Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique
Abstract
:1. Introduction to Genome Editing
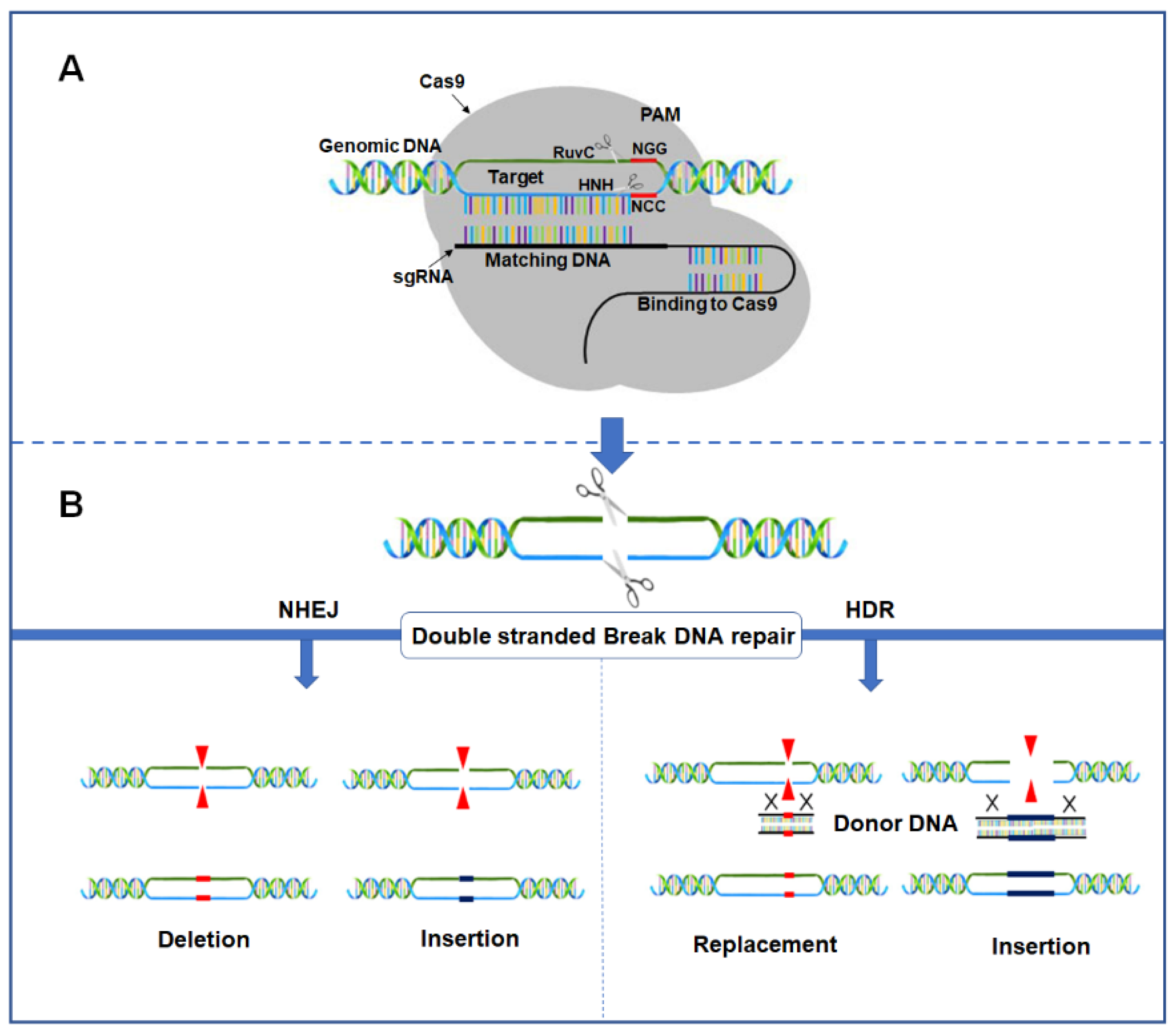
2. Mechanism and Application of the CRISPR/Cas9 System for Genome Editing in Plants
3. Genome Editing in Fruit Crops
4. Genome Editing in Vegetable Crops
5. Genome Editing in Ornamental Crops
6. CRISPR/Cas9 Genome Editing for Generation of Non-Transgenic Horticultural Crops
7. Challenges of CRISPR/Cas9 Genome Editing
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dhaliwal, A.K.; Mohan, A.; Sidhu, G.; Maqbool, R.; Gill, K.S. An ethylmethane sulfonate mutant resource in pre-green revolution hexaploid wheat. PLoS ONE 2015, 10, e0145227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, P.; Chawade, A.; Larsson, M.; Olsson, J.; Olsson, O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int. J. Plant Genomics 2011, 2011, 314829. [Google Scholar] [CrossRef] [Green Version]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pacher, M.; Puchta, H. From classical mutagenesis to nuclease-based breeding–directing natural DNA repair for a natural end-product. Plant J. 2017, 90, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 2004, 56, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Voytas, D.F. Plant genome engineering with sequence-specific nucleases. Annu. Rev. Plant Biol. 2013, 64, 327–350. [Google Scholar] [CrossRef]
- Weinthal, D.; Tovkach, A.; Zeevi, V.; Tzfira, T. Genome editing in plant cells by zinc finger nucleases. Trends Plant Sci. 2010, 15, 308–321. [Google Scholar] [CrossRef]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Huang, S.; Jiang, W.Z.; Wright, D.; Spalding, M.H.; Weeks, D.P.; Yang, B. TAL nucleases (TALNs): Hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain. Nucleic Acids Res. 2010, 39, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, J.D.; Dahlborg, E.J.; Goodwin, M.J.; Cade, L.; Zhang, F.; Cifuentes, D.; Curtin, S.J.; Blackburn, J.S.; Thibodeau-Beganny, S.; Qi, Y. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat. Methods 2010, 8, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Li, T.; Yang, B.; Spalding, M.H. TALEN-mediated genome editing: Prospects and perspectives. Biochem. J. 2014, 462, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Jia, M.; Chen, K.; Kong, X.; Khattak, B.; Xie, C.; Li, A.; Mao, L. CRISPR/Cas9: A powerful tool for crop genome editing. Crop J. 2016, 4, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Khatodia, S.; Bhatotia, K.; Passricha, N.; Khurana, S.; Tuteja, N. The CRISPR/Cas genome-editing tool: Application in improvement of crops. Front. Plant Sci. 2016, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef]
- Malzahn, A.; Lowder, L.; Qi, Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 2017, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Rani, R.; Yadav, P.; Barbadikar, K.M.; Baliyan, N.; Malhotra, E.V.; Singh, B.K.; Kumar, A.; Singh, D. CRISPR/Cas9: A promising way to exploit genetic variation in plants. Biotechnol. Lett. 2016, 38, 1991–2006. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Jansen, R.; van Embden, J.D.A.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, F.J.; Rodriguez-Valera, F. The discovery of CRISPR in archaea and bacteria. FEBS J. 2016, 283, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Karvelis, T.; Gasiunas, G.; Miksys, A.; Barrangou, R.; Horvath, P.; Siksnys, V. crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA Biol. 2013, 10, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Mojica, F.J.; Díez-Villaseñor, C.; García-Martínez, J.; Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 2009, 155, 733–740. [Google Scholar]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Steinert, J.; Schiml, S.; Fauser, F.; Puchta, H. Highly efficient heritable plant genome engineering using Cas9 orthologues from Streptococcus thermophilus and Staphylococcus aureus. Plant J. 2015, 84, 1295–1305. [Google Scholar] [CrossRef]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef]
- Zaidi, S.S.-E.-A.; Mahfouz, M.M.; Mansoor, S. CRISPR-Cpf1: A new tool for plant genome editing. Trends Plant Sci. 2017, 22, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiruvella, K.K.; Liang, Z.; Wilson, T.E. Repair of double-strand breaks by end joining. Cold Spring Harb. Perspect. Biol. 2013, 5, a012757. [Google Scholar] [CrossRef]
