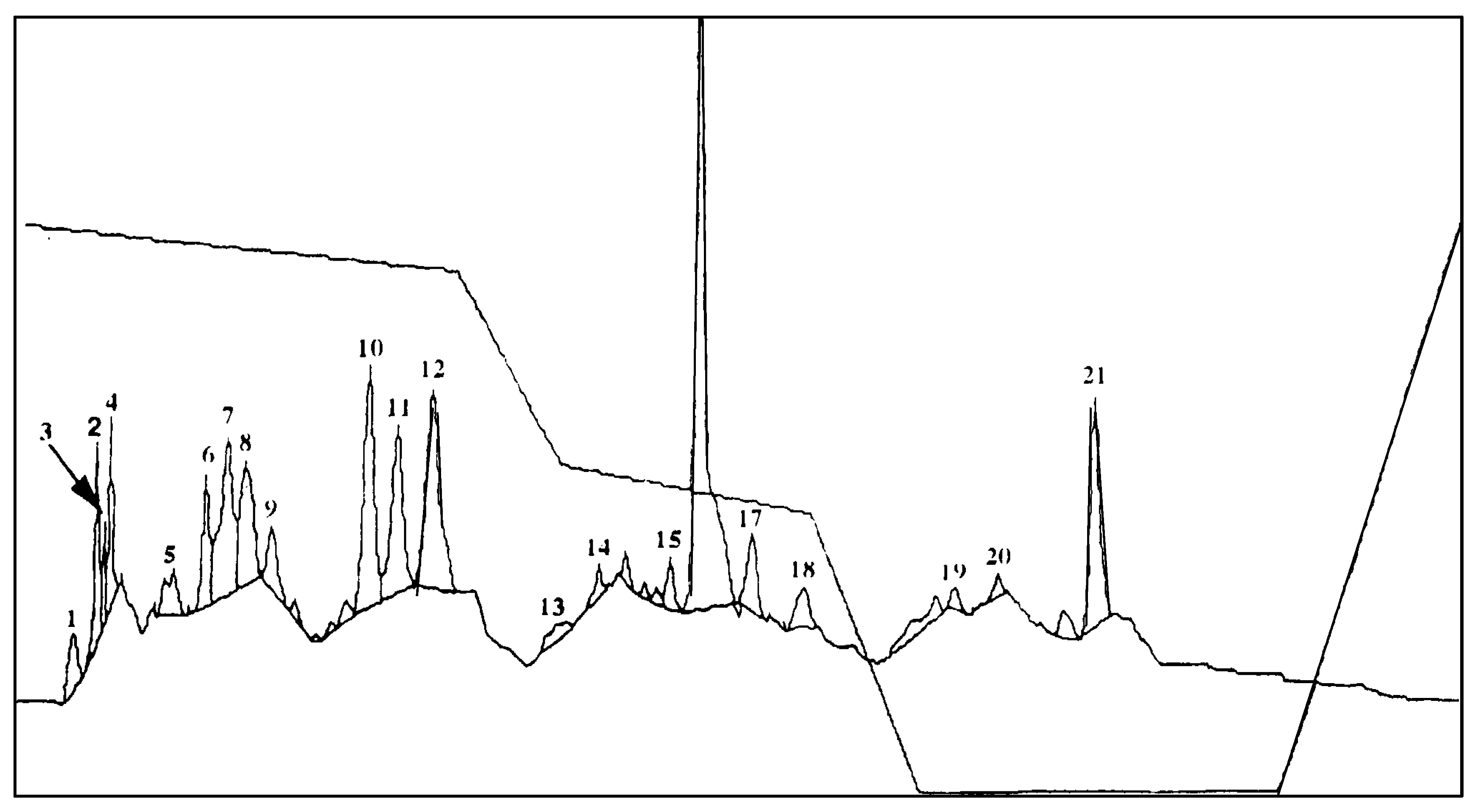
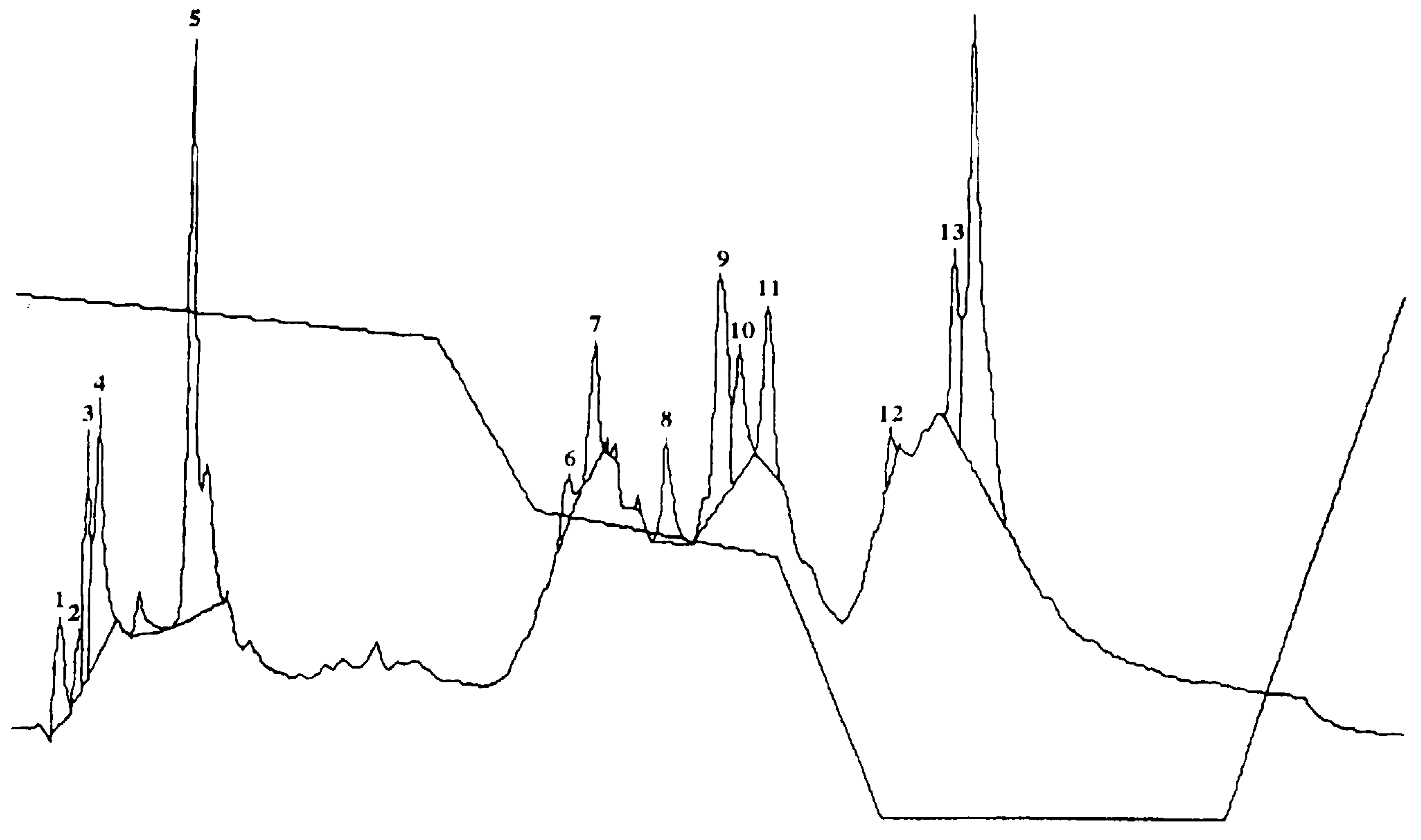

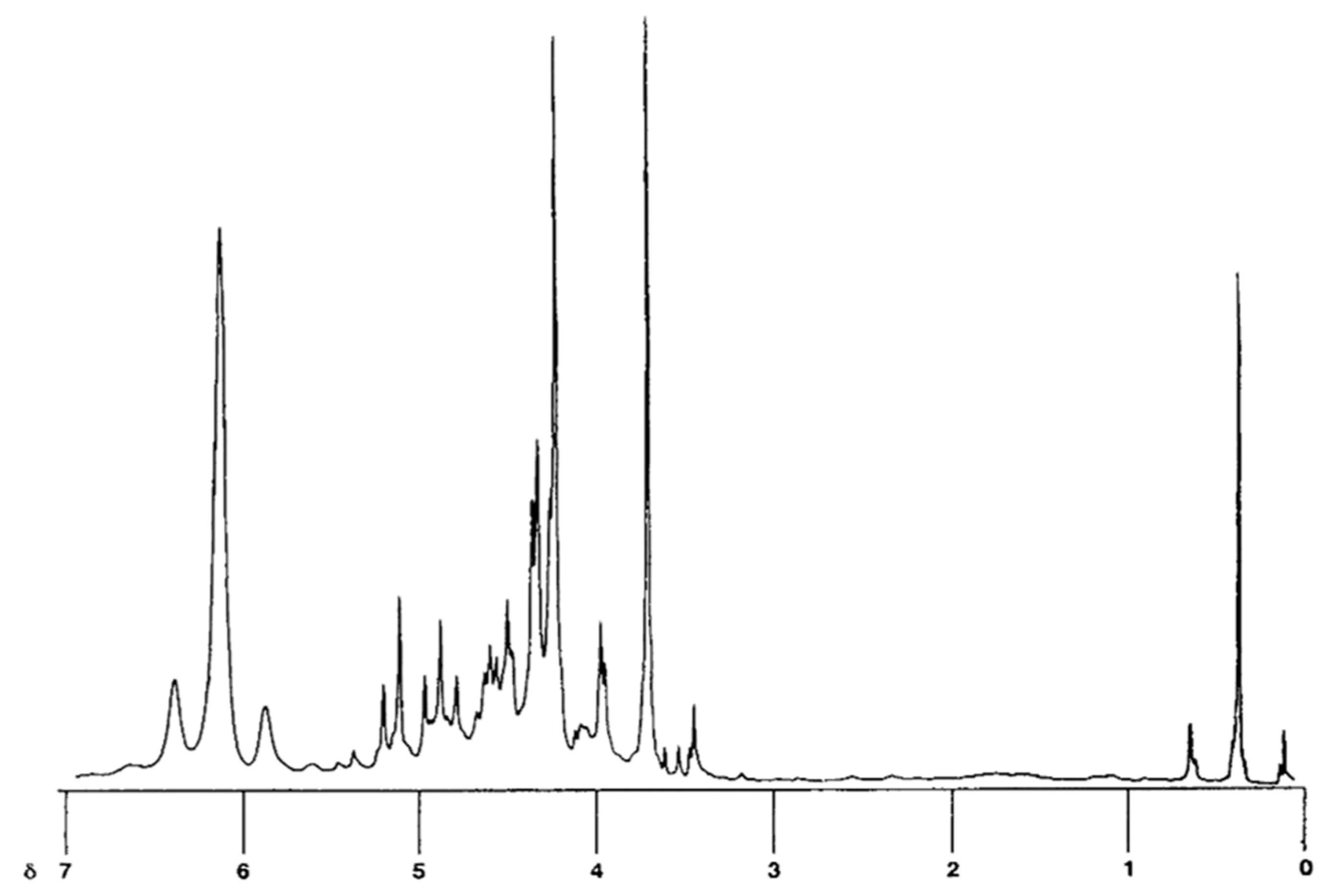
| Treatment | Concentration (%). | Callus Fresh Weight (mg) | ||||
|---|---|---|---|---|---|---|
| Digitaria ciliaris | Chenopodium album | Amaranthus lividus | Portulaca oleracea | Commelina communis | ||
| Control | 230.50 ± 3.50 b | 1130.18 ± 10.00 d | 250.10 ± 8.00 c | 1120.11 ± 15.00 d | 1130.20 ± 12.00 d | |
| MS + Alfalfa | 0.1 | 345.50 ± 4.00 c | 819.30 ± 10.50 c | 125.50 ± 4.00 b | 610.40 ± 5.00 c | 118.70 ± 5.90 c |
| 5 | 115.44 ± 5.00 a | 6.20 ± 0.40 b | 100.50 ± 2.50 a | 100.80 ± 6.80 a | 99.40 ± 3.90 b | |
| 10 | 115.40 ± 4.00 a | 5.20 ± 0.50 a | 125.50 ± 5.00 b | 105.80 ± 5.90 b | 59.90 ± 2.00 a | |
| Name of Phenolic Compounds | Phenolic Compound Concentration (µg/mL) |
|---|---|
| Salicylic acid | 1927.01 ± 12.50 f |
| p-Hydroxybenzoic acid | 372.01 ± 5.00 e |
| Vanillic acid | 137.50 ± 6.50 c |
| Syringic acid | 50.00 ± 3.40 a |
| p-Coumaric acid | 171.50 ± 5.80 d |
| Ferulic acid | 105.00 ± 3.80 b |
| Total | 2762.50 ± 13.50 g |
| Chemical | Concentration (M) | Portulaca oleracea | Chenopodium album | Pinellia ternata | |||
|---|---|---|---|---|---|---|---|
| Fresh Weight (mg) | Fresh Weight (%) | Fresh Weight (mg) | Fresh Weight (%) | Fresh Weight (mg) | Fresh Weight (%) | ||
