The Investigation of Silicon Localization and Accumulation in Citrus
Abstract
1. Introduction
2. Results
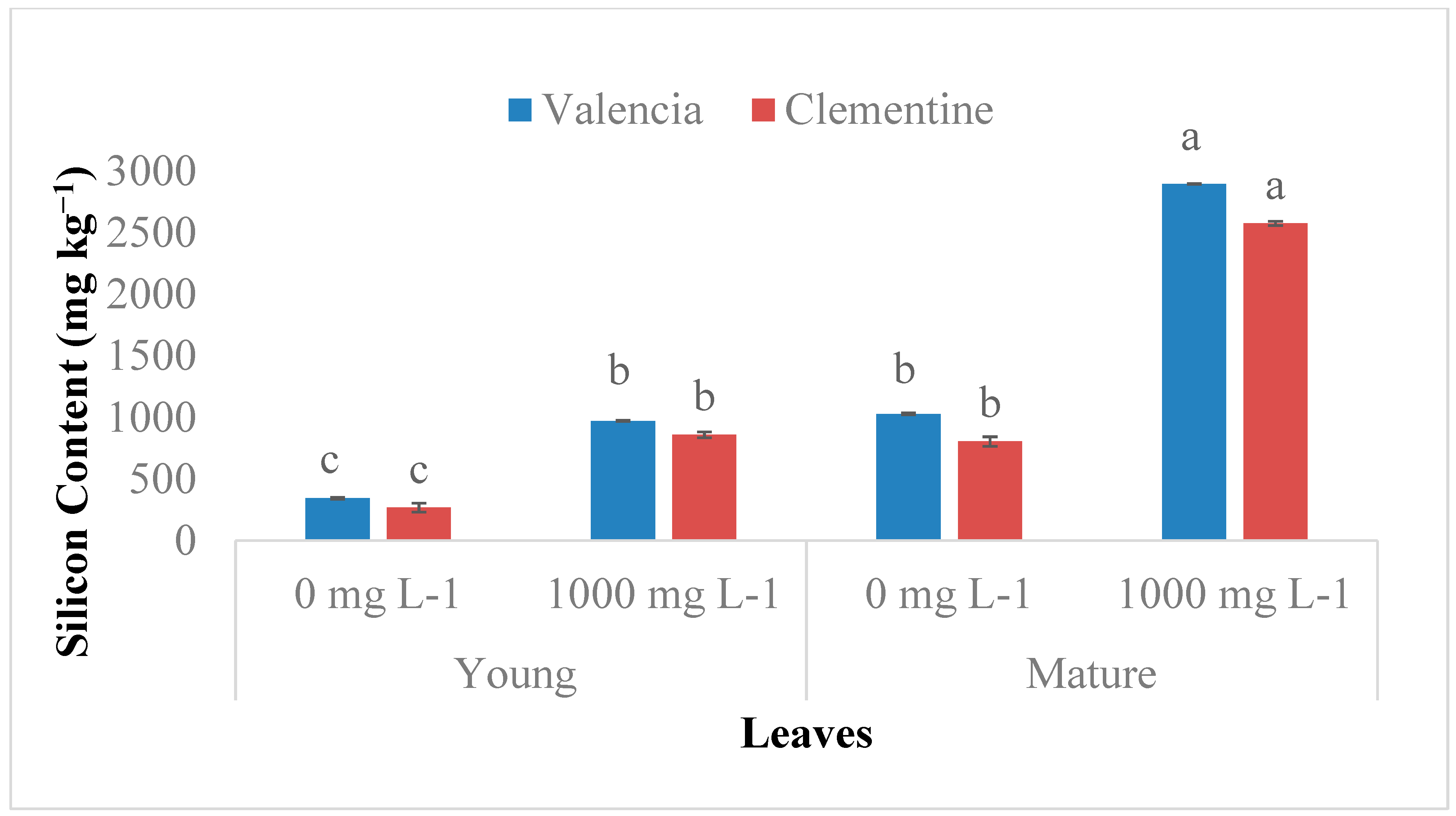
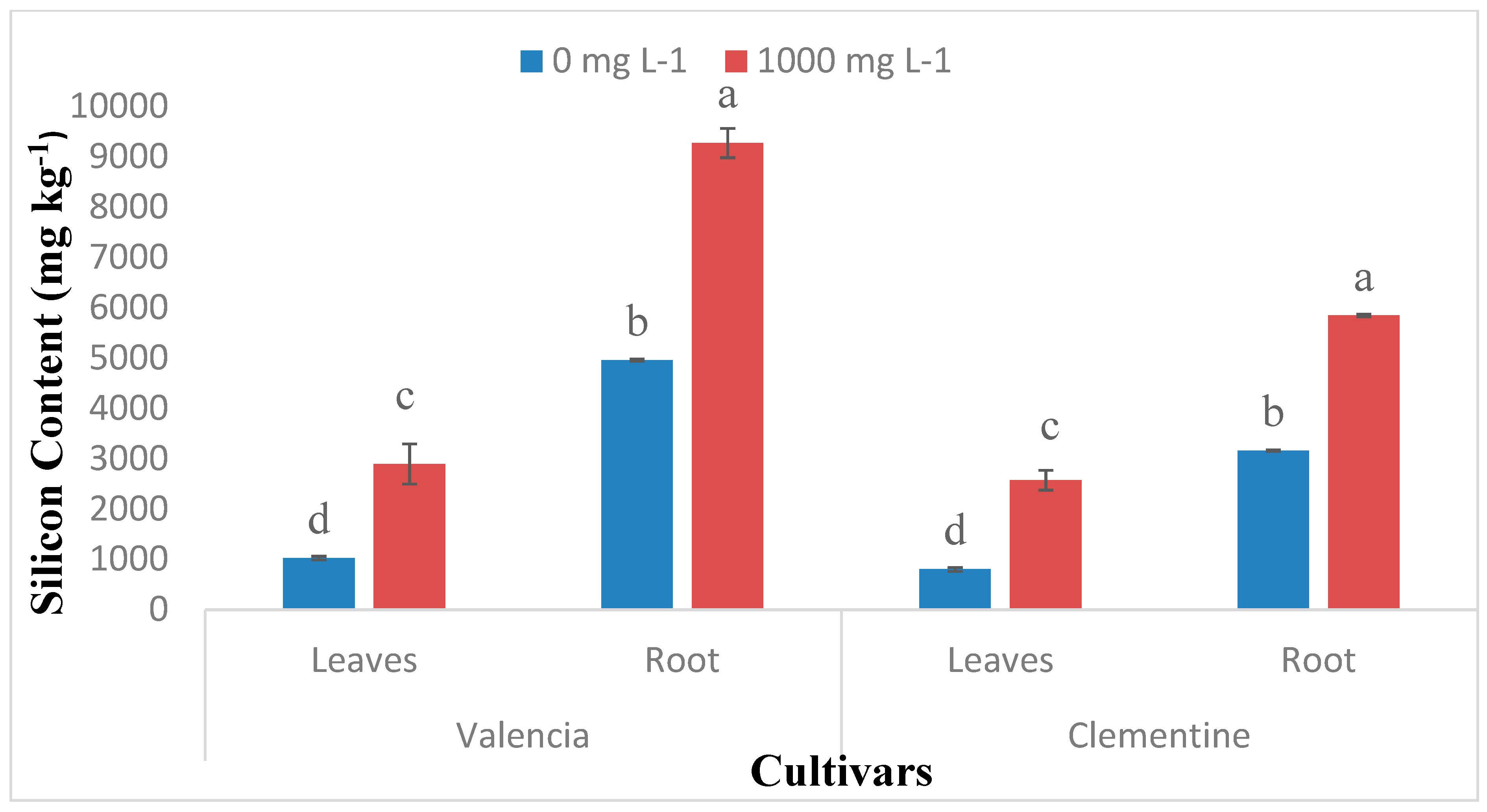
2.1. Si Accumulation in Citrus Species
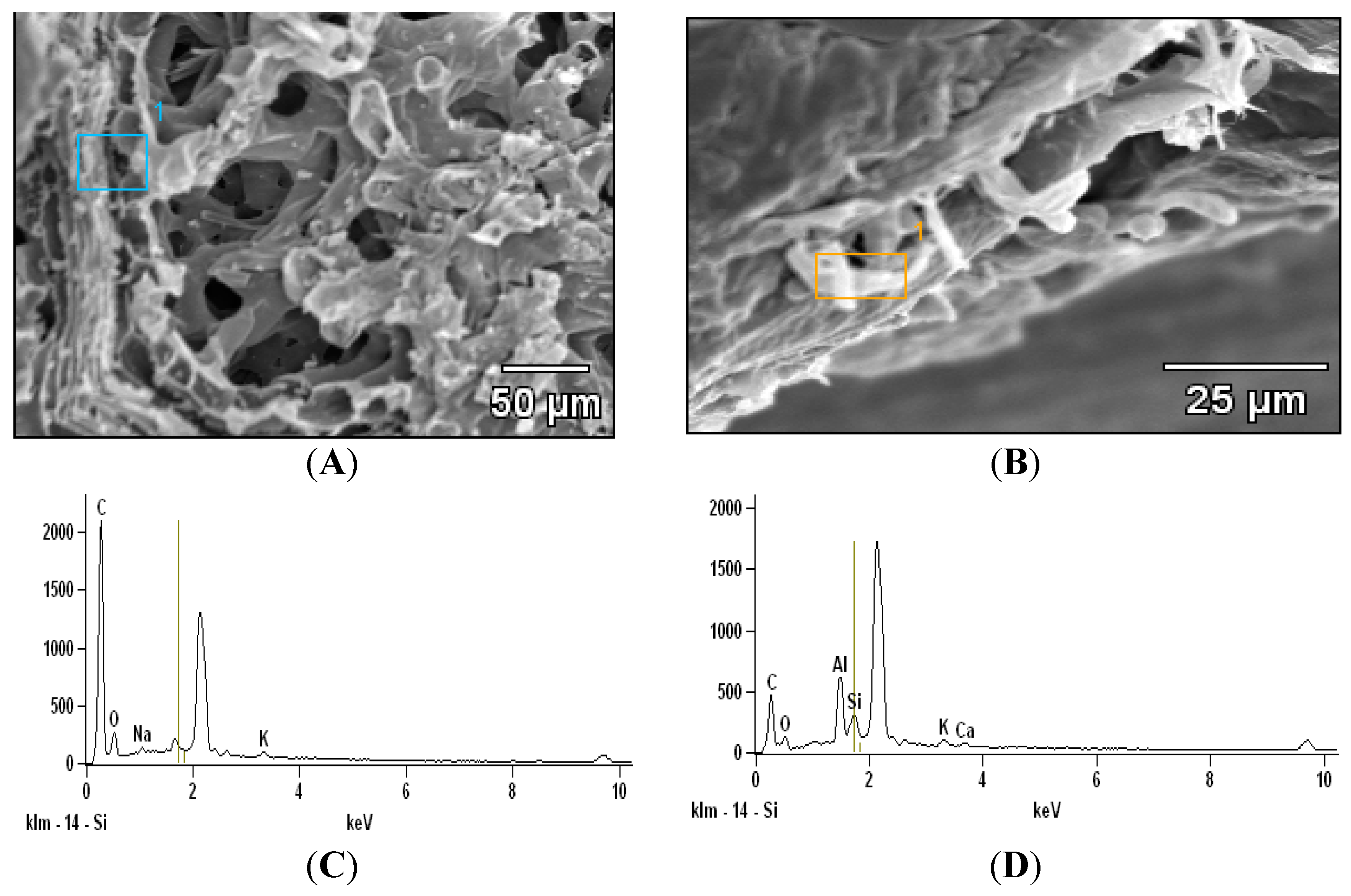
2.2. Scanning Electron Microscopy with Energy Dispersive X-ray Analysis
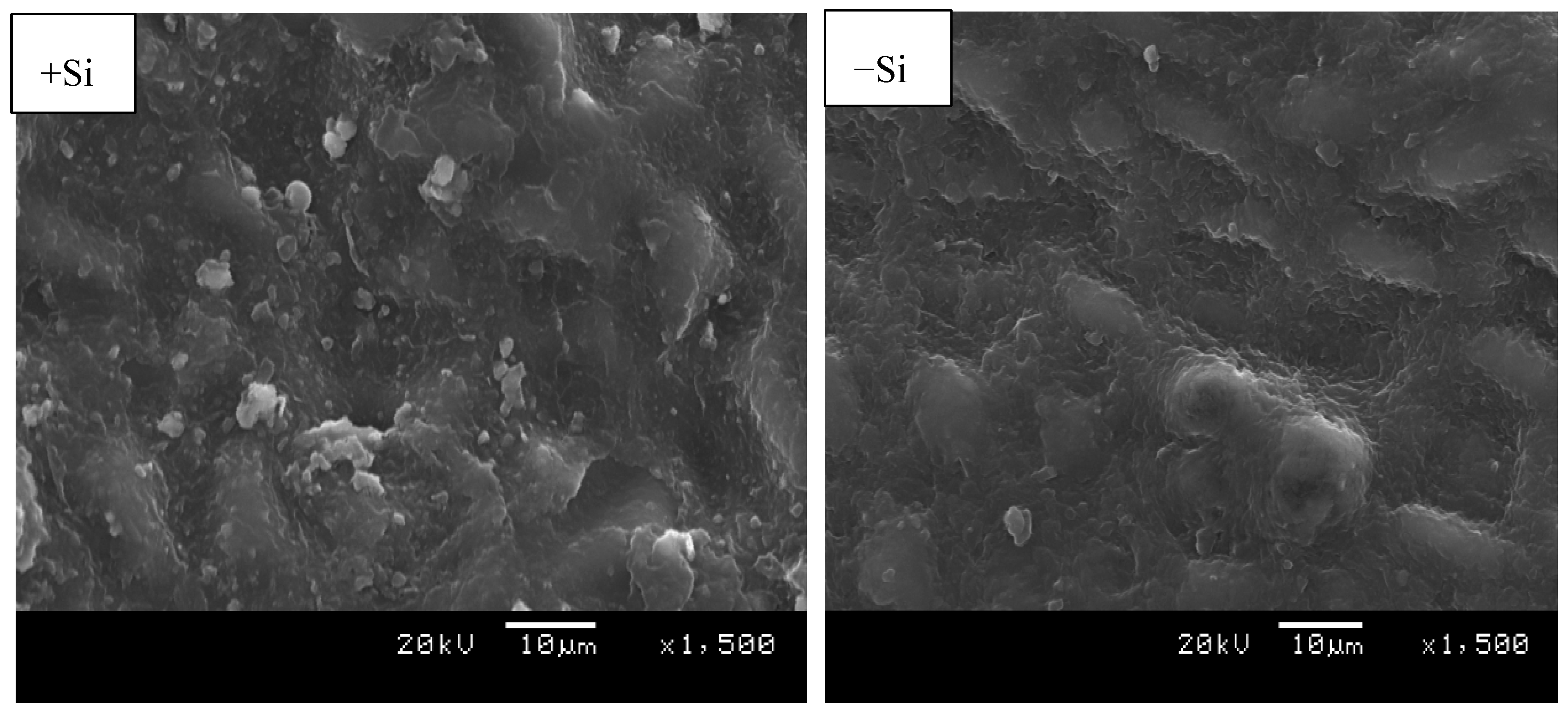
2.3. Environmental Scanning Electron Microscopy (ESEM)
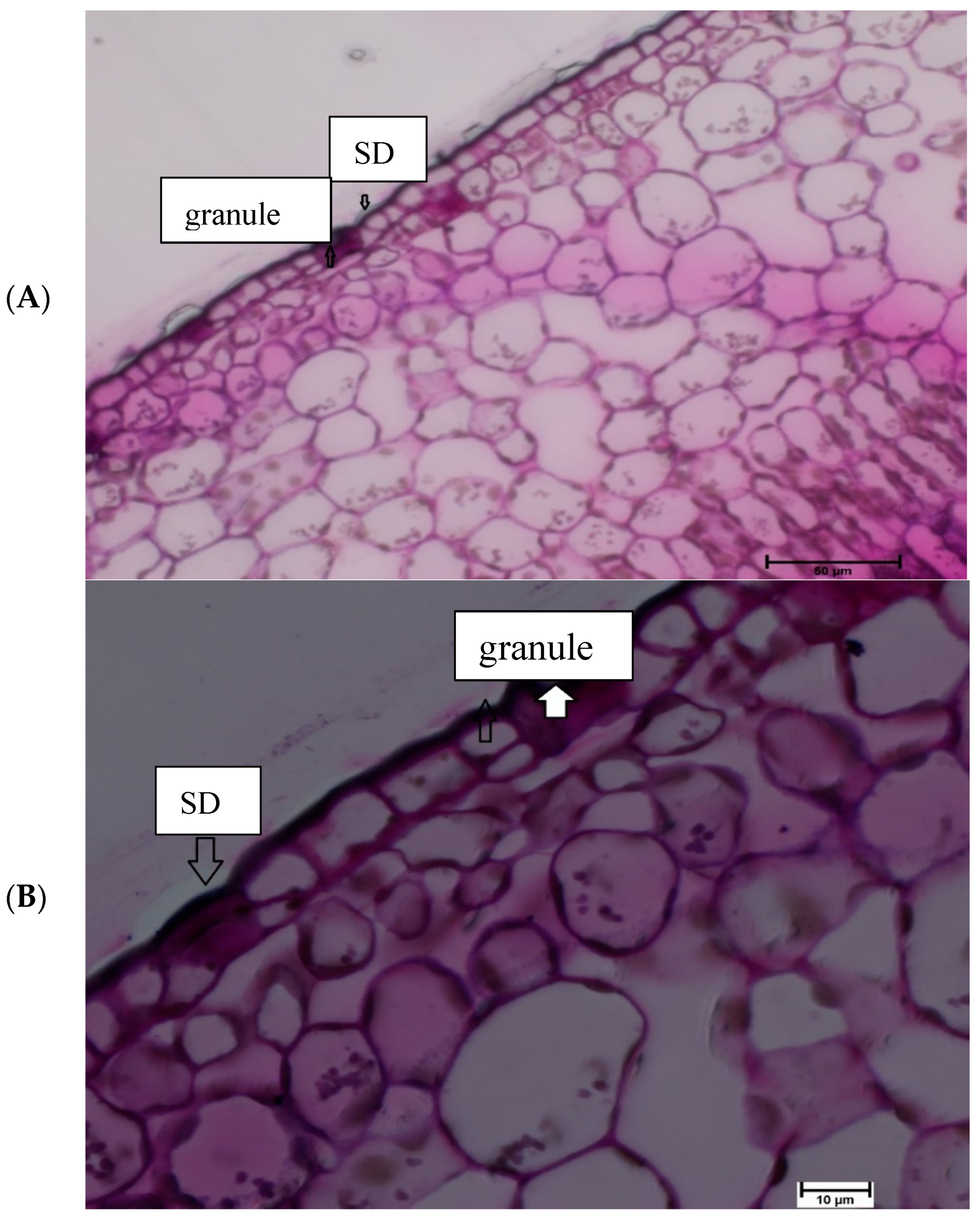
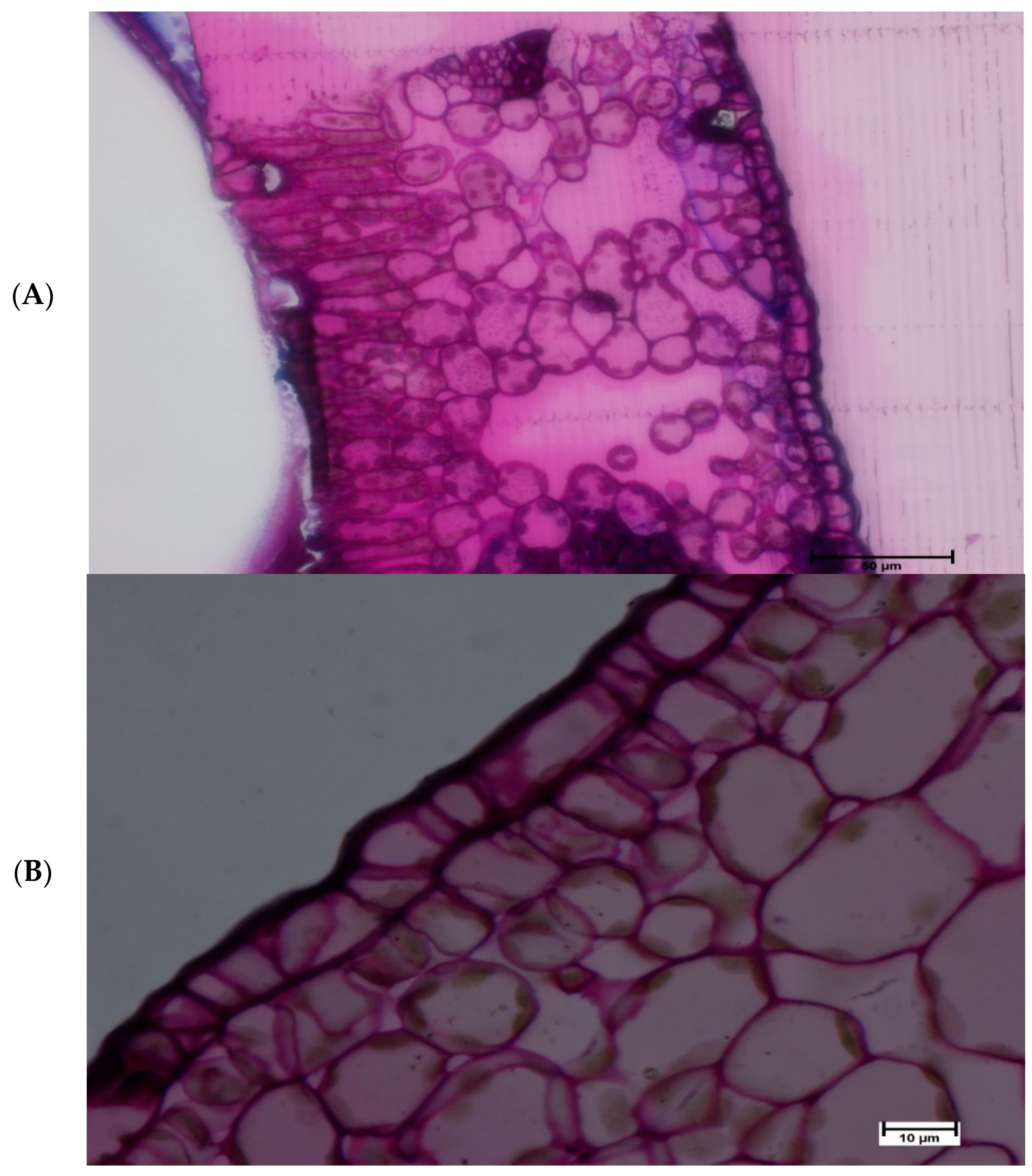
2.4. Light Microscopy
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Silicon Uptake Experiment
4.3. Si Analysis
4.4. Data Collection
4.4.1. Microscopic analysis
Scanning Electron Microscopy (SEM) & EDAX Analysis
Environmental Scanning Electron Microscopy (ESEM)
Light Electron Microscopy
4.4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birchall, J. The essentiality of silicon in biology. Chem. Soc. Rev. 1995, 24, 351–357. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Handreck, K. Silica in soils, plants, and animals. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1967; pp. 107–149. [Google Scholar]
