5-Aminolevulinic Acid and Soil Fertility Enhance the Resistance of Rosemary to Alternaria dauci and Rhizoctonia solani and Modulate Plant Biochemistry
Abstract
1. Introduction
2. Results
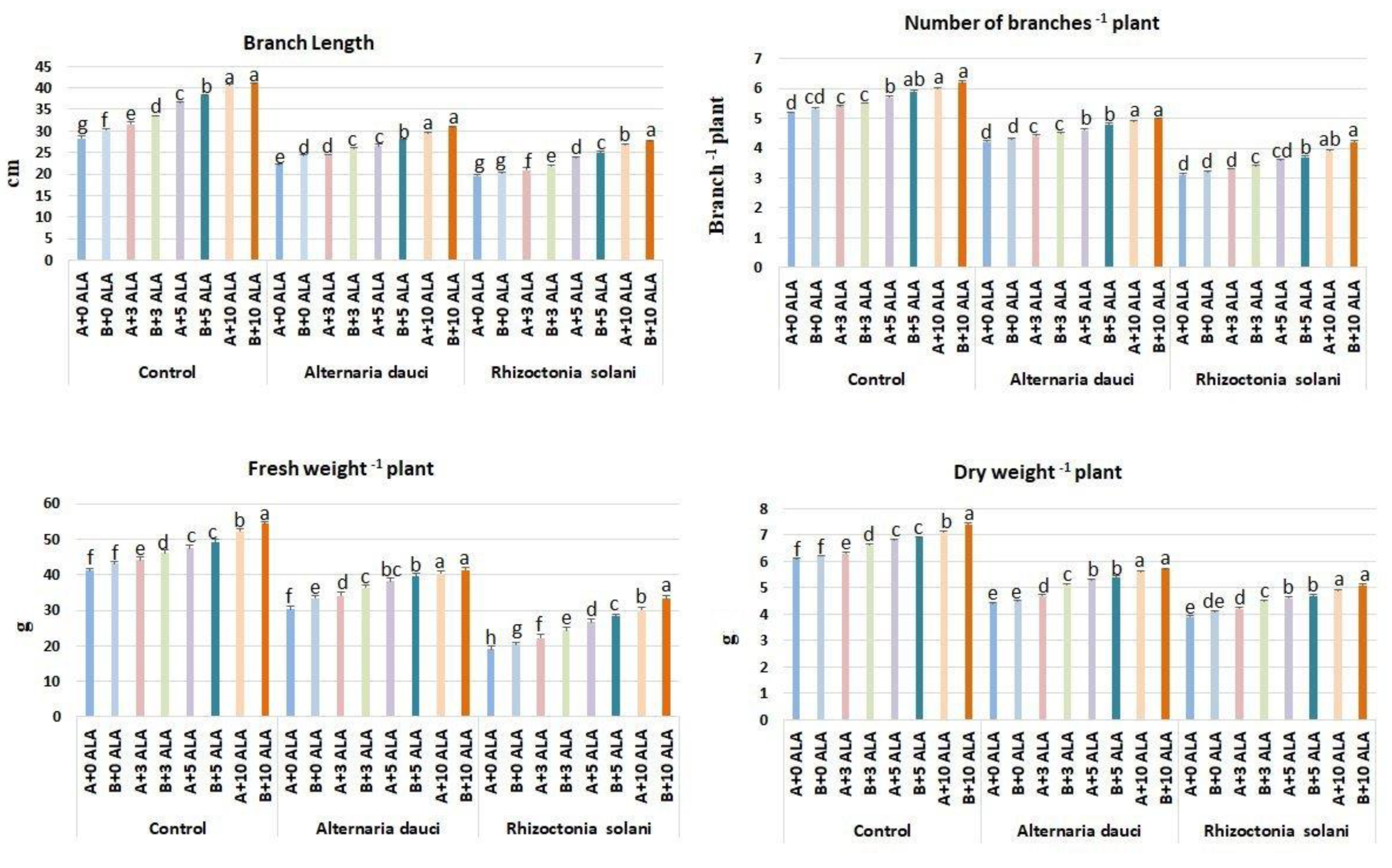
2.1. Vegetative Growth
2.2. Essential Oil Composition
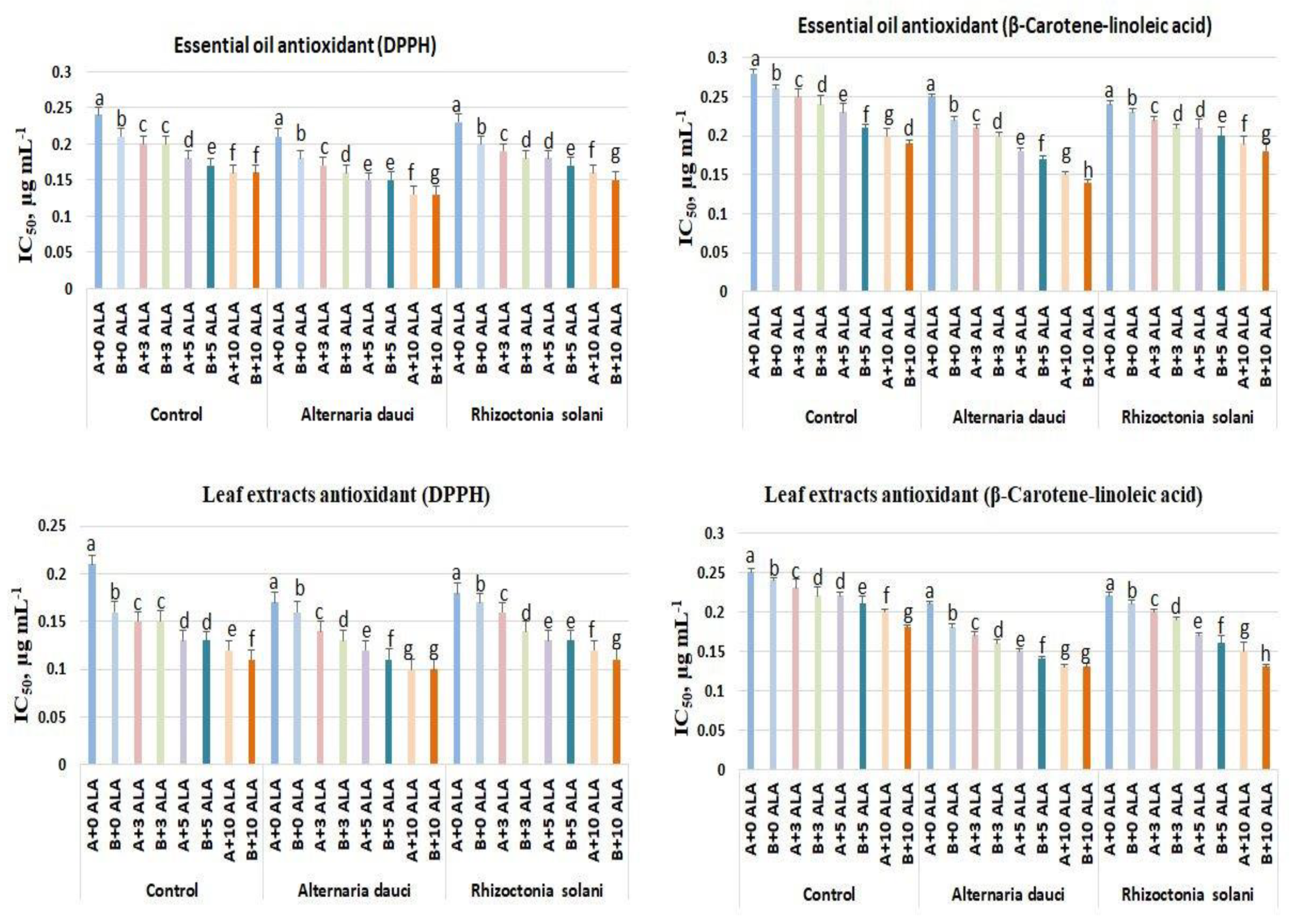
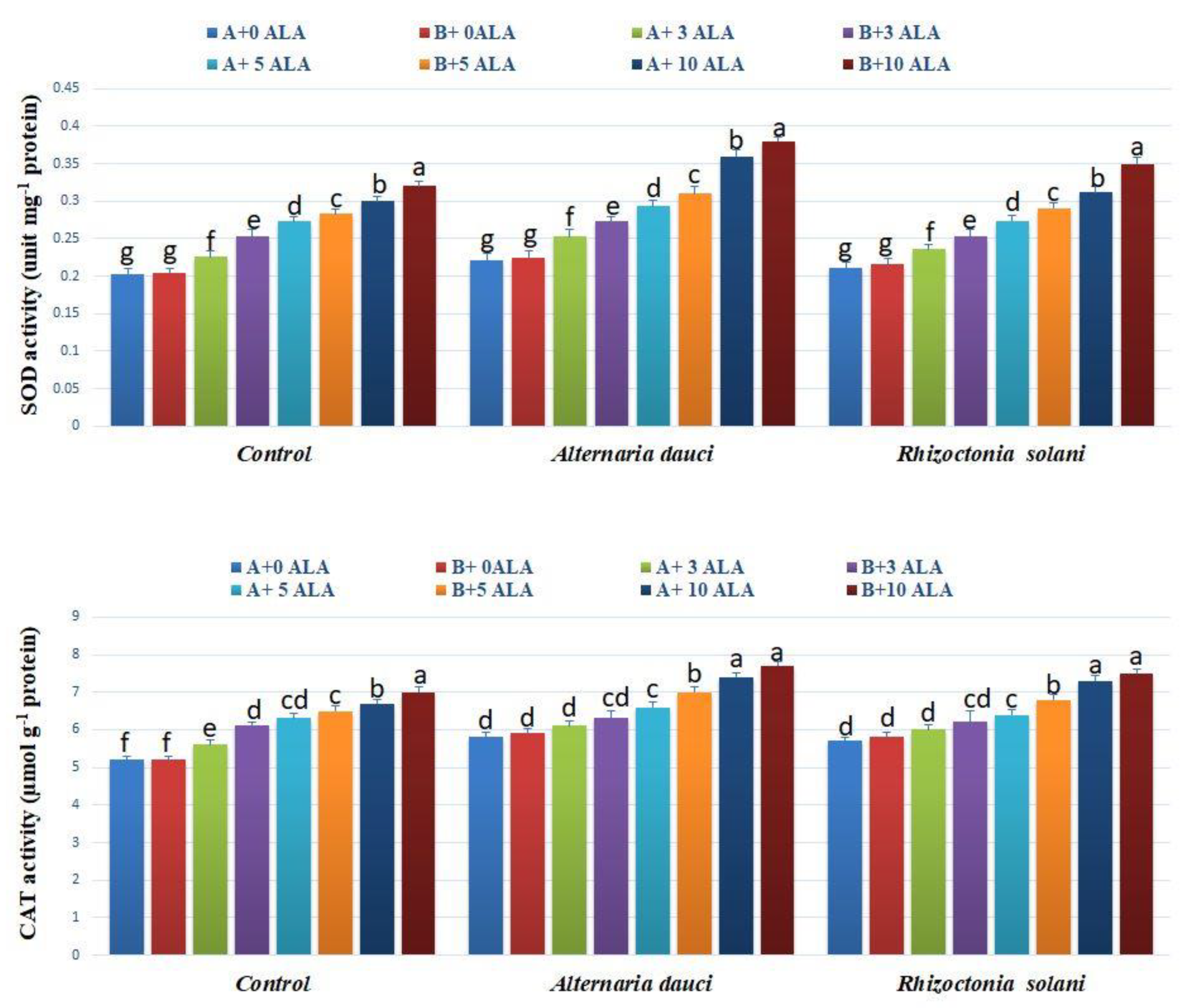
2.3. Antioxidant Activities
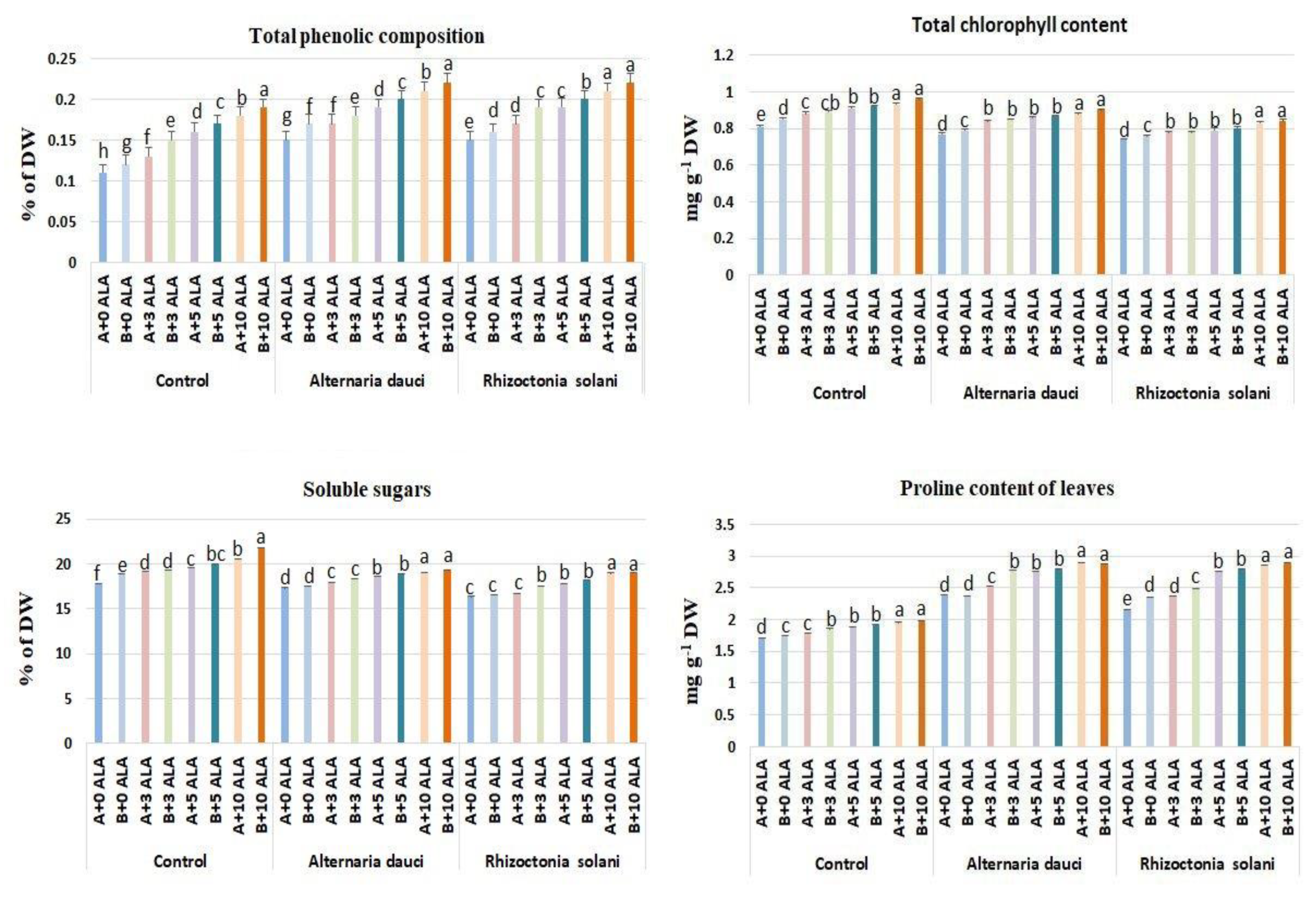
2.4. Chlorophyll, Soluble Sugars, and Proline Compositions
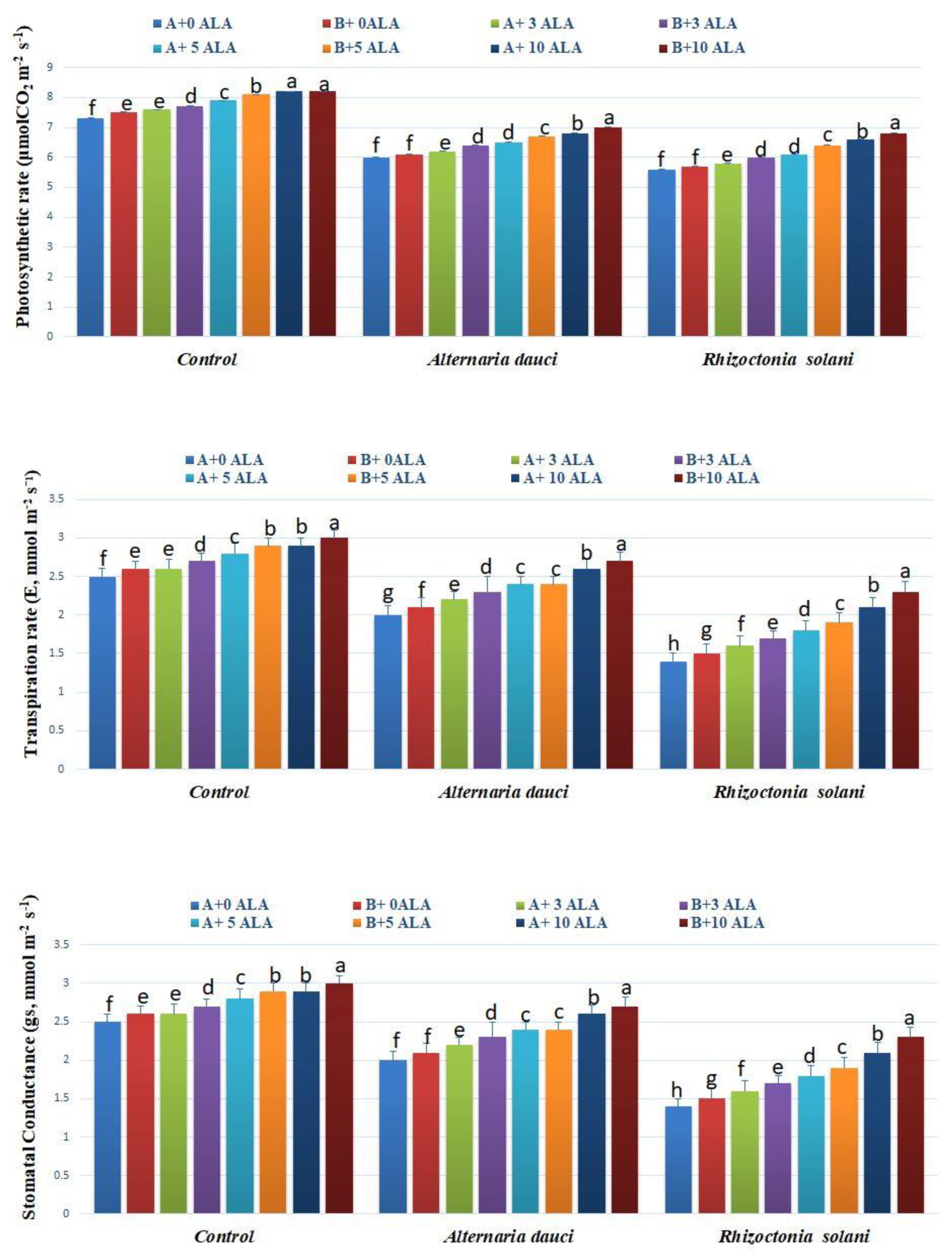
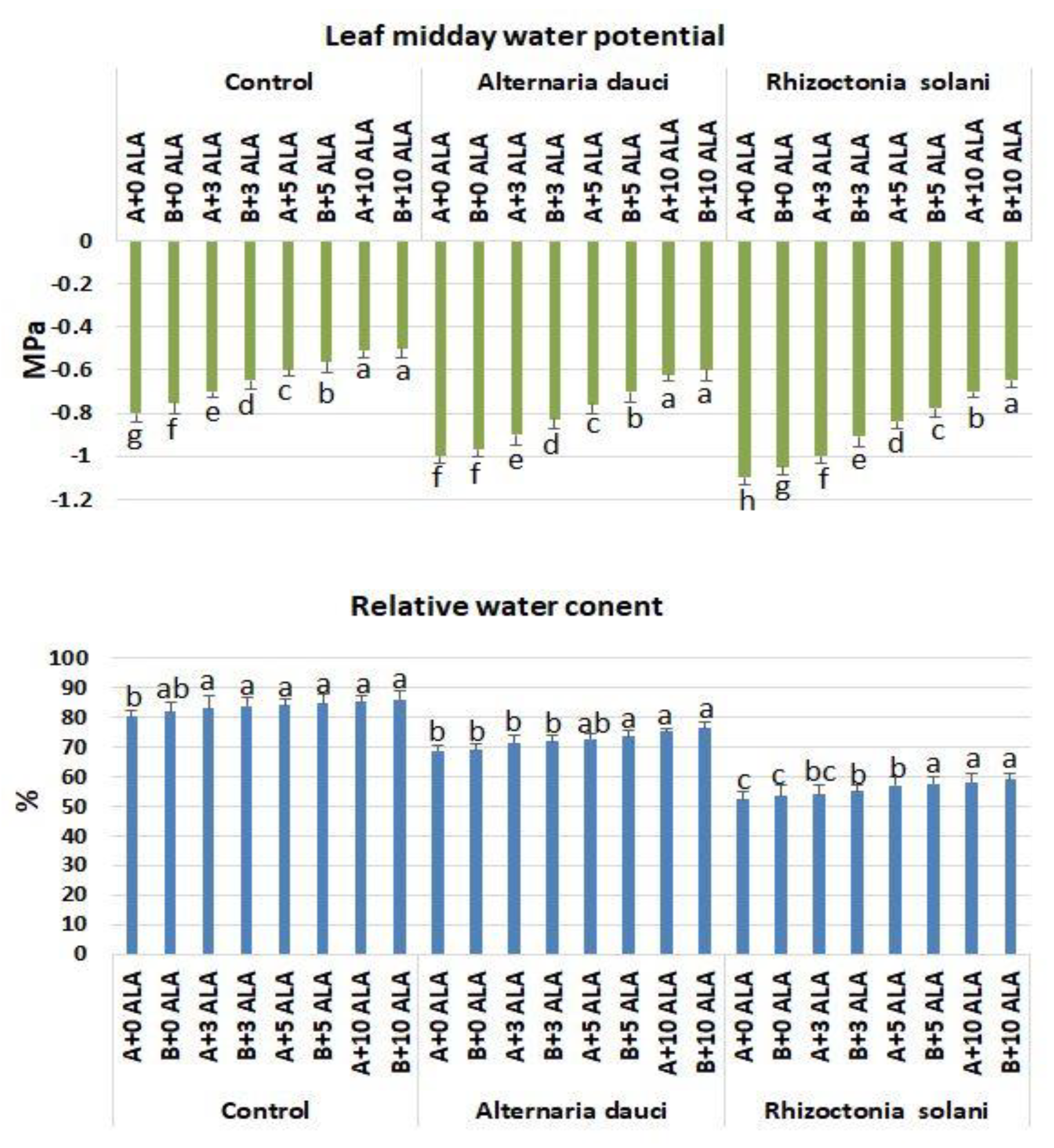
2.5. Gas Exchange and Water Measures
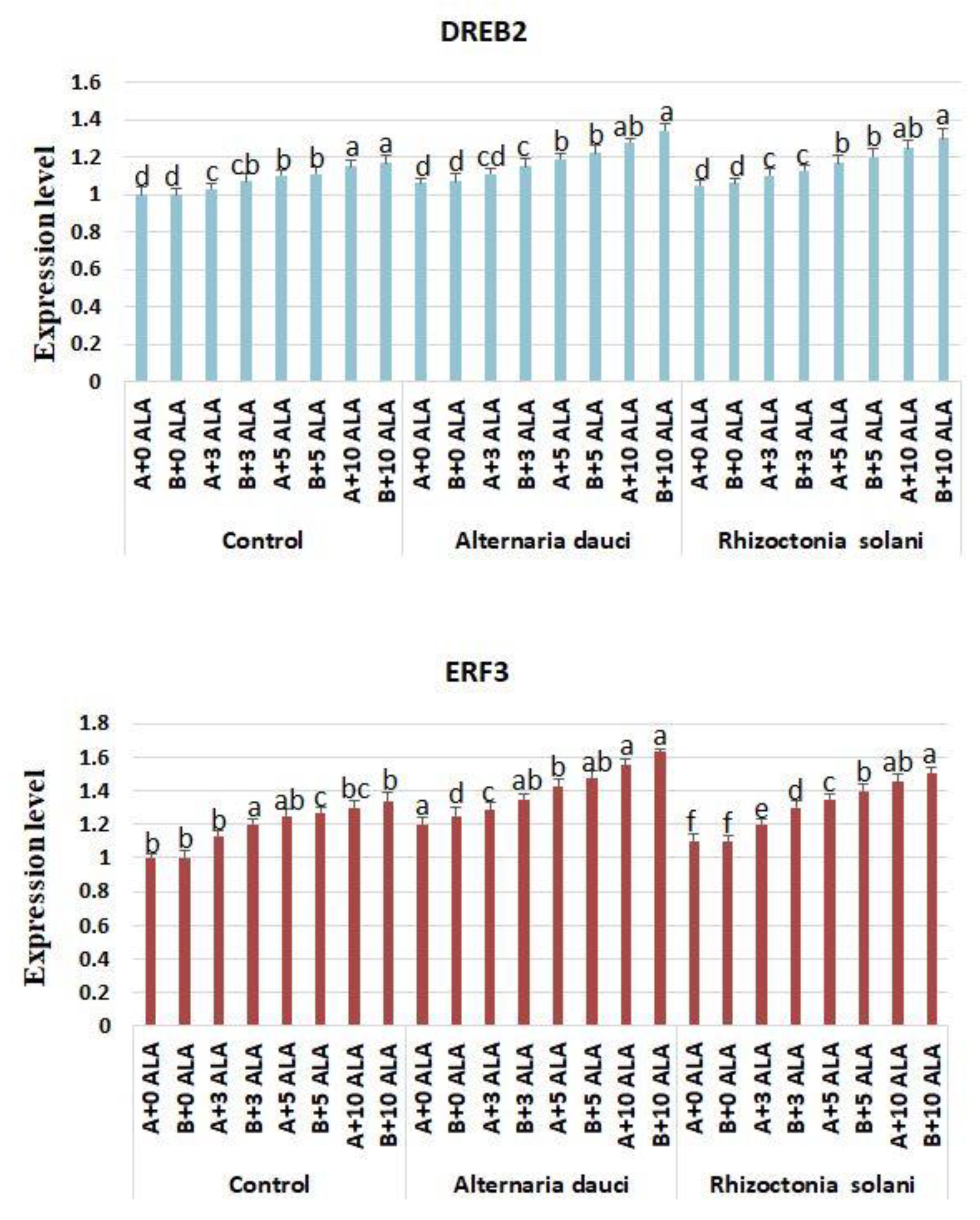
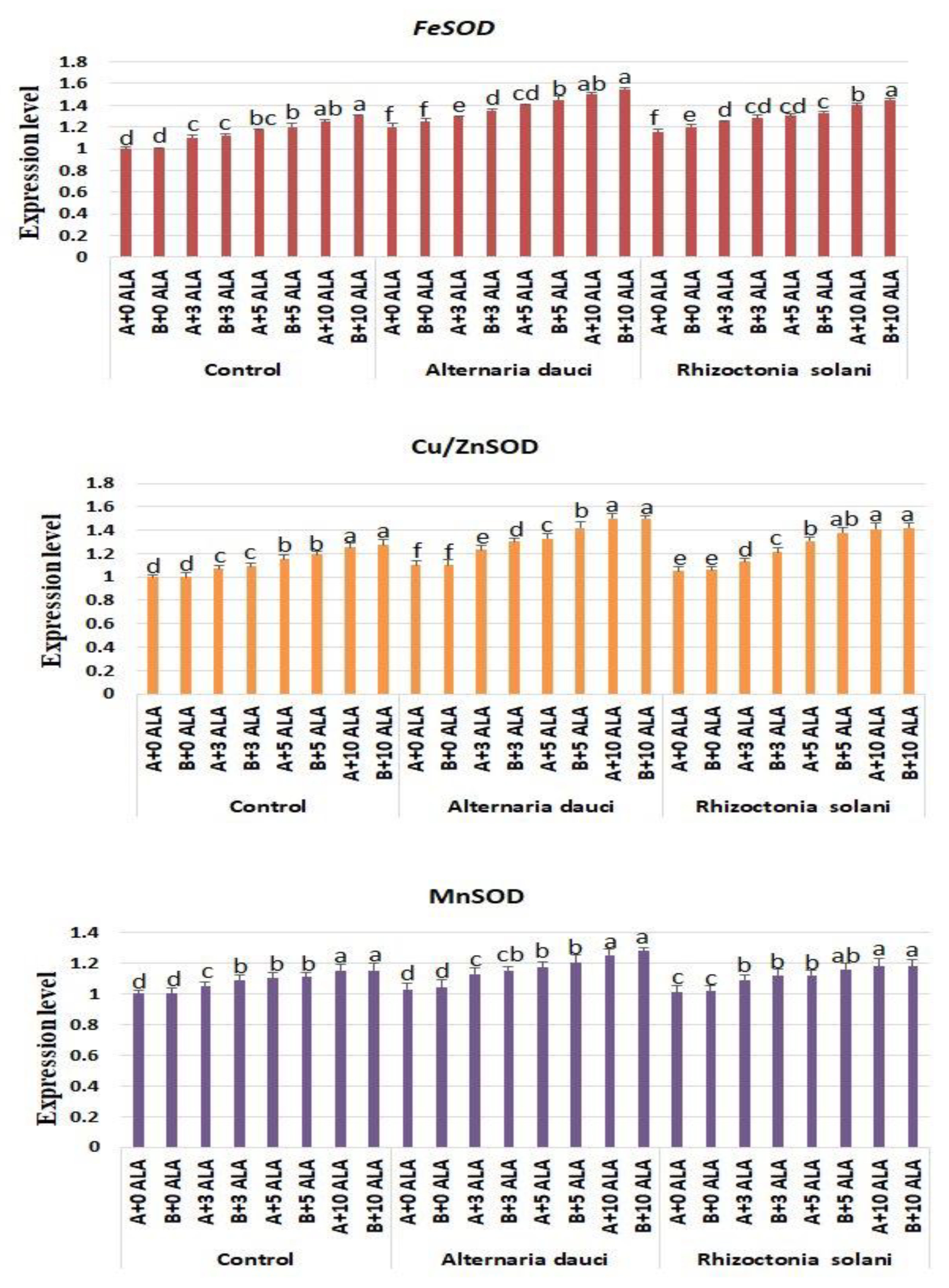
2.6. Gene Expression
3. Discussion
4. Material and Methods
4.1. Plant Material, Soil Mixes, ALA Treatment, and Inoculum Preparation
4.2. Morphological Measurements
4.3. GC-MS of Essential Oil and Preparation of Leaf Extracts
4.4. Antioxidant Activities