| Salicylic acid | 10−5 | 140.10 ± 4.00 n | 19.80 ± 0.90 m | 26.90 ± 2.50 i | 18.50 ± 1.50 i | 160.20 ± 5.50 n | 64.10 ± 1.50 n |
| 10−3 | 30.50 ± 2.00 g | 4.20 ± 0.50 g | 11.70 ± 1.00 e | 8.10 ± 0.50 f | 82.30 ± 2.00 i | 32.90 ± 1.00 i | |
| 10−2 | 7.20 ± 0.50 d | 1.00 ± 0.10 d | 5.70 ± 0.20 b | 3.90 ± 0.20 c | 40.20 ± 1.50 f | 16.10 ± 1.00 f | |
| Syringic acid | 10−5 | 193.30 ± 5.90 p | 27.30 ± 2.00 n | 45.90 ± 2.00 l | 3.20 ± 0.40 b | 187.00 ± 11.00 p | 74.80 ± 4.00 p |
| 10−3 | 48.20 ± 2.50 i | 6.80 ± 0.50 i | 45.20 ± 4.00 k | 3.20 ± 0.20 b | 42.30 ± 2.00 g | 16.90 ± 1.50 g | |
| 10−2 | 4.40 ± 0.50 b | 0.60 ± 0.01 a | 3.70 ± 0.20 a | 2.60 ± 0.10 a | 3.80 ± 0.50 a | 1.80 ± 0.01 a | |
| Ferulic acid | 10−5 | 286.30 ± 10.50 | 4.00 ± 0.50 o | 82.40 ± 4.00 n | 56.70 ± 3.00 l | 190.00 ± 10.00 q | 76.00 ± 4.00 q |
| 10−3 | 45.80 ± 2.00 h | 6.50 ± 1.00 h | 94.70 ± 5.00 p | 65.20 ± 2.00 p | 84.20 ± 3.00 k | 33.70 ± 2.00 k | |
| 10−2 | 4.10 ± 0.30 a | 0.60 ± 0.01 a | 8.60 ± 0.50 c | 5.90 ± 0.30 d | 15.30 ± 1.50 c | 6.10 ± 0.10 c | |
| Vanillic acid | 10−5 | 93.40 ± 3.50 l | 13.20 ± 1.00 k | 128.50 ± 2.00 o | 88.50 ± 4.00 o | 166.00 ± 3.00 o | 66.40 ±2.00 o |
| 10−3 | 90.70 ± 4.50 k | 12.80 ± 1.00 j | 41.80 ± 1.00 j | 28.80 ± 2.00 j | 82.00 ± 2.00 j | 32.60 ± 0.20 j | |
