Transgressivity in Key Functional Traits Rather Than Phenotypic Plasticity Promotes Stress Tolerance in A Hybrid Cordgrass
Abstract
1. Introduction
2. Results
2.1. Phenotypic Expression
2.2. Plant Trait Variability
2.3. Fitness
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Traits
4.3. Phenotypic Expression
4.4. Plant Trait Variability
4.5. Fitness
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Bigot, S.; Buges, J.; Gilly, L.; Jacques, C.; Le Boulch, P.; Berger, M.; Delcros, P.; Domergue, J.-B.; Koehl, A.; Ley-Ngardigal, B.; et al. Pivotal roles of environmental sensing and signaling mechanisms in plant responses to climate change. Glob. Chang. Biol. 2018, 24, 5573–5589. [Google Scholar] [CrossRef] [PubMed]
- Nogués-Bravo, D.; Rodríguez-Sánchez, F.; Orsini, L.; de Boer, E.; Jansson, R.; Morlon, H.; Fordham, D.A.; Jackson, S.T. Cracking the code of biodiversity responses to past climate change. Trends Ecol. Evol. 2018, 33, 765–776. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Archer, M.A.; Wayne, R.K. Transgressive segregation, adaptation and speciation. Heredity 1999, 83, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.C.; Blumenthal, D.; Hufbauer, R.A. Hybridization and invasion: An experimental test with diffuse knapweed (Centaurea diffusa Lam.). Evol. Appl. 2012, 5, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 2013, 14, 471–482. [Google Scholar] [CrossRef]
- Baranwal, V.K.; Mikkilineni, V.; Zehr, U.B.; Tyagi, A.K.; Kapoor, S. Heterosis: Emerging ideas about hybrid vigour. J. Exp. Bot. 2012, 63, 6309–6314. [Google Scholar] [CrossRef]
- Hovick, S.M.; Whitney, K.D. Hybridisation is associated with increased fecundity and size in invasive taxa: Meta-analytic support for the hybridisation-invasion hypothesis. Ecol. Lett. 2014, 17, 1464–1477. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Royauté, R.; Wright, J.W.; Hodgskiss, P.; Thomas Ledig, F. Genetic conservation and management of the California endemic, Torrey pine (Pinus torreyana Parry): Implications of genetic rescue in a genetically depauperate species. Ecol. Evol. 2017, 7, 7370–7381. [Google Scholar] [CrossRef]
- Janes, J.K.; Hamilton, J.A. Mixing It Up: The role of hybridization in forest management and conservation under climate change. Forests 2017, 8, 237. [Google Scholar] [CrossRef]
- Gramlich, S.; Sagmeister, P.; Dullinger, S.; Hadacek, F.; Hörandl, E. Evolution in situ: Hybrid origin and establishment of willows (Salix L.) on alpine glacier forefields. Heredity 2016, 116, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, Y.; Chen, K.; Kong, M.; Song, W.; Lu, B.; Shi, Y.; Zhao, Y.; Zhao, J. Mapping of quantitative trait loci for seedling salt tolerance in maize. Mol. Breed. 2019, 39, 64. [Google Scholar] [CrossRef]