4.5. Chlorophyll, Soluble Sugars, and Proline Compositions
4.6. Gas Exchange and Water Measures
4.7. Gene Expression
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senge, O.M.; Ryan, A.A.; Letchford, A.K.; MacGowan, A.S.; Mielke, T. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry 2014, 6, 781–843. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) Alleviated Salinity Stress in Cucumber Seedlings by Enhancing Chlorophyll Synthesis Pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Liu, J.; Cao, R.X.; Huang, Y.J.; Hall, A.M.; Guo, C.B.; Wang, L.J. Effects of 5-aminolevulinic acid treatment on photosynthesis of strawberry. Photosynthetica 2017, 55, 276–284. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant. Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, M.F.; Wu, F.; Li, W.L.; Li, S.P. 5-Aminolevulinic acid affects fruit coloration, growth, and nutrition quality of Litchi chinensis Sonn. cv. Feizixiao in Hainan, tropical China. Sci. Hortic-Amst. 2015, 193, 188–194. [Google Scholar] [CrossRef]
- An, Y.; Li, J.; Duan, C.; Liu, L.; Sun, Y.; Cao, R.; Wang, L. 5-Aminolevulinic Acid Thins Pear Fruits by Inhibiting Pollen Tube Growth via Ca(2+)-ATPase-Mediated Ca(2+) Efflux. Front. Plant Sci. 2016, 7, 121. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of Cadmium-Induced Proteomic and Metabolic Changes by 5-Aminolevulinic Acid in Leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. J. Plant. Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Koike, S.T.; Smith, R.F.; Cahn, M.D.; Pryor, B.M. Association of the carrot pathogen Alternaria dauci with new diseases, alternaria leaf speck, of lettuce and celery in California. Plant. Health Progress 2017, 18, 136–143. [Google Scholar] [CrossRef]
- Sturrock, C.J.; Woodhall, J.; Brown, M.; Walker, C.; Mooney, S.J.; Ray, R.V. Effects of damping-off caused by Rhizoctonia solani anastomosis group 2-1 on roots of