| 10−2 | 6.20 ± 1.00 c | 0.90 ± 0.10 c | 10.60 ± 0.50 d | 7.30 ± 0.50 e | 5.40 ± 0.40 b | 2.20 ± 0.10 b | |
| p-Coumaric acid | 10−5 | 95.60 ± 5.00 m | 13.50 ± 1.00 l | 82.80 ± 5.00 n | 57.00 ± 3.00 m | 85.50 ± 3.00 l | 34.20 ± 2.00 l |
| 10−3 | 23.20 ± 2.00 f | 3.30 ± 0.50 f | 14.30 ± 1.00 g | 9.90 ± 0.80 g | 32.10 ± 2.00 e | 12.80 ± 1.00 e | |
| 10−2 | 21.80 ± 2.00 e | 3.10 ± 0.50 e | 13.10 ± 0.80 f | 9.00 ± 1.00 g | 20.50 ± 2.00 d | 8.20 ± 0.50 d | |
| p-Hydroxybenzoic acid | 10−5 | 183.50 ± 6.80 o | 26.0 ± 2.50 n | 87.50 ± 2.00 o | 60.30 ± 3.00 n | 102.30 ± 6.50 m | 40.90 ± 3.00 m |
| 10−3 | 82.20 ± 3.90 j | 11.60 ± 1.00 j | 78.70 ± 3.00 m | 54.20 ± 4.00 k | 53.50 ± 2.00 h | 21.40 ± 1.50 h | |
| 10−2 | 22.60 ± 2.00 f | 3.20 ± 0.50 e | 20.00 ± 1.00 h | 13.80 ± 1.00 h | 21.30 ± 1.00 d | 8.50 ± 0.50 d | |
| Control | - | 707.8 ± 15.60 q | - | 145.2 ± 5.00 q | 250.0 ± 8.00 r | ||
| Fraction | Concentration (mg/mL) | Germination Percentage (%) | * TL (cm) | ** TW (mg) |
|---|---|---|---|---|
| Control | 90.00 ± 3.50 p | 8.60 ± 1.00 q | 2.04 ± 0.01 j | |
| 80% MeOH extract | 5 | 92.50 ± 4.00 r | 8.40 ± 1.10 o | 2.50 ± 0.02 j |
| 10 | 68.01 ± 2.00 k | 5.70 ± 0.50 l | 1.62 ± 0.01 g | |
| Organic phase (nonpolar compounds) | 5 | 85.50 ± 5.00 o | 6.50 ± 0.40 m | 1.79 ± 0.01 h |
| 10 | 65.30 ± 4.00 i | 5.10 ± 0.30 h | 1.54 ± 0.01 f | |