- Sen, M.; De, D.K. Studies on combining ability in high yielding drought tolerant mungbean genotypes under West Bengal condition. Legum. Res. 2018, 41, 795–803. [Google Scholar] [CrossRef]
- Martínez, L.M.; Fernández-Ocaña, A.; Rey, P.J.; Salido, T.; Amil-Ruiz, F.; Manzaneda, A.J. Variation in functional responses to water stress and differentiation between natural allopolyploid populations in the Brachypodium distachyon species complex. Ann. Bot. 2018, 121, 1369–1382. [Google Scholar] [CrossRef]
- Ainouche, M.L.; Jenczewski, E. Focus on polyploidy. New Phytol. 2010, 186, 1–4. [Google Scholar] [CrossRef]
- Cara, N.; Marfil, C.F.; Masuelli, R.W. Epigenetic patterns newly established after interspecific hybridization in natural populations of Solanum. Ecol. Evol. 2013, 3, 3764–3779. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 1206, 35–55. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; JHU Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Jackson, S.; Chen, Z.J. Genomic and Expression Plasticity of Polyploidy. Curr. Opin. Plant Biol. 2011, 13, 153–159. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Krebs, C.; Bossdorf, O. Hybridization increases invasive knotweed success. Evol. Appl. 2014, 7, 413–420. [Google Scholar] [CrossRef]
- Taylor, S.A.; Larson, E.L.; Harrison, R.G. Hybrid zones: Windows on climate change. Trends Ecol. Evol. 2015, 30, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 389–410. [Google Scholar] [CrossRef]
- Hall, R.J.; Hastings, A.; Ayres, D.R. Explaining the explosion: Modelling hybrid invasions. Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.M.; Ayres, D.R.; Leira-Doce, P.; Bailey, J.; Blum, M.; Strong, D.R.; Luque, T.; Figueroa, E. The production of hybrids with high ecological amplitude between exotic Spartina densiflora and native S. maritima in the Iberian Peninsula. Divers. Distrib. 2010, 16, 547–558. [Google Scholar] [CrossRef]
- Gallego-Tévar, B.; Rubio-Casal, A.E.; de Cires, A.; Figueroa, E.; Grewell, B.J.; Castillo, J.M. Phenotypic plasticity of polyploid plant species promotes transgressive behaviour in their hybrids. AoB PLANTS 2018, 10, ply055. [Google Scholar] [CrossRef]
- Thompson, J.D. The Biology of an invasive plant: What makes Spartina anglica so successful? Bioscience 1991, 41, 393–401. [Google Scholar] [CrossRef]
- IPCC. Summary Chapter for Policymakers. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2015; p. 31. ISBN 9781107661820. [Google Scholar]