wheat and oil seed rape quantified using X-ray Computed Tomography and real-time PCR. Front. Plant Sci. 2015, 6, 461. [Google Scholar] [CrossRef]
- Molla, K.A.; Karmakar, S.; Chanda, P.K.; Ghosh, S.; Sarkar, S.N.; Datta, S.K.; Datta, K. Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia solani) in transgenic rice. Mol. Plant. Pathol. 2013, 14, 910–922. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic Acid-Regulated Antioxidant Mechanisms and Gene Expression Enhance Rosemary Performance under Saline Conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement. Altern. Med. eCAM 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.-H.; Jung, S. Perturbed porphyrin biosynthesis contributes to differential herbicidal symptoms in photodynamically stressed rice (Oryza sativa) treated with 5-aminolevulinic acid and oxyfluorfen. Pestic. Biochem. Physiol. 2014, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.-U. Herbicidal activity of δ-aminolevulinic acid on several plants as affected by application methods. Korean J. Crop. Sci. Hortic. 2003, 48, 50–55. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Abad, M.J.; Ansuategui, M.; Bermejo, P. Active antifungal substances from natural sources. Arkivoc 2007, 7, 116–145. [Google Scholar]
- Gwinn, K.D.; Ownley, B.H.; Greene, S.E.; Clark, M.M.; Taylor, C.L.; Springfield, T.N.; Trently, D.J.; Green, J.F.; Reed, A.; Hamilton, S.L. Role of Essential Oils in Control of Rhizoctonia Damping-Off in Tomato with Bioactive Monarda Herbage. Phytopathology 2010, 100, 493–501. [Google Scholar] [CrossRef]
- Liu, D.; Kong, D.D.; Fu, X.K.; Ali, B.; Xu, L.; Zhou, W.J. Influence of exogenous 5-aminolevulinic acid on chlorophyll synthesis and related gene expression in oilseed rape de-etiolated cotyledons under water-deficit stress. Photosynthetica 2016, 54, 468–474. [Google Scholar] [CrossRef]
