Intraspecific Fine-Root Trait-Environment Relationships across Interior Douglas-Fir Forests of Western Canada
Abstract
1. Introduction
2. Results
2.1. Fine-root Trait Response to Abiotic Factors
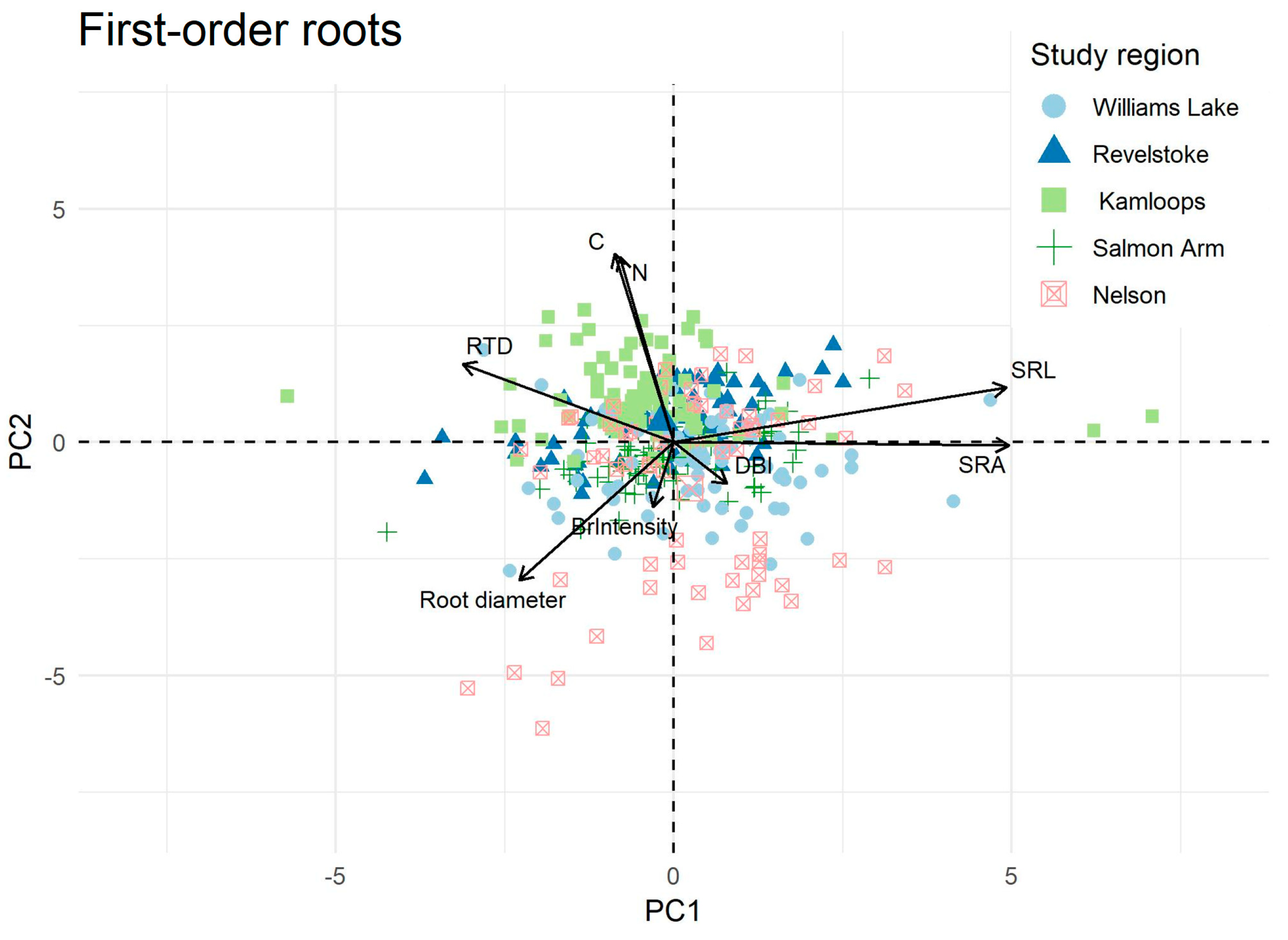
2.2. Ordination and Trait Correlation
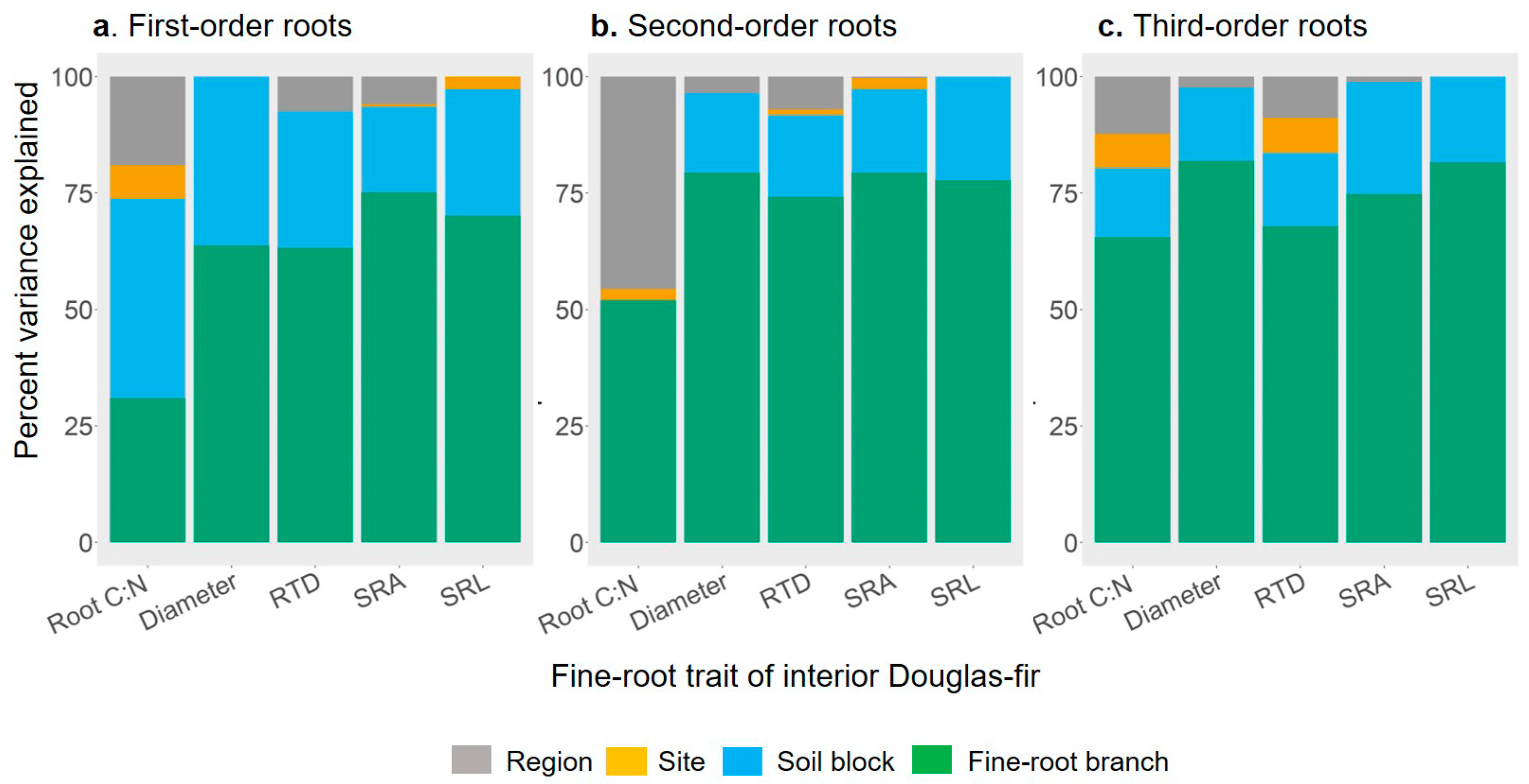
2.3. Partition of Fine-root Trait Variance
3. Discussion
3.1. Fine-root Functional Traits Relates to the Environment
3.1.1. Fine-root Morphology
3.1.2. Fine Root Chemistry and Architecture
3.2. Intraspecific Root Trait Variation
4. Conclusions
5. Materials and Methods
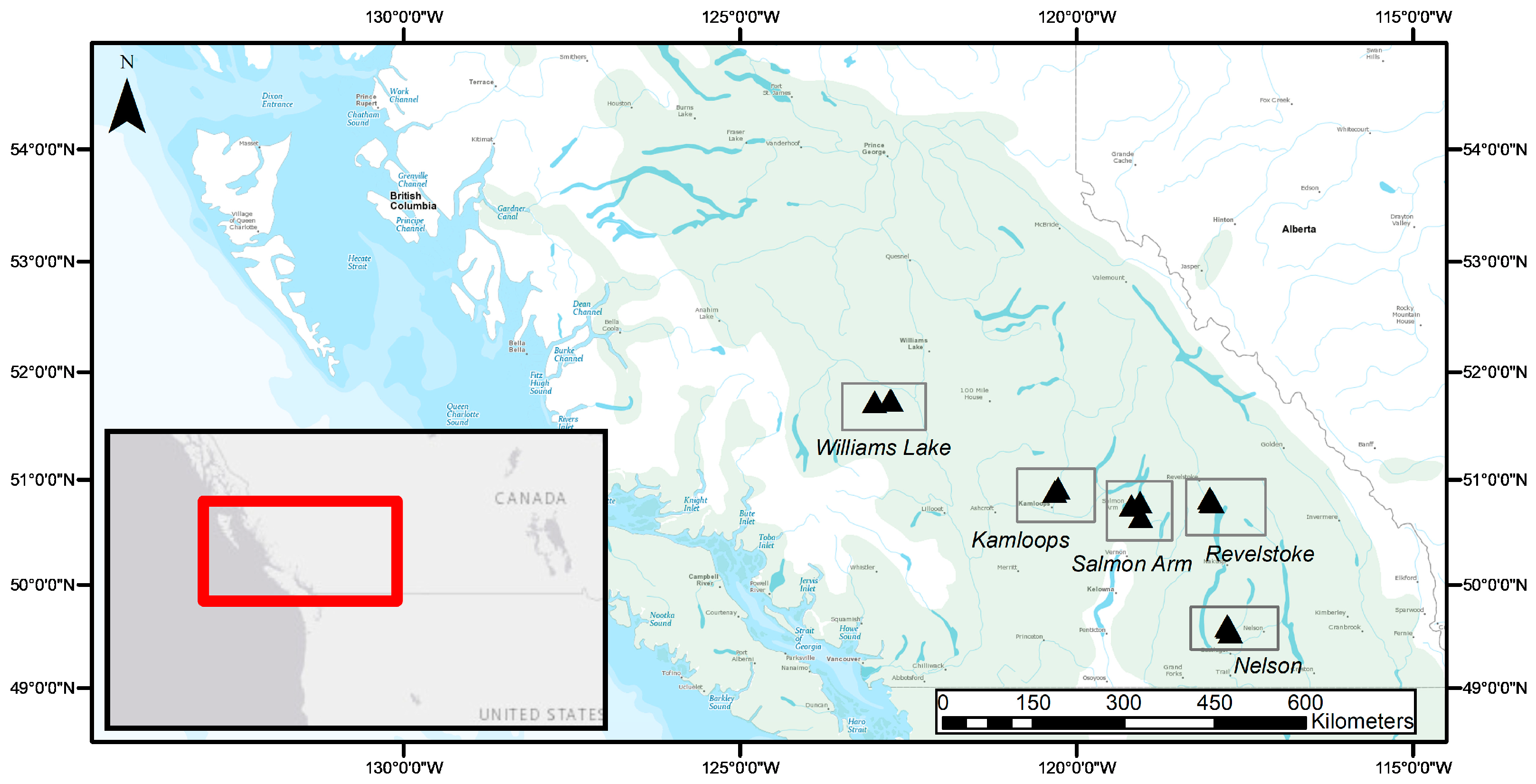
5.1. Biogeographic Gradient
5.2. Fine-Root Sampling and Processing
5.3. Fine-Root Traits Measurements
5.4. Climate and Soil Data
5.5. Data Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H.; et al. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ostonen, I.; Truu, M.; Helmisaari, H.-S.; Lukac, M.; Borken, W.; Vanguelova, E.; Godbold, D.L.; Lõhmus, K.; Zang, U.; Tedersoo, L.; et al. Adaptive root foraging strategies along a boreal-temperate forest gradient. New Phytol. 2017, 215, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; McCormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests: Soil-climate interactions explain functional trait variation. Glob. Ecol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Freschet, G.T.; Roumet, C.; Blackwood, C.B. A worldview of root traits: The influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol. 2017, 215, 1562–1573. [Google Scholar] [CrossRef]
- McCormack, L.M.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Q.; Zhao, N.; Xu, Z.; Zhu, X.; Jiao, C.; Yu, G.; He, N. Different phylogenetic and environmental controls of first-order root morphological and nutrient traits: Evidence of multidimensional root traits. Funct. Ecol. 2018, 32, 29–39. [Google Scholar] [CrossRef]