- Sutter, L.A.; Chambers, R.M.; Perry, J.E. Seawater intrusion mediates species transition in low salinity, tidal marsh vegetation. Aquat. Bot. 2015, 122, 32–39. [Google Scholar] [CrossRef]
- Thorne, K.; MacDonald, G.; Guntenspergen, G.; Ambrose, R.; Buffington, K.; Dugger, B.; Freeman, C.; Janousek, C.; Brown, L.; Rosencranz, J.; et al. U.S. Pacific coastal wetland resilience and vulnerability to sea-level rise. Sci. Adv. 2018, 4, eaao3270. [Google Scholar] [CrossRef]
- Pennings, S.C.; Grant, M.B.; Bertness, M.D. Plant zonation in low-latitude salt marshes: Disentangling the roles of flooding, salinity and competition. J. Ecol. 2005, 93, 159–167. [Google Scholar] [CrossRef]
- Janousek, C.N.; Mayo, C. Plant responses to increased inundation and salt exposure: Interactive effects on tidal marsh productivity. Plant Ecol. 2013, 214, 917–928. [Google Scholar] [CrossRef]
- Watson, E.B.; Wigand, C.; Davey, E.W.; Andrews, H.M.; Bishop, J.; Raposa, K.B. Wetland loss patterns and inundation-productivity relationships prognosticate widespread salt marsh loss for Southern New England. Estuaries Coasts 2017, 40, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Favre, A.; Karrenberg, S. Stress tolerance in closely related species and their first-generation hybrids: A case study of Silene. J. Ecol. 2011, 99, 1415–1423. [Google Scholar] [CrossRef]
- Karrenberg, S.; Edelist, C.; Lexer, C.; Rieseberg, L. Response to salinity in the homoploid hybrid species Helianthus paradoxus and its progenitors H. annuus and H. petiolaris. New Phytol. 2006, 170, 615–629. [Google Scholar] [CrossRef]
- Lee, A.K.; Ayres, D.R.; Pakenham-Walsh, M.R.; Strong, D.R. Responses to salinity of Spartina hybrids formed in San Francisco Bay, California (S. alterniflora × foliosa and S. densiflora × foliosa). Biol. Invasions 2016, 18, 2207–2219. [Google Scholar] [CrossRef]
- Boers, A.M.; Zedler, J.B. Stabilized water levels and Typha invasiveness. Wetlands 2008, 28, 676–685. [Google Scholar] [CrossRef]
- Waldren, S.; Etherigton, J.R.; Davies, M.S. Comparative studies of plant growth and distribution in relation to waterlogging. XV. The effect of waterlogging on growth of various populations of and hybrids between Geum rivale L. and Geum urbanum L. New Phytol. 1988, 109, 97–106. [Google Scholar] [CrossRef]
- Ayres, D.R.; Grotkopp, E.; Zaremba, K.; Sloop, C.M.; Blum, M.J.; Bailey, J.P.; Anttila, C.K.; Strong, D.R. Hybridization between invasive Spartina densiflora (Poaceae) and native S. foliosa in San Francisco Bay, California, USA. Am. J. Bot. 2008, 95, 713–719. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tévar, B.; Figueroa, E.; Grewell, B.J.; Vallet, D.; Rousseau, H.; Keller, J.; Lima, O.; Dréano, S.; Salmon, A.; et al. Low genetic diversity contrasts with high phenotypic variability in heptaploid Spartina densiflora populations invading the Pacific coast of North America. Ecol. Evol. 2018, 8, 4992–5007. [Google Scholar] [CrossRef]