- Unniyampurath, U.; Pilankatta, R.; Krishnan, M. RNA interference in the age of CRISPR: Will CRISPR interfere with RNAi? Int. J. Mol. Sci. 2016, 17, 291. [Google Scholar] [CrossRef]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 9, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H.; Dujon, B.; Hohn, B. Two different but related mechanisms are used in plants for the repair of genomic double-strand breaks by homologous recombination. Proc. Natl. Acad. Sci. USA 1996, 93, 5055–5060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Meng, X.; Zong, Y.; Chen, K.; Zhang, H.; Liu, J.; Li, J.; Gao, C. Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nat. Plants 2016, 2, 16139. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Liu, T.; Li, C.; Jiao, B.; Li, S.; Hou, Y.; Luo, K. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci. Rep. 2015, 5, 12217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, B.; Weeks, D.P.; Spalding, M.H.; Yang, B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014, 42, 10903–10914. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Gao, C.; Qiu, J.-L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 17107. [Google Scholar] [CrossRef] [PubMed]
- Soda, N.; Verma, L.; Giri, J. CRISPR-Cas9 based plant genome editing: Significance, opportunities and recent advances. Plant Physiol. Biochem. 2018, 131, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, J.J.; Tang, T.; Liu, K.D.; Dai, C. CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci. Rep. 2017, 7, 7489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xingliang, M.; Yaoguang, L. CRISPR/Cas9-based genome editing systems and the analysis of targeted genome mutations in plants. Hereditas (Beijing) 2015, 38, 118–125. [Google Scholar]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Peterson, B.A.; Haak, D.C.; Nishimura, M.T.; Teixeira, P.J.; James, S.R.; Dangl, J.L.; Nimchuk, Z.L. Genome-wide assessment of efficiency and specificity in CRISPR/Cas9 mediated multiple site targeting in Arabidopsis. PLoS ONE 2016, 11, e0162169. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Mikami, M.; Toki, S. Biallelic gene targeting in rice. Plant Physiol. 2016, 170, 667–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svitashev, S.; Young, J.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted mutagenesis, precise gene editing and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Ni, H.; Xu, Y.; Chen, Q.; Jiang, L. CRISPR/Cas9-mediated base-editing system efficiently generates gain-of-function mutations in Arabidopsis. Sci. China Life Sci. 2017, 60, 520–523. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, H.; Xu, N.; Zhang, B.; Gou, F.; Zhu, J.-K. Application of the CRISPR–Cas system for efficient genome engineering in plants. Mol. Plant 2013, 6, 2008–2011. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, G.; Ma, S.; Xie, X.; Wu, X.; Zhang, X.; Wu, Y.; Zhao, P.; Xia, Q. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 2015, 87, 99–110. [Google Scholar] [CrossRef]
- Qin, G.; Gu, H.; Ma, L.; Peng, Y.; Deng, X.W.; Chen, Z.; Qu, L.J. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007, 17, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Alok, A.; Shivani; Kaur, N.; Pandey, P.; Awasthi, P.; Tiwari, S. CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Funct. Integr. Genomics 2018, 18, 89–99. [Google Scholar] [CrossRef]
- Naim, F.; Dugdale, B.; Kleidon, J.; Brinin, A.; Shand, K.; Waterhouse, P.; Dale, J. Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9. Transgenic Res. 2018, 27, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, F.M.; Harrison, K.; Armitage, A.D.; Simkin, A.J.; Harrison, R.J. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry. Plant Methods 2019, 15, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; LeBlanc, C.; Irish, V.F.; Jacob, Y. Rapid and efficient CRISPR/Cas9 gene editing in Citrus using the YAO promoter. Plant Cell Rep. 2017, 36, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Vergne, E.; Dousset, N.J.-P.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient targeted mutagenesis in apple and first time edition of pear using the CRISPR-Cas9 system. Front. Plant Sci. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitani, C.; Hirai, N.; Komori, S.; Wada, M.; Okada, K.; Osakabe, K.; Yamamoto, T.; Osakabe, Y. Efficient genome editing in apple using a CRISPR/Cas9 system. Sci. Rep. 2016, 6, 31481. [Google Scholar] [CrossRef]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS ONE 2017, 12, e0177966. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, S.; Li, D.; Zhang, Q.; Li, L.; Zhong, C.; Liu, Y.; Huang, H. Optimized paired-sgRNA/Cas9 cloning and expression cassette triggers high-efficiency multiplex genome editing in kiwifruit. Plant Biotechnol. J. 2018, 16, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zheng, X.; Huang, Y.; Ye, J.; Chen, P.; Zhang, C.; Zhao, F.; Xie, Z.; Zhang, S.; Wang, N. Genome sequencing and CRISPR/Cas9 gene editing of an early flowering Mini-Citrus (Fortunella hindsii). Plant Biotechnol. J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Furr, J.; Cooper, W.; Reece, P. An investigation of flower formation in adult and juvenile citrus trees. Am. J. Bot. 1947, 34, 1–8. [Google Scholar] [CrossRef]