- Epstein, E. Silicon. Ann Rev Plant Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Raven, J.A. Cycling silicon–The role of accumulation in plants. New Phytol. 2003, 158, 419–421. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef]
- Ma, F.J.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B 2011, 87, 377–385. [Google Scholar] [CrossRef]
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Ma, J.; Miyake, Y. The possibility of silicon as an essential element for higher plants. Comments Agric. Food Chem. 1990, 2, 99–102. [Google Scholar]
- Yamaji, N.; Chiba, Y.; Mitani-Ueno, N.; Ma, J.F. Functional characterization of a silicon transporter gene implicated in silicon distribution in barley. Plant Physiol. 2012, 160, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.B.; Susmitha, P. Silicon uptake, transportation and accumulation in Rice. J. Pharmacogn. Phytochem. 2017, 6, 290–293. [Google Scholar]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 is a silicon influx transporter in barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Rémus-Borel, W.; Belzile, F.; Ma, J.F.; Bélanger, R.R. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Motomura, H.; Fujii, T.; Suzuki, M. Distribution of silicified cells in the leaf blades of Pleioblastus chino (Franchet et Savatier) Makino (Bambusoideae). Ann. Bot. 2000, 85, 751–757. [Google Scholar] [CrossRef]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carriere) Rehder (Poaceae: Bambusoideae). Ann. Bot. 2004, 93, 235–248. [Google Scholar] [CrossRef] [PubMed]
- De Melo, S.P.; Monteiro, F.A.; De Bona, F.D. Silicon distribution and accumulation in shoot tissue of the tropical forage grass Brachiaria brizantha. Plant Soil. 2010, 336, 241–249. [Google Scholar] [CrossRef]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in abaxial epidermis before the opening of leaf blades of Pleioblastus chino (Poaceae, Bambusoideae). Ann. Bot. 2006, 97, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J. Silicon: A beneficial substance. Better Crops 2013, 97, 14–16. [Google Scholar]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Dayanandan, P.; Franklin, C.; Takeoka, Y. Structure and function of silica bodies in the epidermal system of grass shoots. Ann. Bot. 1985, 55, 487–507. [Google Scholar] [CrossRef]