- Sloop, C.M.; McGray, H.G.; Blum, M.J.; Strong, D.R. Characterization of 24 additional microsatellite loci in Spartina species (Poaceae). Conserv. Genet. 2005, 6, 1049–1052. [Google Scholar] [CrossRef]
- Gallego-Tévar, B.; Grewell, B.J.; Futrell, C.J.; Drenovsky, R.E.; Castillo, J.M. Interactive effects of salinity and inundation on native Spartina foliosa, invasive S. densiflora, and their hybrid from San Francisco Estuary, California. Ann. Bot. 2019, mcz170. [Google Scholar] [CrossRef]
- Bassene, J.B.; Froelicher, Y.; Dubois, C.; Ferrer, R.M.; Navarro, L.; Ollitrault, P.; Ancillo, G. Non-additive gene regulation in a citrus allotetraploid somatic hybrid between C. reticulata Blanco and C. limon (L.) Burm. Heredity 2010, 105, 299–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hochholdinger, F.; Hoecker, N. Towards the molecular basis of heterosis. Trends Plant Sci. 2007, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Rupe, M.A.; Danilevskaya, O.N.; Yang, X.; Hu, Z. Genome-wide mRNA profiling reveals heterochronic allelic variation and a new imprinted gene in hybrid maize endosperm. Plant J. 2003, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.S.; Husband, B.C. Maternal and paternal contributions to the fitness of hybrids between red and white mulberry (Morus, Moraceae). Am. J. Bot. 2004, 91, 1802–1808. [Google Scholar] [CrossRef]
- Groszmann, M.; Greaves, I.K.; Fujimoto, R.; James Peacock, W.; Dennis, E.S. The role of epigenetics in hybrid vigour. Trends Genet. 2013, 29, 684–690. [Google Scholar] [CrossRef]
- Salmon, A.; Ainouche, M.L.; Wendel, J.F. Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae). Mol. Ecol. 2005, 14, 1163–1175. [Google Scholar] [CrossRef]
- Ni, Z.; Kim, E.D.; Ha, M.; Lackey, E.; Liu, J.; Zhang, Y.; Sun, Q.; Chen, Z.J. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 2009, 457, 327–331. [Google Scholar] [CrossRef]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M.; Pyšek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Kurashige, N.S.; Callahan, H.S. Evolution of active and passive forms of plasticity: Insights from artificially selected Arabidopsis. Evol. Ecol. Res. 2007, 9, 935–945. [Google Scholar]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 1–21. [Google Scholar] [CrossRef]