- Nishikawa, F. Regulation of floral induction in citrus. J. Jpn. Soc. Hortic. Sci. 2013, 82, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Pillitteri, L.J.; Lovatt, C.J.; Walling, L.L. Isolation and characterization of a TERMINAL FLOWER homolog and its correlation with juvenility in citrus. Plant Physiol. 2004, 135, 1540–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liljegren, S.J.; Gustafson-Brown, C.; Pinyopich, A.; Ditta, G.S.; Yanofsky, M.F. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 1999, 11, 1007–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.; Gleave, A.P.; Allan, A.C. Mutagenesis of kiwifruit CENTRORADIALIS-like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottwald, T.R.; Graham, J.H.; Schubert, T.S. Citrus canker: The pathogen and its impact. Plant Health Prog. 2002, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, J.; Jia, H.; Sosso, D.; Li, T.; Frommer, W.B.; Yang, B.; White, F.F.; Wang, N.; Jones, J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA 2014, 111, E521–E529. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4:dCsLOB1.3 infection. Plant Biotechnol. J. 2016, 14, 1291–1301. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Dorantes-Acosta, A.E.; Sánchez-Hernández, C.V.; Arteaga-Vazquez, M.A. Biotic stress in plants: Life lessons from your parents and grandparents. Front. Genet. 2012, 3, 256. [Google Scholar] [CrossRef] [Green Version]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, B. Banana Streak Badnavirus Infection in Musa: Epidemiology, Diagnosis and Control; ASPAC Food & Fertilizer Technology Center: Taipei, Taiwan, 1995; Volume 143. [Google Scholar]
- Tripathi, J.N.; Ntui, V.O.; Ron, M.; Muiruri, S.K.; Britt, A.; Tripathi, L. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019, 2, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xie, J.; Duan, Y.; Hu, H.; Hu, Y.; Li, W. Genome-wide identification and expression profiling reveal tissue-specific expression and differentially-regulated genes involved in gibberellin metabolism between Williams banana and its dwarf mutant. BMC Plant Biol. 2016, 16, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene Cs LOB 1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Xu, J.; Orbović, V.; Zhang, Y.; Wang, N. Editing citrus genome via SaCas9/sgRNA system. Front. Plant Sci. 2017, 8, 2135. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Peng, A.; Xie, Z.; He, Y.; Zou, X. CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange (Citrus sinensis (L.) Osbeck). Plant Biotechnol. Rep. 2019, 13, 501–510. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Q.; Duan, Y.; Hall, D.; Gupta, G.; Stover, E. Regulation of citrus DMR6 via RNA interference and CRISPR/Cas9-mediated gene editing to improve Huanglongbing tolerance. In Proceedings of the Biotechnology and Genetic Enginneering-Odd, Fort Pierce, FL, USA, 30 July 2018; p. 13. [Google Scholar]
- Ren, F.; Ren, C.; Zhang, Z.; Duan, W.; Lecourieux, D.; Li, S.H.; Liang, Z.C. Efficiency optimization of CRISPR/Cas9-Mediated targeted mutagenesis in grape. Front. Plant Sci. 2019, 10, 612. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M. CRISPR–Cas9-mediated genome editing in apple and grapevine. Nat. Protoc. 2018, 13, 2844. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef]
- Martín-Pizarro, C.; Triviño, J.C.; Posé, D. Functional analysis of the TM6 MADS-box gene in the octoploid strawberry by CRISPR/Cas9-directed mutagenesis. J. Exp. Bot. 2018, 70, 885–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, G.; Liu, Z. Efficient genome editing of wild strawberry genes, vector development and validation. Plant Biotechnol. J. 2018, 16, 1868–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, B. Improvement of horticultural crops for abiotic stress tolerance: An introduction. HortScience 2011, 46, 1068–1069. [Google Scholar] [CrossRef] [Green Version]
- Lintas, C. Nutritional aspects of fruits and vegetables consumption. Options Mediterr. 1992, 19, 79–87. [Google Scholar]