- Taranovskaia, V.G. The silicification of subtropics greenhouse and plantations. Sov. Subtrop. 1939, 7, 32–37. [Google Scholar]
- Matichenkov, V.; Calvert, D.; Snyder, G. Silicon fertilizers for citrus in Florida. Proc. Fla. State Hortic. Soc. 1999, 112, 5–8. [Google Scholar]
- Matichenkov, V.; Bocharnikova, E.; Calvert, D. Response of citrus to silicon soil amendments. Proc. Fla. State Hortic. Soc. 2001, 114, 94–97. [Google Scholar]
- Wutscher, H. Growth and mineral nutrition of young orange trees grown with high levels of silicon. HortScience. 1989, 24, 3. [Google Scholar]
- Heine, G. Silicon Nutrition and Resistance against Pythium Aphanidermatum of Lycopersicon Esculentum and Mormodica Charantia. Ph.D. Thesis, University of Hannover, Hannover, Germany, 2005. [Google Scholar]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Marodin, J.C.; Resende, J.T.; Morales, R.G.; Silva, M.L.; Galvão, A.G.; Zanin, D.S. Yield of tomato fruits in relation to silicon sources and rates. Hortic. Bras. 2014, 32, 220–224. [Google Scholar] [CrossRef]
- Adatia, M.; Besford, R. The effects of silicon on cucumber plants grown in recirculating nutrient solution. Ann. Bot. 1986, 58, 343–351. [Google Scholar] [CrossRef]
- Liang, Y.; Hua, H.; Zhu, Y.G.; Zhang, J.; Cheng, C.; Römheld, V. Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol. 2006, 172, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Henriet, C.; Draye, X.; Oppitz, I.; Swennen, R.; Delvaux, B. Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil 2006, 287, 359–374. [Google Scholar] [CrossRef]
- Motomura, H.; Mita, N.; Suzuki, M. Silica accumulation in long-lived leaves of Sasa veitchii (Carrière) Rehder (Poaceae–Bambusoideae). Ann. Bot. 2002, 90, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Handreck, K. Uptake of silica by Trifolium incarnatum in relation to the concentration in the external solution and to transpiration. Plant Soil. 1969, 30, 71–80. [Google Scholar] [CrossRef]
- Ma, J.F.; Mitani, N.; Nagao, S.; Konishi, S.; Tamai, K.; Iwashita, T.; Yano, M. Characterization of the silicon uptake system and molecular mapping of the silicon transporter gene in rice. Plant Physiol. 2004, 136, 3284–3289. [Google Scholar] [CrossRef]