- Castillo, J.M.; Redondo, S.; Wharmby, C.; Figueroa, M.E.; Luque, T.; Castellanos, E.M.; Davy, A.J. Environmental determination of shoot height in populations of the cordgrass Spartina maritima. Estuaries 2005, 28, 761–766. [Google Scholar] [CrossRef]
- Naidoo, G.; Mundree, S.G. Relationship between morphological and physiological responses to waterlogging and salinity in Sporobolus virginicus (L.) Kunth. Oecologia 1993, 93, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Fievét, J.B.; Nidelet, T.; Dillmann, C.; de Vienne, D. Heterosis is a Systemic Property Emerging from Nonlinear Genotype-Phenotype Relationships: Evidence from in Vitro Genetics and Computer Simulations. Front. Genet. 2018, 9, 9–159. [Google Scholar] [CrossRef]
- Vasseur, F.; Fouqueau, L.; de Vienne, D.; Nidelet, T.; Violle, C.; Weigel, D. Nonlinear phenotypic variation uncovers the emergence of heterosis in Arabidopsis thaliana. PLoS Biol. 2019, 17, e3000214. [Google Scholar] [CrossRef]
- Castillo, J.M.; Grewell, B.J.; Pickart, A.; Bortolus, A.; Peña, C.; Figueroa, E.; Sytsma, M. Phenotypic plasticity of invasive Spartina densiflora (Poaceae) along a broad latitudinal gradient on the pacific coast of North America. Am. J. Bot. 2014, 101, 448–458. [Google Scholar] [CrossRef]
- Grewell, B.J.; Castillo, J.M.; Skaer Thomason, M.J.; Drenovsky, R.E. Phenotypic plasticity and population differentiation in response to salinity in the invasive cordgrass Spartina densiflora. Biol. Invasions 2016, 18, 2175–2187. [Google Scholar] [CrossRef]
- Lippman, Z.B.; Zamir, D. Heterosis: Revisiting the magic. Trends Genet. 2007, 23, 60–66. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Zhang, Q.; De Gruyter, F.; Martens, S.; Huber, H. Shade affects responses to drought and flooding—Acclimation to multiple stresses in bittersweet (Solanum dulcamara L.). Plant Biol. 2016, 18, 112–119. [Google Scholar] [CrossRef]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- Abbott, R.J. Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol. Evol. 1992, 7, 401–405. [Google Scholar] [CrossRef]
- Gray, A.J.; Marshall, D.F.; Raybould, A.F. A Century of Evolution in Spartina anglica. Adv. Ecol. Res. 1991, 21, 1–62. [Google Scholar]
- Kittelson, P.; Milton, J.B. Mechanisms of Expasion for an introduces species of cordgrass Spartina densiflora, in Humboldt Bay, California. Estuaries 1997, 20, 770–778. [Google Scholar] [CrossRef]
- Nieva, F.J.; Díaz-Espejo, A.; Castellanos, E.M.; Figueroa, M.E. Field Variability of Invading Populations of Spartina densiflora Brong. in Different Habitats of the Odiel Marshes (SW Spain). Estuar. Coast. Shelf Sci. 2001, 52, 515–527. [Google Scholar] [CrossRef]