- Unnevehr, L.J. Food safety issues and fresh food product exports from LDCs. Agric. Econ. 2000, 23, 231–240. [Google Scholar] [CrossRef]
- León, J.S.; Jaykus, L.A.; Moe, C.L. Food safety issues and the microbiology of fruits and vegetables. Microbiol. Safe Foods 2009, 255–281. [Google Scholar] [CrossRef]
- Ma, C.; Liu, M.; Li, Q.; Si, J.; Ren, X.; Song, H. Efficient BoPDS Gene Editing in Cabbage by the CRISPR/Cas9 System. Hortic. Plant J. 2019, 5, 164–169. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Gao, Q.; Zhang, J.; Zong, M.; Zhang, H.; Ren, Y.; Guo, S.; Gong, G.; Liu, F. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2017, 36, 399–406. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zheng, A.; Jiang, M.; Xue, S.; Yuan, Q.; Jiang, L.; Chen, Q.; Li, M.; Wang, Y.; Zhang, Y. CRISPR/Cas9-mediated mutagenesis of homologous genes in Chinese kale. Sci. Rep. 2018, 8, 16786. [Google Scholar] [CrossRef]
- Martinelli, F.; Uratsu, S.L.; Reagan, R.L.; Chen, Y.; Tricoli, D.; Fiehn, O.; Rocke, D.M.; Gasser, C.S.; Dandekar, A.M. Gene regulation in parthenocarpic tomato fruit. J. Exp. Bot. 2009, 60, 3873–3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueta, R.; Abe, C.; Watanabe, T.; Sugano, S.S.; Ishihara, R.; Ezura, H.; Osakabe, Y.; Osakabe, K. Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci. Rep. 2017, 7, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Moneruzzaman, K.; Hossain, A.; Sani, W.; Saifuddin, M.; Alenazi, M. Effect of harvesting and storage conditions on the post-harvest quality of tomato (Lycopersicon esculentum Mill) cv. Roma VF. Aust. J. Crop Sci. 2009, 3, 113–121. [Google Scholar]
- Yu, Q.-H.; Wang, B.; Li, N.; Tang, Y.; Yang, S.; Yang, T.; Xu, J.; Guo, C.; Yan, P.; Wang, Q. CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci. Rep. 2017, 7, 11874. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef] [Green Version]
- Nakayasu, M.; Akiyama, R.; Lee, H.J.; Osakabe, K.; Osakabe, Y.; Watanabe, B.; Sugimoto, Y.; Umemoto, N.; Saito, K.; Muranaka, T.; et al. Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol. Biochem. 2018, 131, 70–77. [Google Scholar] [CrossRef]
- Wang, R.; da Rocha Tavano, E.C.; Lammers, M.; Martinelli, A.P.; Angenent, G.C.; de Maagd, R.A. Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Sci. Rep. 2019, 9, 1696. [Google Scholar] [CrossRef]
- Soyk, S.; Müller, N.; Ju Park, S.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.B.; Lippman, Z. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Veillet, F.; Perrot, L.; Chauvin, L.; Kermarrec, M.-P.; Guyon-Debast, A.; Chauvin, J.-E.; Nogué, F.; Mazier, M. Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 2019, 20, 402. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Liu, C.; Zhao, R.; Wang, L.; Chen, L.; Yu, W.; Zhang, S.; Sheng, J.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Toledo Thomazella, D.P.; Brail, Q.; Dahlbeck, D.; Staskawicz, B. CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. bioRxiv 2016, 064824. [Google Scholar]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Liu, W.; Jiang, J.; Xu, L.; Huang, L.; Cao, J. Efficient genome editing of Brassica campestris based on the CRISPR/Cas9 system. Mol. Genet. Genomics 2019, 294, 1251–1261. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, C.; Zheng, M.; Liu, M.; Zhang, D.; Liu, B.; Li, Q.; Si, J.; Ren, X.; Song, H. CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic. Res. 2019, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front. Plant Sci. 2018, 9, 1594. [Google Scholar] [CrossRef]
- Stajič, E.; Kiełkowska, A.; Murovec, J.; Bohanec, B. Deep sequencing analysis of CRISPR/Cas9 induced mutations by two delivery methods in target model genes and the CENH3 region of red cabbage (Brassica oleracea var. capitata f. rubra). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 139, 227–235. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.G.; Qi, Y.; Baranski, R. Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.-S.; Feng, K.; Xiong, A.-S. CRISPR/Cas9-mediated multiply targeted mutagenesis in orange and purple carrot plants. Mol. Biotechnol. 2019, 61, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.; Gagneul, D.; Alves Dos Santos, H.; Etienne, A.; Hilbert, J.-L.; Rambaud, C. Efficient Genome Editing Using CRISPR/Cas9 Technology in Chicory. Int. J. Mol. Sci. 2019, 20, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Bertier, L.D.; Ron, M.; Huo, H.; Bradford, K.J.; Britt, A.B.; Michelmore, R.W. High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-induced modifications of NCED4 in lettuce (Lactuca sativa). G3 Genes Genomes Genet. 2018, 8, 1513–1521. [Google Scholar]