- Erktan, A.; Roumet, C.; Bouchet, D.; Stokes, A.; Pailler, F.; Munoz, F. Two dimensions define the variation of fine root traits across plant communities under the joint influence of ecological succession and annual mowing. J. Ecol. 2018, 106, 2031–2042. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Violle, C.; Pérez-Ramos, I.M.; Marañón, T.; Navarro-Fernández, C.M.; Olmo, M.; Villar, R. A Multidimensional Functional Trait Approach Reveals the Imprint of Environmental Stress in Mediterranean Woody Communities. Ecosystems 2018, 21, 248–262. [Google Scholar] [CrossRef]
- Zhou, M.; Bai, W.; Zhang, Y.; Zhang, W.-H. Multi-dimensional patterns of variation in root traits among coexisting herbaceous species in temperate steppes. J. Ecol. 2018, 106, 2320–2331. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 2018, 99, 1024–1030. [Google Scholar] [CrossRef]
- Zadworny, M.; McCormack, M.L.; Mucha, J.; Reich, P.B.; Oleksyn, J. Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol. 2016, 212, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; McCormack, M.L.; Żytkowiak, R.; Karolewski, P.; Mucha, J.; Oleksyn, J. Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob. Change Biol. 2017, 23, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Tanikawa, T.; Miyatani, K.; Hirano, Y. Intraspecific variation in morphological traits of root branch orders in Chamaecyparis obtusa. Plant Soil 2017, 416, 503–513. [Google Scholar] [CrossRef]