- Raven, P.H.; Tai, W. Observations of chromosomes in Ludwigia (Onagraceae). Ann. Mo. Bot. Gard. 1979, 66, 862–879. [Google Scholar] [CrossRef]
- Zardini, E.M.; Gu, H.; Raven, P.H. On the Separation of Two Species within the Ludwigia uruguayensis Complex (Onagraceae). Syst. Bot. 1991, 16, 242–244. [Google Scholar] [CrossRef]
- Singh, R.J. (Ed.) Plant Cytogenetics; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4398-8418-8. [Google Scholar]
- Osmond, C.B.; Björkman, O.; Anderson, D.J. Physiological Processes in Plant Ecology. In Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1980; Volume 36, ISBN 978-3-642-67639-0. [Google Scholar]
- Kercher, S.M.; Zedler, J.B. Flood tolerance in wetland angiosperms: A comparison of invasive and noninvasive species. Aquat. Bot. 2004, 80, 89–102. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Fujita, K.; Ogata, S. Water Stress and Potassium Fertilization in Field Grown Maize (Zea mays L.): Effects on leaf water relations and leaf rolling. J. Agron. Crop Sci. 1993, 170, 195–201. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Castillo, J.M.; Leira-Doce, P.; Carrión-Tacuri, J.; Muñoz-Guacho, E.; Arroyo-Solís, A.; Curado, G.; Doblas, D.; Rubio-Casal, A.E.; Álvarez-López, A.; Redondo-Gómez, S.; et al. Contrasting strategies to cope with drought by invasive and endemic species of Lantana in Galapagos. Biodivers. Conserv. 2007, 16, 2123–2136. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Swank, J.C.; Below, F.E.; Lambert, R.J.; Hageman, R.H. Interaction of carbon and nitrogen metabolism in the productivity of maize. Plant Physiol. 1982, 70, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. A photometric adaptation of the somogyi method for determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar]
- Castillo, J.M.; Leira-Doce, P.; Rubio-Casal, A.E.; Figueroa, E. Spatial and temporal variations in aboveground and belowground biomass of Spartina maritima (small cordgrass) in created and natural marshes. Estuar. Coast. Shelf Sci. 2008, 78, 819–826. [Google Scholar] [CrossRef]
- Martin, E.; Hine, R. A Dictionary of Biology; Oxford University Press: Oxford, UK, 2008; ISBN 9780199204625. [Google Scholar]
- Zörgö, E.; Gjuvsland, A.; Cubillos, F.A.; Louis, E.J.; Liti, G.; Blomberg, A.; Omholt, S.W.; Warringer, J. Life history shapes trait heredity by accumulation of loss-of-function alleles in yeast. Mol. Biol. Evol. 2012, 29, 1781–1789. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Ainouche, M.; Chelaifa, H.; Ferreira, J.; Bellot, S.; Ainouche, A.; Salmon, A. Polyploid evolution in spartina: Dealing with highly redundant hybrid genomes. In Polyploidy and Genome Evolution; Pamela, S., Douglas, E.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 225–243. ISBN 9783642314421. [Google Scholar]