- Butler, N.M.; Atkins, P.A.; Voytas, D.F.; Douches, D.S. Generation and Inheritance of Targeted Mutations in Potato (Solanum tuberosum L.) Using the CRISPR/Cas System. PLoS ONE 2015, 10, e0144591. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Turesson, H.; Nicolia, A.; Fält, A.-S.; Samuelsson, M.; Hofvander, P. Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 2017, 36, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, S.; Wang, W.; Xiong, X.; Meng, F.; Cui, X. Efficient targeted mutagenesis in potato by the CRISPR/Cas9 system. Plant Cell Rep. 2015, 34, 1473–1476. [Google Scholar] [CrossRef] [Green Version]
- Tuncel, A.; Corbin, K.R.; Ahn-Jarvis, J.; Harris, S.; Hawkins, E.; Smedley, M.A.; Harwood, W.; Warren, F.J.; Patron, N.J.; Smith, A.M. Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol. J. 2019, 17, 2259–2271. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-mediated mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in tomato provides resistance against the parasitic weed Phelipanche aegyptiaca. Sci. Rep. 2019, 9, 11438. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Park, S.J.; Van Eck, J.; Lippman, Z.B. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 2016, 30, 2048–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. CRISPR/Cas9-mediated mutagenesis of lncRNA1459 alters tomato fruit ripening. Plant J. 2018, 94, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhi, V.; Bhattacharya, A.; Choudhary, S.; Pathak, R.; Gawade, V.; Palan, B.; Alamalakala, L.; Mikkilineni, V.; Char, B. Demonstration of CRISPR-cas9-mediated pds gene editing in a tomato hybrid parental line. Indian J. Genet. 2018, 78, 132–137. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, K.; Datsenka, T.U.; Liu, W.; Lv, S.; Lang, Z.; Wang, X.; Gao, J.; Wang, W.; Nie, W. Critical function of DNA methyltransferase 1 in tomato development and regulation of the DNA methylome and transcriptome. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef]
- Wang, D.; Samsulrizal, N.H.; Yan, C.; Allcock, N.S.; Craigon, J.; Blanco-Ulate, B.; Ortega-Salazar, I.; Marcus, S.E.; Bagheri, H.M.; Fons, L.P. Characterization of CRISPR Mutants Targeting Genes Modulating Pectin Degradation in Ripening Tomato. Plant Physiol. 2019, 179, 544–557. [Google Scholar]
- Scovel, G.; Ben-Meir, H.; Zuker, A.; Shklarman, E.; Ovadis, M.; Neta-Sharir, I.; Ben-Yephet, Y.; Weiss, D.; Watad, A.; Vainstein, A. Genetic engineering of agronomic and ornamental traits in carnation. In IV International Symposium on in Vitro Culture and Horticultural Breeding 560; ISHS: Tampere, Finland, 2001; pp. 91–94. [Google Scholar]
- Lema-Rumińska, J.; Zalewska, M. Changes in flower colour among Lady Group of Chrysanthemum × grandiflorum/Ramat/Kitam. as a result of mutation breeding. Folia Hortic. 2005, 17, 61–72. [Google Scholar]
- Sedgley, M. Cultivar development of ornamental members of the Proteaceae. In III International Protea Research Symposium 387; ISHS: Harare, Zimbabwe, 1995; pp. 163–170. [Google Scholar]
- Van Huylenbroeck, J. Ornamental Crops; Springer: Cham, Switzerland, 2018; Volume 11. [Google Scholar]
- Yan, R.; Wang, Z.; Ren, Y.; Li, H.; Liu, N.; Sun, H. Establishment of Efficient Genetic Transformation Systems and Application of CRISPR/Cas9 Genome Editing Technology in Lilium pumilum DC. Fisch. and Lilium longiflorum White Heaven. Int. J. Mol. Sci. 2019, 20, 2920. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yang, X.; Yang, C.; Li, M.; Guo, Y. Exploiting the CRISPR/Cas9 System for Targeted Genome Mutagenesis in Petunia. Sci. Rep. 2016, 6, 20315. [Google Scholar] [CrossRef]
- Chandler, S.F.; Sanchez, C. Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotechnol. J. 2012, 10, 891–903. [Google Scholar] [CrossRef]
- Tong, C.G.; Wu, F.H.; Yuan, Y.H.; Chen, Y.R.; Lin, C.S. High-efficiency CRISPR/Cas-based editing of Phalaenopsis orchid MADS genes. Plant Biotechnol. J. 2019. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Liao, D.C.; Chang, W.J.; Chou, M.L.; Huang, Y.T.; Chen, J.J.; Ko, S.S.; Chan, M.T.; Shih, M.C. Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla. Plant Biotechnol. J. 2016, 14, 284–298. [Google Scholar] [CrossRef]