| Aqueous phase | 5 | 64.50 ± 3.90 g | 4.90 ± 0.20 g | 1.29 ± 0.01 c |
| 10 | 50.40 ± 2.00 a | 3.50 ± 0.11 a | 1.01 ± 0.01 a | |
| Organic phase (aglycones) | 5 | 90.10 ± 3.50 q | 8.30 ± 1.00 n | 1.96 ± 0.02 i |
| 10 | 60.60 ± 4.59 f | 4.10 ± 0.40 d | 1.33 ± 0.01 d | |
| Aqueous phase (glycosides) | 5 | 79.50 ± 5.00 n | 6.50 ± 0.60 m | 1.58 ± 0.01 f |
| 10 | 76.50 ± 3.00 m | 5.60 ± 0.20 k | 1.50 ± 0.01 f | |
| Organic phase (alkaline hydrolysis) | 5 | 79.50 ± 6.00 n | 5.30 ± 0.40 i | 1.75 ± 0.01 h |
| 10 | 53.60 ± 3.00 c | 3.60 ± 0.11 b | 1.16 ± 0.01 b | |
| Aqueous phase (alkaline hydrolysis) | 5 | 65.00 ± 3.50 h | 4.90 ± 0.20 g | 1.46 ± 0.01 e |
| 10 | 54.50 ± 2.50 d | 3.70 ± 0.30 c | 1.25 ± 0.01 c | |
| Organic phase (acid hydrolysis) | 5 | 92.50 ± 7.50 r | 8.50 ± 0.90 p | 2.04 ± 0.02 j |
| 10 | 65.70 ± 5.00 j | 4.80 ± 0.10 f | 1.50 ± 0.01 f | |
| Aqueous phase (acid hydrolysis) | 5 | 68.50 ± 3.50 l | 5.50 ± 0.40 j | 1.54 ± 0.01 f |
| 10 | 53.50 ± 0.05 b | 4.70 ± 0.40 e | 1.33 ± 0.01 d |
| Phenolic Compound | Germination Percentage (%) | Seedling Length (cm) | Seedling Weight (mg) |
|---|---|---|---|
| Salicylic acid | 74.00 ± 2.00 c | 5.40 ± 0.20 b | 1.60 ± 0.1 b |
| Scopoletin | 69.50 ± 1.50 b | 5.60 ± 0.30 c | 1.63 ± 0.1 c |
| Rutin | 82.30 ± 2.50 d | 5.90 ± 0.20 d | 1.69 ± 0.1 d |