- Van Der Sman, A.J.M.; Joosten, N.N.; Blom, C.W.P.M. Flooding regimes and life-history characteristics of short-lived species in river forelands. J. Ecol. 1993, 81, 121. [Google Scholar] [CrossRef]

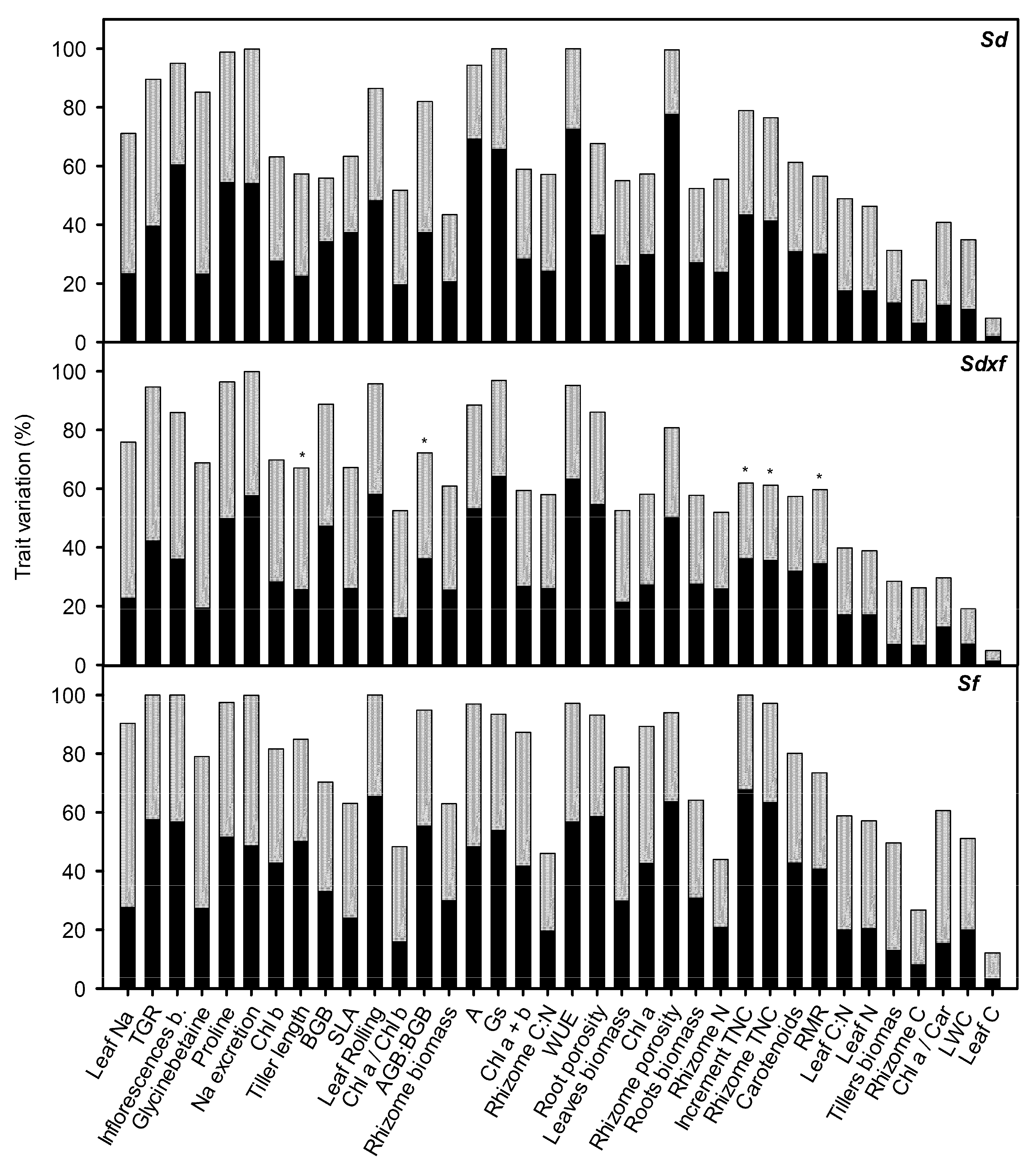
 ) and its parental species S. densiflora (
) and its parental species S. densiflora ( ), Spartina foliosa (
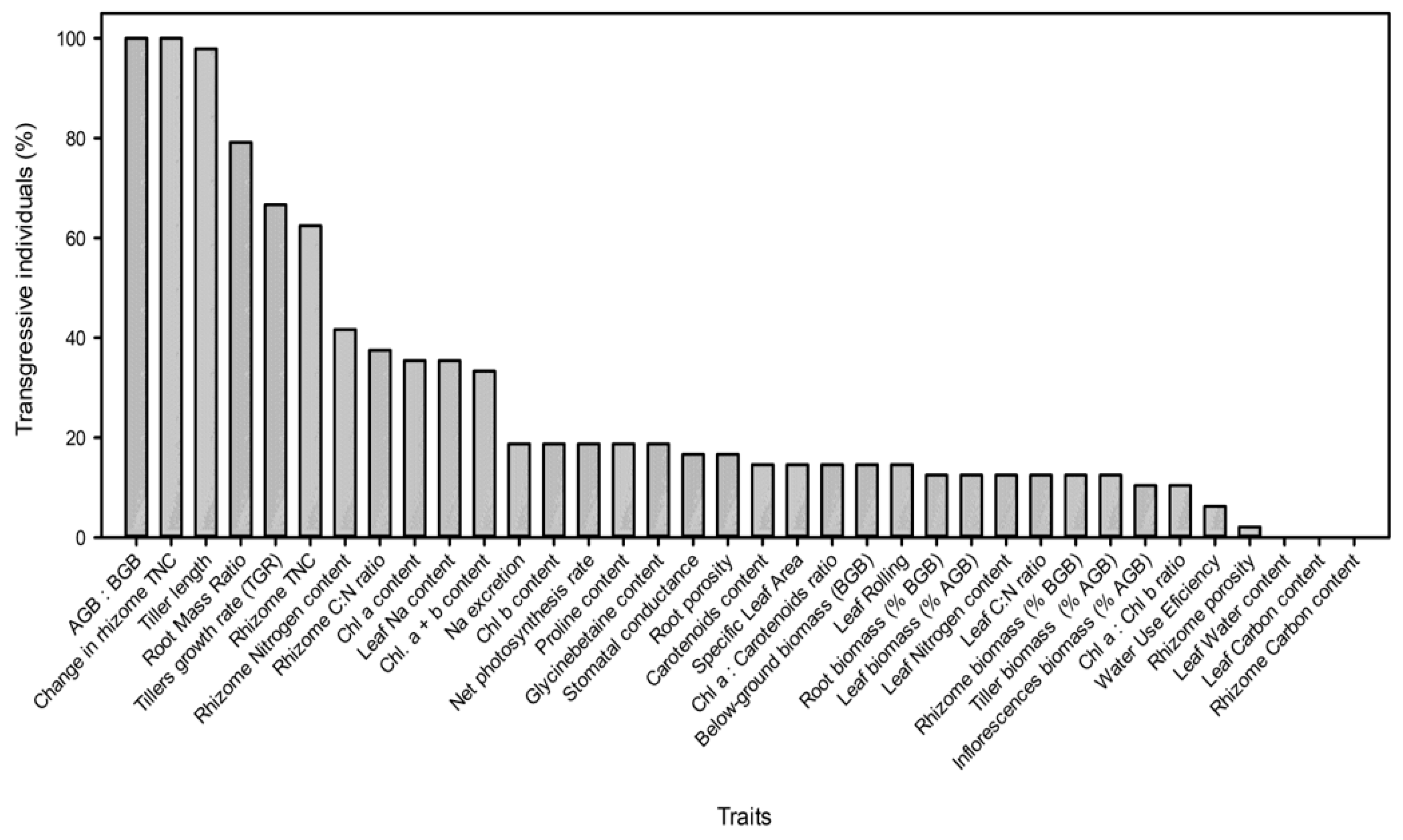
), Spartina foliosa ( ) at different salinities (0.5, 10, 20, and 40 ppt) and inundation depths (SI, shallow inundation (4.4 cm deep); II, intermediate inundation (35.5 cm deep); DI, deep inundation (55.0 cm deep)). Values are mean ± SEM (n = 4). The percentages of hybrid individuals with transgressive traits (including worst- and best-parent heterosis) (
) at different salinities (0.5, 10, 20, and 40 ppt) and inundation depths (SI, shallow inundation (4.4 cm deep); II, intermediate inundation (35.5 cm deep); DI, deep inundation (55.0 cm deep)). Values are mean ± SEM (n = 4). The percentages of hybrid individuals with transgressive traits (including worst- and best-parent heterosis) ( ) and percentage of traits showing worst- or best-parent heterosis at the hybrid population (
) and percentage of traits showing worst- or best-parent heterosis at the hybrid population ( ) for 36 key functional traits are represented in panel B.
) for 36 key functional traits are represented in panel B.
 ) and its parental species S. densiflora (
) and its parental species S. densiflora ( ), Spartina foliosa (
), Spartina foliosa ( ) at different salinities (0.5, 10, 20, and 40 ppt) and inundation depths (SI, shallow inundation (4.4 cm deep); II, intermediate inundation (35.5 cm deep); DI, deep inundation (55.0 cm deep)). Values are mean ± SEM (n = 4). The percentages of hybrid individuals with transgressive traits (including worst- and best-parent heterosis) (
) at different salinities (0.5, 10, 20, and 40 ppt) and inundation depths (SI, shallow inundation (4.4 cm deep); II, intermediate inundation (35.5 cm deep); DI, deep inundation (55.0 cm deep)). Values are mean ± SEM (n = 4). The percentages of hybrid individuals with transgressive traits (including worst- and best-parent heterosis) ( ) and percentage of traits showing worst- or best-parent heterosis at the hybrid population (
) and percentage of traits showing worst- or best-parent heterosis at the hybrid population ( ) for 36 key functional traits are represented in panel B.