- Pandey, S.; Ranade, S.; Nagar, P.; Kumar, N. Role of polyamines and ethylene as modulators of plant senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Noordegraaf, C. Production and marketing of high quality plants. In International Workshop on Floriculture & Nursery Industries and Environment 353; ISHS: Vertemate con Minoprio, Italy, 1994; pp. 134–148. [Google Scholar]
- John, P. Ethylene biosynthesis: The role of 1-aminocyclopropane-1-carboxylate (ACC) oxidase, and its possible evolutionary origin. Physiol. Plant. 1997, 100, 583–592. [Google Scholar] [CrossRef]
- Xu, J.; Kang, B.C.; Naing, A.H.; Bae, S.J.; Kim, J.S.; Kim, H.; Kim, C.K. CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 (ACO 1) enhances Petunia flower longevity. Plant Biotechnol. J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Res. 2018, 27, 25–38. [Google Scholar] [CrossRef]
- Nishihara, M.; Higuchi, A.; Watanabe, A.; Tasaki, K. Application of the CRISPR/Cas9 system for modification of flower color in Torenia fournieri. BMC Plant Biol. 2018, 18, 331. [Google Scholar] [CrossRef] [Green Version]
- Kishi-Kaboshi, M.; Aida, R.; Sasaki, K. Generation of Gene-Edited Chrysanthemum morifolium Using Multi-Copy Transgenes as Targets and Markers. Plant Cell Physiol. 2017, 58, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Subburaj, S.; Chung, S.J.; Lee, C.; Ryu, S.-M.; Kim, D.H.; Kim, J.-S.; Bae, S.; Lee, G.-J. Site-directed mutagenesis in Petunia× hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 2016, 35, 1535–1544. [Google Scholar] [CrossRef]
- Sun, L.; Kao, T.-H. CRISPR/Cas9-mediated knockout of PiSSK1 reveals essential role of S-locus F-box protein-containing SCF complexes in recognition of non-self S-RNases during cross-compatible pollination in self-incompatible Petunia inflata. Plant Reprod. 2018, 31, 129–143. [Google Scholar] [CrossRef]
- Anderson, N.O. Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Bisognin, D.A. Breeding vegetatively propagated horticultural crops. Crop Breed. Appl. Biotechnol. 2011, 11, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.M. Mutagenesis in crop improvement under the climate change. Rom. Biotechnol. Lett. 2010, 15, 88–106. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Gupta, R.; Singh, R.L. Biotechnological Tools to Enhance Sustainable Production. In Biotechnology for Sustainable Agriculture; Elsevier: Waltham, MA, USA, 2018; pp. 19–66. [Google Scholar]
- Hunter, P. “Genetically Modified Lite” placates public but not activists. EMBO Rep. 2014, 15, 138–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, K.; Podevin, N.; Breyer, D.; Carroll, D.; Herman, P. Engineering nucleases for gene targeting: Safety and regulatory considerations. New Biotechnol. 2014, 31, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S. Gene-edited plants on the plate: The ‘CRISPR cabbage story’. Physiol. Plant. 2018, 164, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Schaart, J.G.; van de Wiel, C.C.; Lotz, L.A.; Smulders, M.J. Opportunities for products of new plant breeding techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef]
- Chagné, D. Whole genome sequencing of fruit tree species. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 74, pp. 1–37. [Google Scholar]
- Hashimoto, R.; Ueta, R.; Abe, C.; Osakabe, Y.; Osakabe, K. Efficient multiplex genome editing induces precise, and self-ligated type mutations in tomato plants. Front. Plant Sci. 2018, 9, 916. [Google Scholar] [CrossRef]
- Labuhn, M.; Adams, F.F.; Ng, M.; Knoess, S.; Schambach, A.; Charpentier, E.M.; Schwarzer, A.; Mateo, J.L.; Klusmann, J.-H.; Heckl, D. Refined sgRNA efficacy prediction improves large-and small-scale CRISPR–Cas9 applications. Nucleic Acids Res. 2017, 46, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Zhang, H.; Lou, D.; Yu, D. Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci. Rep. 2016, 6, 21451. [Google Scholar] [CrossRef]
- Parmar, N.; Singh, K.H.; Sharma, D.; Singh, L.; Kumar, P.; Nanjundan, J.; Khan, Y.J.; Chauhan, D.K.; Thakur, A.K. Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: A comprehensive review. 3 Biotech 2017, 7, 239. [Google Scholar] [CrossRef]
- Zhao, H.; Wolt, J.D. Risk associated with off-target plant genome editing and methods for its limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar]
- Lassoued, R.; Macall, D.M.; Smyth, S.J.; Phillips, P.W.B.; Hesseln, H. Risk and safety considerations of genome edited crops: Expert opinion. Curr. Res. Biotechnol. 2019, 1, 11–21. [Google Scholar] [CrossRef]

| Species | Target Gene | Target Trait | Delivery Method | Reference |
|---|---|---|---|---|
| Apple | DIPM-1, DIPM-2, DIPM-4 | Fire blight disease resistance | PEG-mediated protoplast transfection | [85] |