- Johnson, D.; Martin, F.; Cairney, J.W.G.; Anderson, I.C. The importance of individuals: Intraspecific diversity of mycorrhizal plants and fungi in ecosystems: Tansley review. New Phytol. 2012, 194, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Defrenne, C.E.; Philpott, T.J.; Guichon, S.H.A.; Roach, W.J.; Pickles, B.J.; Simard, S.W. Shifts in ectomycorrhizal fungal communities and exploration types relate to the environment and fine-root traits across interior Douglas-fir forests of western Canada. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Liese, R.; Alings, K.; Meier, I.C. Root Branching Is a Leading Root Trait of the Plant Economics Spectrum in Temperate Trees. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Leites, L.P.; Bradley St Clair, J.; Jaquish, B.C.; Sáenz-Romero, C.; López-Upton, J.; Joyce, D.G. Comparative genetic responses to climate in the varieties of Pinus ponderosa and Pseudotsuga menziesii: Clines in growth potential. For. Ecol. Manag. 2014, 324, 138–146. [Google Scholar] [CrossRef]
- Šmilauerová, M.; Šmilauer, P. Morphological responses of plant roots to heterogeneity of soil resources. New Phytol. 2002, 154, 703–715. [Google Scholar] [CrossRef]
- Beidler, K.V.; Taylor, B.N.; Strand, A.E.; Cooper, E.R.; Schönholz, M.; Pritchard, S.G. Changes in root architecture under elevated concentrations of CO2 and nitrogen reflect alternate soil exploration strategies. New Phytol. 2015, 205, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Iversen, C.M. Physical and functional constraints on viable belowground acquisition strategies. 2019; Submitted. [Google Scholar]
- Beiler, K.J.; Durall, D.M.; Simard, S.W.; Maxwell, S.A.; Kretzer, A.M. Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytol. 2010, 185, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.S.; Simard, S.W.; Jones, M.D.; Durall, D.M. Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 2013, 172, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chen, W.; Adams, T.S.; Wei, X.; Li, L.; McCormack, M.L.; DeForest, J.L.; Koide, R.T.; Eissenstat, D.M. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 2016, 97, 2815–2823. [Google Scholar] [CrossRef]