) for 36 key functional traits are represented in panel B.
| Intra-Population Trait Variability | S. densiflora x foliosa | ||||||||
| Sd | Sdxf | Intra | PP | T | D-Sd | D-Sf | |||
| Sd | 0.794 | 0.89 | Inter | 0.911 | 0.785 | Inter (Sd) | 0.193 | −0.0899 | 0.112 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.26 | 0.602 | 0.514 | |||
| Sf | 0.817 | Intra | 0.461 | Intra (Sd) | 0.0293 | 0.0756 | 0.16 | ||
| <0.0001 | <0.0001 | 0.865 | 0.661 | 0.352 | |||||
| Inter-Treatment Trait Variability | S. densiflora | PP (Sd) | 0.384 | −0.34 | −0.0336 | ||||
| Sd | Sdxf | Intra | PP | 0.0207 | 0.0424 | 0.846 | |||
| Sd | 0.874 | 0.9 | Inter | 0.904 | 0.636 | ||||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | Inter (Sf) | 0.357 | −0.178 | 0.0249 | ||
| 0.0327 | 0.299 | 0.885 | |||||||
| Sf | 0.83 | Intra | 0.245 | ||||||
| <0.0001 | 0.15 | Intra (Sf) | 0.422 | −0.269 | −0.0366 | ||||
| 0.0103 | 0.112 | 0.832 | |||||||
| Phenotypic Plasticity | S. foliosa | ||||||||
| Sd | Sdxf | Intra | PP | PP (Sf) | 0.0532 | 0.0826 | 0.127 | ||
| Sd | 0.643 | 0.722 | Inter | 0.914 | 0.653 | 0.758 | 0.632 | 0.46 | |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Inter (Sdxf) | 0.159 | −0.0795 | 0.122 | ||||||
| Sf | 0.652 | Intra | 0.29 | 0.354 | 0.645 | 0.479 | |||
| <0.0001 | 0.086 | ||||||||
| Intra (Sdxf) | 0.103 | −0.0395 | 0.162 | ||||||
| 0.548 | 0.819 | 0.346 | |||||||
| PP (Sdxf) | 0.188 | −0.112 | 0.0198 | ||||||
| 0.273 | 0.516 | 0.909 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Tévar, B.; Grewell, B.J.; Drenovsky, R.E.; Castillo, J.M. Transgressivity in Key Functional Traits Rather Than Phenotypic Plasticity Promotes Stress Tolerance in A Hybrid Cordgrass. Plants 2019, 8, 594. https://doi.org/10.3390/plants8120594
Gallego-Tévar B, Grewell BJ, Drenovsky RE, Castillo JM. Transgressivity in Key Functional Traits Rather Than Phenotypic Plasticity Promotes Stress Tolerance in A Hybrid Cordgrass. Plants. 2019; 8(12):594. https://doi.org/10.3390/plants8120594
Chicago/Turabian StyleGallego-Tévar, Blanca, Brenda J. Grewell, Rebecca E. Drenovsky, and Jesús M. Castillo. 2019. "Transgressivity in Key Functional Traits Rather Than Phenotypic Plasticity Promotes Stress Tolerance in A Hybrid Cordgrass" Plants 8, no. 12: 594. https://doi.org/10.3390/plants8120594
APA StyleGallego-Tévar, B., Grewell, B. J., Drenovsky, R. E., & Castillo, J. M. (2019). Transgressivity in Key Functional Traits Rather Than Phenotypic Plasticity Promotes Stress Tolerance in A Hybrid Cordgrass. Plants, 8(12), 594. https://doi.org/10.3390/plants8120594