| Apple | PDS | Albino phenotypes | Agrobacterium-mediated leaf discs transformation | [65] |
| Apple and Pear | PDS | Albino phenotypes | vacuum-infiltration in a suspension of Agrobacterium tumefaciens | [64] |
| TFL1 | Early flowering | |||
| Banana | PDS | Albino phenotype | Agrobacterium-mediated suspension cells transformation | [61] |
| Banana | PDS | Albino phenotypes | Agrobacterium- mediated embryogenic cell suspension cultures transformation | [60] |
| Banana | MaGA20ox2 | semi—dwarfing size | Agrobacterium-mediated suspension cells transformation | [84] |
| Banana | eBSV | Control of virus pathogenesis | Agrobacterium-mediated suspension cells transformation | [82] |
| Cacao | TcNPR3 | Phytophthora tropicalis resistance | Agrobacterium-mediated transient leaf transformation | [80] |
| Citrus (Carrizo Citrange) | PDS | Albino phenotypes | Agrobacterium-mediated epicotyl transformation | [63] |
| Citrus (Grapefruit) | CsLOB1 | Canker disease resistance | Agrobacterium-mediated epicotyl transformation | [86] |
| Citrus (Grapefruit) | PDS | Albino phenotype | Agrobacterium-mediated epicotyl transformation | [87] |
| Citrus (Kumquat) | PDS | Albino phenotypes | Agrobacterium-mediated epicotyl transformation | [68] |
| Citrus (Sweet Orange) | CsLOB1 | Canker disease resistance | Agrobacterium-mediated epicotyl transformation | [77] |
| Citrus (Sweet Orange) | CsWRKY22 | Canker disease resistance | Agrobacterium-mediated epicotyl transformation | [88] |
| Citrus (Sweet Orange) | DMR6 | Huanglongbing resistance | Agrobacterium-mediated epicotyl transformation | [89] |
| Grape | PDS | Albino phenotypes | Agrobacterium- mediated callus transformation | [66] |
| Grape | PDS | Albino phenotype | Agrobacterium-mediated suspension cells transformation | [90] |
| Grape and Apple | IdnDH | Biosynthesis of tartaric acid | Agrobacterium-mediated suspension cells transformation | [91] |
| Grape | L-idonate dehydrogenase gene (IdnDH) | Tartaric acid content | Agrobacterium-mediated suspension cells transformation | [92] |
| Grape | VvWRKY52 | Botrytis cinerea resistance | Agrobacterium-mediated somatic embryos transformation | [78] |
| Kiwifruit | PDS | Albino phenotype | Agrobacterium-mediated transformation | [67] |
| Strawberry | APETALA3 (AP3) | Flowering control | Agrobacterium-mediated leaf disk | [93] |
| Strawberry | Auxin Response Factor 8 (FvARF8) and Auxin biosynthesis gene (FveTAA1) | Auxin biosynthesis | Agrobacterium-mediated transformation | [94] |
| Strawberry | PDS | Albino phenotypes | Agrobacterium-mediated leaf and petiole transformation | [62] |
| Species | Target Gene | Target Trait | Delivery Method | Reference |
|---|---|---|---|---|
| Brassica campestris | pectin-methylesterase genes Bra003491, Bra007665, and Bra014410 | methylation of pectin | Agrobacterium-mediated transformation | [99] |
| Brassica oleracea var. capitata | Phytoene desaturase gene BoPDS, the S-receptor kinase gene BoSRK, and the male-sterility-associated gene BoMS1 | Albino phenotypes, Male sterility, self-incompatibility | Agrobacterium-mediated transformation | [100] |
| Cabbage | BoPDS | Albino phenotypes | Agrobacterium-mediated hypocotyl transformation | [101] |
| Cabbage | PDS and FRI | Albino phenotype and flowering | PEG-mediated protoplast transfection | [102] |
| Red cabbage | centromere-specific histone H3 (CENH3) | haploid lines induction | protoplast transformation and Agro infiltration | [103] |
| Carrot | Flavanone-3-hydroxylase (DcF3H) | Anthocyanin biosynthesis blockage | Agrobacterium-mediated callus transformation | [104] |
| Carrot | DcMYB113-like | Anthocyanin biosynthesis | Agrobacterium-mediated transformation | [105] |
| Chicory | CiPDS | Albino phenotype | Agrobacterium-mediated leaf sections and protoplast transfection | [106] |
| Cucumber | Eukaryotic translation initiation factor 4E (eIF4E) | Virus resistance | Agrobacterium-mediated cotyledon transformation | [107] |
| Kale | PDS | Albino phenotypes | Agrobacterium-mediated transformation | [108] |
| Lettuce | LsBIN2 | Impaired brassinosteroid response | PEG-mediated protoplast transfection | [109] |
| Lettuce | LsNCED4 | Thermo-inhibition of seed germination | Agrobacterium-mediated cotyledon segments transformation | [110] |
| Potato | Acetolactate synthase1 (StALS1) | Herbicide resistance | Agrobacterium-mediated leaf transformation | [111] |
| Potato | 16α-hydroxylation (St16DOX) | Steroidal glycoalkaloids (SGAs) biosynthesis | Agrobacterium- mediated shoots transformation | [112] |