- Twieg, B.D.; Durall, D.M.; Simard, S.W. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 2007, 176, 437–447. [Google Scholar] [CrossRef]
- Agerer, R. Fungal relationships and structural identity of their ectomycorrhizae. Mycol. Prog. 2006, 5, 67–107. [Google Scholar] [CrossRef]
- Tobner, C.M.; Paquette, A.; Messier, C. Interspecific coordination and intraspecific plasticity of fine root traits in North American temperate tree species. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Messier, J.; Lechowicz, M.J.; McGill, B.J.; Violle, C.; Enquist, B.J. Interspecific integration of trait dimensions at local scales: The plant phenotype as an integrated network. J. Ecol. 2017, 105, 1775–1790. [Google Scholar] [CrossRef]
- Trocha, L.K.; Mucha, J.; Eissenstat, D.M.; Reich, P.B.; Oleksyn, J. Ectomycorrhizal identity determines respiration and concentrations of nitrogen and non-structural carbohydrates in root tips: A test using Pinus sylvestris and Quercus robur saplings. Tree Physiol. 2010, 30, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Pickles, B.J.; Genney, D.R.; Potts, J.M.; Lennon, J.J.; Anderson, I.C.; Alexander, I.J. Spatial and temporal ecology of Scots pine ectomycorrhizas. New Phytol. 2010, 186, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Pickles, B.J.; Gorzelak, M.A.; Green, D.S.; Egger, K.N.; Massicotte, H.B. Host and habitat filtering in seedling root-associated fungal communities: Taxonomic and functional diversity are altered in ‘novel’ soils. Mycorrhiza 2015, 25, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Meidinger, D.V.; Pojar, J. Ecosystems of British Columbia; Research Branch, Ministry of Forests: Victoria, BC, Canada, 1991; ISBN 978-0-7718-8997-4. [Google Scholar]