- Heine, G.; Tikum, G.; Horst, W.J. Silicon nutrition of tomato and bitter gourd with special emphasis on silicon distribution in root fractions. J. Plant Nutr. Soil Sci. 2005, 168, 600–606. [Google Scholar] [CrossRef]
- Casey, W.; Kinrade, S.; Knight, C.; Rains, D.; Epstein, E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 2004, 27, 51–54. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, MA, USA, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Zhang, C.; Wang, L.; Zhang, W.; Zhang, F. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant Soil. 2013, 372, 137–149. [Google Scholar] [CrossRef]
- Coradin, T.; Lopez, P.J. Biogenic silica patterning: Simple chemistry or subtle biology? ChemBioChem 2003, 4, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Sakai, W.S.; Sanford, W. A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscopy and energy dispersive X-ray analysis. Am. J. Bot. 1984, 71, 1315–1322. [Google Scholar] [CrossRef]
- Takahashi, N.; Kato, Y.; Isogai, A.; Kurata, K. Silica distribution on the husk epidermis at different parts of the panicle in rice (Oryza sativa L.) determined by X-ray microanalysis. Plant Prod. Sci. 2006, 9, 168–171. [Google Scholar] [CrossRef][Green Version]
- Naidoo, P.; McFarlane, S.; Keeping, M.; Caldwell, P. Deposition of silicon in leaves of sugarcane (Saccharum spp. hybrids) and its effect on the severity of brown rust caused by Puccinia melanocephala. In Proceedings of the Annual Congress-South African Sugar Technologists’ Association, Durban, South Africa, 2009. [Google Scholar]
- Schurt, D.A.; Cruz, M.F.; Nascimento, K.J.; Filippi, M.C.; Rodrigues, F.A. Silicon potentiates the activities of defense enzymes in the leaf sheaths of rice plants infected by Rhizoctonia solani. Trop. Plant Pathol. 2014, 39, 457–463. [Google Scholar] [CrossRef]
- Inanaga, S.; Okasaka, A.; Tanaka, S. Does silicon exist in association with organic compounds in rice plant? Soil Sci. Plant Nutr. 1995, 41, 111–117. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathol. 2002, 92, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.; Sangster, A. Silica deposition in the inflorescence bracts of wheat (Triticum aestivum). II. X-ray microanalysis and backscattered electron imaging. Can. J. Bot. 1989, 67, 281–287. [Google Scholar] [CrossRef]
- Saunt, J. Citrus Varieties of the World, 2nd ed.; Sinclair International Limited: Norwich, UK, 2000. [Google Scholar]
- FSSA-MVSA. Fertiliser Handbook of South Africa, 6th ed.; The Fertilizer Society of South Africa: Lynnwood Ridge, South Africa, 2007; ISBN 090-907-186-1. [Google Scholar]
- Lux, A.L.M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 5. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mvondo-She, M.A.; Marais, D. The Investigation of Silicon Localization and Accumulation in Citrus. Plants 2019, 8, 200. https://doi.org/10.3390/plants8070200
Mvondo-She MA, Marais D. The Investigation of Silicon Localization and Accumulation in Citrus. Plants. 2019; 8(7):200. https://doi.org/10.3390/plants8070200
Chicago/Turabian StyleMvondo-She, Mireille Asanzi, and Diana Marais. 2019. "The Investigation of Silicon Localization and Accumulation in Citrus" Plants 8, no. 7: 200. https://doi.org/10.3390/plants8070200
APA StyleMvondo-She, M. A., & Marais, D. (2019). The Investigation of Silicon Localization and Accumulation in Citrus. Plants, 8(7), 200. https://doi.org/10.3390/plants8070200