| Potato | Granule-bound starch synthase (StGBSS) | Starch quality | PEG-mediated protoplast transfection | [113] |
| Potato | StIAA2 | Aux/IAA protein | Agrobacterium- mediated stem segments transformation | [114] |
| Potato | SBE1, SBE2 | Starch quality | PEG-mediated protoplast transfection | [115] |
| Tomato * | Aux/IAA9 (SlIAA9) | Parthenocarpic Fruits | Agrobacterium-mediated leaf disk transformation | [116] |
| Tomato | Carotenoid cleavage dioxygenase 8 | Resistance against Phelipanche aegyptiaca | Agrobacterium- mediated transformation | [117] |
| Tomato | SGR1, lycopene ε-cyclase, beta-lycopene cyclase, lycopene β-cyclase1, and LCY-B2 | Lycopene content | Agrobacterium-mediated transformation | [118] |
| Tomato | SlAGAMOUS-LIKE 6 (SlAGL6) | Parthenocarpic Fruits | Agrobacterium-mediated transformation | [119] |
| Tomato | Ripening inhibitor (RIN) | MADS-box transcription factor regulating fruit ripening | Agrobacterium-mediated transformation | [120] |
| Tomato | Self-pruning 5G (SlSP5G) | Day-length-sensitive flowering | Agrobacterium-mediated transformation | [121] |
| Tomato | Blade-on-petiole (SlBOP) | Inflorescence architecture | Agrobacterium-mediated cotyledon segments transformation | [122] |
| Tomato | Mildew Resistant Locus 1 (SlMlo1) | Powdery mildew resistance | Agrobacterium-mediated transformation | [123] |
| Tomato | Alcobaca (SLALC) | Long-shelf Life | Agrobacterium-mediated hypocotyls transformation | [124] |
| Tomato | lncRNA1459 | Fruit ripening repress | Agrobacterium-mediated transformation | [125] |
| Tomato | PDS | Albino phenotypes | Agrobacterium-mediated transformation | [126] |
| Tomato | SlyPDS, SlyGABA–TP1, SlyGABA–TP2, SlyGABA–TP3, SlyCAT9, and SlySSADH | Albino phenotype; γ–aminobutyric acid (GABA) | Agrobacterium-mediated transformation | [127] |
| Tomato | (Methyltransferase 1) SlMET1 | DNA methylation | Agrobacterium-mediated transformation | [128] |
| Tomato | enzymes pectate lyase (PL), polygalacturonase 2a (PG2a), and β-galactanase (TBG4) | Pectin Degradation control | Agrobacterium-mediated transformation | [129] |
| Tomato | NPR1 | drought tolerance | Agrobacterium-mediated cotyledon segments transformation | [130] |
| Tomato and potato | SlALS2 | Herbicide resistance | Agrobacterium-mediated transformation | [131] |
| Watermelon | PDS | Albino phenotype | Agrobacterium-mediated callus transformation | [132] |
| Species | Target Gene | Target Trait | Delivery Method | References |
|---|---|---|---|---|
| Chrysanthemum morifolium | Yellowish-green fluorescent (CpYGFP) | Fluorescence protein disruption | Agrobacterium-mediated leaf sections transformation | [154] |
| Ipomoea nil | carotenoid cleavage dioxygenase (CCD) | carotenoid accumulation regulation | Agrobacterium-mediated immature embryo | [152] |
| Lilium longiflorum, Lilium pumilum | LpPDS | Albino phenotype | Agrobacterium-mediated callus transformation | [143] |
| Petunia | Phytoene desaturase (PhPDS) | Albino phenotype | Agrobacterium-mediated leaf discs transformation | [144] |
| Petunia | Nitrate reductase (PhNR) | Deficiency in nitrate assimilation | PEG-mediated protoplast transfection | [155] |
| Petunia | PhACO genes (PhACO1, PhACO3, and PhACO4) | flower longevity | PEG-mediated protoplast transfection | [151] |
| Petunia | PiSSK1 | Self-incompatibility | Agrobacterium-mediated transformation | [156] |
| Phalaenopsis orchid | MADS | Floral initiation and development | Agrobacterium-mediated protocorms transformation | [146] |
| Torenia fournieri | flavanone 3-hydroxylase gene (F3H) | flavonoid biosynthesis | Agrobacterium-mediated leaf sections | [153] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erpen-Dalla Corte, L.; M. Mahmoud, L.; S. Moraes, T.; Mou, Z.; W. Grosser, J.; Dutt, M. Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants 2019, 8, 601. https://doi.org/10.3390/plants8120601
Erpen-Dalla Corte L, M. Mahmoud L, S. Moraes T, Mou Z, W. Grosser J, Dutt M. Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants. 2019; 8(12):601. https://doi.org/10.3390/plants8120601
Chicago/Turabian StyleErpen-Dalla Corte, Lígia, Lamiaa M. Mahmoud, Tatiana S. Moraes, Zhonglin Mou, Jude W. Grosser, and Manjul Dutt. 2019. "Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique" Plants 8, no. 12: 601. https://doi.org/10.3390/plants8120601
APA StyleErpen-Dalla Corte, L., M. Mahmoud, L., S. Moraes, T., Mou, Z., W. Grosser, J., & Dutt, M. (2019). Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants, 8(12), 601. https://doi.org/10.3390/plants8120601