- Soil Classification Working Group. The Canadian System of Soil Classification; 3rd (revised); Agriculture and Agri-Food Canada Publication 1646: Ottawa, Canada, 1998. [Google Scholar]
- BC Ministry of Forests and Range. Field manual for describing terrestrial ecosystems; B.C. Ministry of Forests and Range: Victoria, Canada, 2010; ISBN 978-0-7726-6356-6.
- Delory, B.M.; Weidlich, E.W.A.; Meder, L.; Lütje, A.; van Duijnen, R.; Weidlich, R.; Temperton, V.M. Accuracy and bias of methods used for root length measurements in functional root research. Methods Ecol. Evol. 2017, 8, 1594–1606. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. FINE ROOT ARCHITECTURE OF NINE NORTH AMERICAN TREES. Ecol. Monogr. 2002, 72, 17. [Google Scholar] [CrossRef]
- Zadworny, M.; Eissenstat, D.M. Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytol. 2011, 190, 213–221. [Google Scholar] [CrossRef]
- Rose, L. Pitfalls in Root Trait Calculations: How Ignoring Diameter Heterogeneity Can Lead to Overestimation of Functional Traits. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally Downscaled and Spatially Customizable Climate Data for Historical and Future Periods for North America. PLOS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef]
- Kalra, Y.; Maynard, D. Methods Manual for Forest Soil and Plant Analysis; nformation Report NOR-X-319; Forestry Canada, Northwest region, Northern Forestry Center: Edmonton, AB, Canada, 1991; Available online: http://cfs.nrcan.gc.ca/pubwarehouse/pdfs/11845.pdf (accessed on May 1 2019).