| Quercetin | 57.30 ± 2.10 a | 4.90 ± 0.10 a | 1.42 ± 0.2 a |
| Control | 91.30 ± 1.50 e | 8.40 ± 0.50 e | 1.98 ± 0.1 e |
| Saponin | Concentration (ppm) | Germination (%) |
|---|---|---|
| Medicagenic acid | 500 | 68.7 ± 3.50 b |
| Soyasaponin I | 500 | 75.6 ± 6.80 c |
| 3-Glc-Glc, 28 AraRhaXyl medicagenic acid | 500 | 0 a |
| 3-Glc, 28-Glc medicagenic acid | 500 | 87.3 ± 5.50 e |
| 3-Glc, 28 AraRhaXyl medicagenic acid | 500 | 92.4 ± 8.90 f |
| 3-GlcA, 28 AraRha medicagenic acid | 500 | 86.7 ± 6.50 e |
| Zanhic acid tridesmoside | 500 | 85.4 ± 5.00 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.-M. Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals. Plants 2019, 8, 233. https://doi.org/10.3390/plants8070233
Ghimire BK, Ghimire B, Yu CY, Chung I-M. Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals. Plants. 2019; 8(7):233. https://doi.org/10.3390/plants8070233
Chicago/Turabian StyleGhimire, Bimal Kumar, Balkrishna Ghimire, Chang Yeon Yu, and Ill-Min Chung. 2019. "Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals" Plants 8, no. 7: 233. https://doi.org/10.3390/plants8070233
APA StyleGhimire, B. K., Ghimire, B., Yu, C. Y., & Chung, I.-M. (2019). Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals. Plants, 8(7), 233. https://doi.org/10.3390/plants8070233