- Hendershot, W.H.; Duquette, M. A Simple Barium Chloride Method for Determining Cation Exchange Capacity and Exchangeable Cations1. Soil Sci. Soc. Am. J. 1986, 50, 605. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. lme4: Linear Mixed-Effects Models using “Eigen” and S4. 2018. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on May 1 2019).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S.; Statistics and Computing, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. 2019; Available online: http://cran.r-project.org/package=sjPlot (accessed on 29 June 2019).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
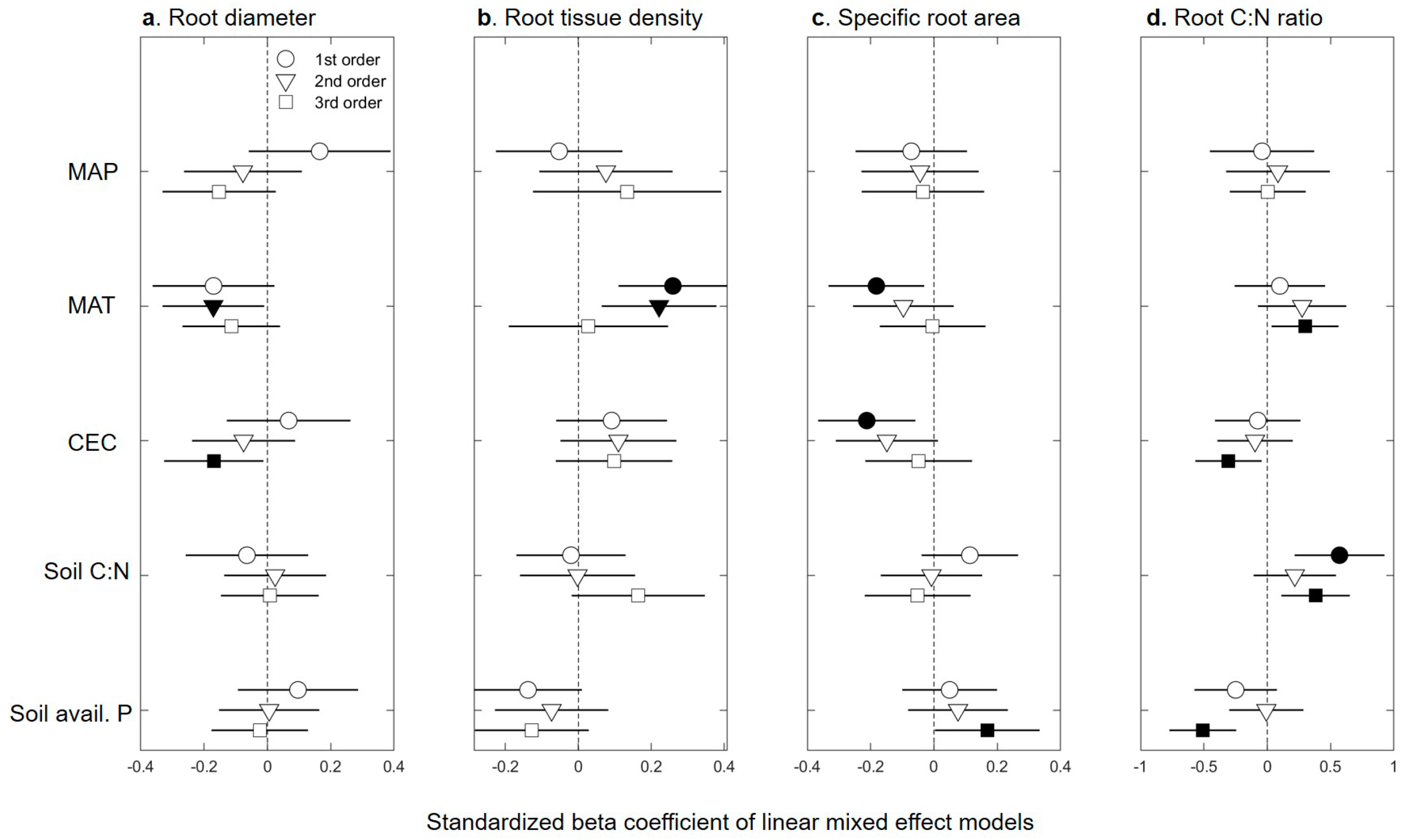
| Data Trans. | LR 1 χ2 | LR d.f. | p-Value | Model Fit | Predictor | Estimate | Standard Error | Wald χ2 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marginal R2 | Conditional R2 | ||||||||||
| Root diameter | |||||||||||
| 1st | NA | ||||||||||
| 2nd | Log10 | 5.43 | 1 | 0.02 | 0.03 | 0.19 | MAT 2 | −0.01045 | 0.00397 | 7.16 | 0.01 |
| 3rd | Log10 | 7.50 | 2 | 0.02 | 0.03 | 0.18 | MAP 3 | −0.00007 | 0.00003 | 6.82 | 0.01 |
| CEC 4 | −0.00173 | 0.00076 | 5.19 | 0.02 | |||||||
| Specific root area | |||||||||||
| 1st | Log10 | 9.03 | 2 | 0.01 | 0.05 | 0.19 | MAT | −0.00857 | 0.00572 | 5.56 | 0.02 |
| CEC | −0.00177 | 0.00059 | 7.33 | <0.01 | |||||||
| 2nd | Log10 | 3.83 | 1 | 0.05 | 0.02 | 0.18 | CEC | −0.00114 | 0.00056 | 4.10 | 0.04 |
| 3rd | Log10 | 4.81 | 1 | 0.03 | 0.02 | 0.22 | Soil avail. P 5 | 0.00029 | 0.00006 | 5.03 | 0.02 |
| Root tissue density | |||||||||||
| 1st | Log10 | 8.34 | 2 | 0.01 | 0.05 | 0.34 | MAT | 0.01826 | 0.00629 | 8.44 | <0.01 |
| Soil avail. P | −0.00010 | 0.00005 | 3.98 | 0.04 | |||||||
| 2nd | Log10 | 5.61 | 1 | 0.02 | 0.04 | 0.20 | MAT | 0.01464 | 0.00560 | 6.82 | <0.01 |
| 3rd | NA | ||||||||||
| Root C:N | |||||||||||
| 1st | Log10 | 9.19 | 1 | <0.01 | 0.28 | 0.60 | Soil C:N 6 | 0.00854 | 0.00236 | 13.12 | <0.01 |
| 2nd | NA | ||||||||||
| 3rd (multiple linear regression) | Log10 | F-value = 7.17 | 4 | <0.01 | Multiple R2 = 0.35 | Adjusted R2 = 0.30 | MAT | 0.02098 | 0.00965 | 4.72 | 0.03 |
| CEC | −0.00218 | 0.00087 | 6.3 | 0.02 | |||||||
| Soil C:N | 0.00538 | 0.00173 | 9.66 | <0.01 | |||||||
| Soil avail. P | −0.00028 | 0.00008 | 13.2 | <0.01 | |||||||
| Root branching intensity | Log10 | 3.97 | 1 | 0.05 | 0.02 | 0.18 | Soil avail. P | 0.00027 | 0.00013 | 4.07 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defrenne, C.E.; McCormack, M.L.; Roach, W.J.; Addo-Danso, S.D.; Simard, S.W. Intraspecific Fine-Root Trait-Environment Relationships across Interior Douglas-Fir Forests of Western Canada. Plants 2019, 8, 199. https://doi.org/10.3390/plants8070199
Defrenne CE, McCormack ML, Roach WJ, Addo-Danso SD, Simard SW. Intraspecific Fine-Root Trait-Environment Relationships across Interior Douglas-Fir Forests of Western Canada. Plants. 2019; 8(7):199. https://doi.org/10.3390/plants8070199
Chicago/Turabian StyleDefrenne, Camille E., M. Luke McCormack, W. Jean Roach, Shalom D. Addo-Danso, and Suzanne W. Simard. 2019. "Intraspecific Fine-Root Trait-Environment Relationships across Interior Douglas-Fir Forests of Western Canada" Plants 8, no. 7: 199. https://doi.org/10.3390/plants8070199
APA StyleDefrenne, C. E., McCormack, M. L., Roach, W. J., Addo-Danso, S. D., & Simard, S. W. (2019). Intraspecific Fine-Root Trait-Environment Relationships across Interior Douglas-Fir Forests of Western Canada. Plants, 8(7), 199. https://doi.org/10.3390/plants8070199