- Zhen, A.; Bie, Z.L.; Huang, Y.; Liu, Z.X.; Fan, M.L. Effects of 5-aminolevulinic acid on the H2O2-content and antioxidative enzyme gene expression in NaCl-treated cucumber seedlings. Biol. Plant. 2012, 56, 566–570. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.; Zhang, J.; Zhang, J.; Du, Q.; Li, J. H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant Activity of the Essential Oil and its Major Terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2018, 13 (Suppl. 4), S875–S880. [Google Scholar]
- Seol, G.-H.; Kang, P.; Lee, H.S.; Seol, G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Bai, G.; Sun, J.; Li, Y. Effect of 5-aminolevulinic acid and genistein on accumulation of polyphenol and anthocyanin in ‘Qinyang’ apples. Anim. Plant Sci. 2015, 25 (Suppl. 1), 68–79. [Google Scholar]
- Mohamed, A.A.; Adel, D.A.Q. Promotive effects of 5-aminolevulinic acid on growth of young ‘Barhee’ tissue culture- derived date palm (Phoenix dactylifera L.) trees in a newly established orchard. Food Agric. Environ. 2011, 9, 783–786. [Google Scholar]
- De Assis Gomes, M.d.M.; Lagôa, A.M.M.A.; Medina, C.L.; Machado, E.C.; Machado, M.A. Interactions between leaf water potential, stomatal conductance and abscisic acid content of orange trees submitted to drought stress. Braz. J. Plant Physiol. 2004, 16, 155–161. [Google Scholar] [CrossRef]
- Emamgholizadeh, S.; Esmaeilbeiki, F.; Babak, M.; zarehaghi, D.; Maroufpoor, E.; Rezaei, H. Estimation of the organic carbon content by the pattern recognition method. Commun. Soil Sci. Plant Anal. 2018, 49, 2143–2154. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Abdel-Lateef, A.M.; Mahmoud, H.H.; Helal, A.I. Determination of trace elements in water and sediment samples from Ismaelia Canal using ion chromatography and atomic absorption spectroscopy. Chem. Speciat. Bioavailab. 2012, 24, 31–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. A simple digestion procedure for estimation of total nitrogen in soil and sediments. J. Environ. Qual. 1972, 1, 423–425. [Google Scholar] [CrossRef]
- Neal, C.; Neal, M.; Wickham, H. Phosphate measurement in natural waters: Two examples of analytical problems associated with silica interference using phosphomolybdic acid methodologies. Sci. Total Environ. 2000, 251–252, 511–522. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skaicka-Wozniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crops Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured pub Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Zin El-Abedin, T.K. Omeprazole alleviates water stress in peppermint and modulates the expression of menthol biosynthesis genes. Plant. Physiol. Biochem. 2019, 139, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wolyn, D.J. Freezing tolerance assessment for seedlings of three asparagus cultivars grown under controlled conditions. Can. J. Plant. Sci. 2014, 95, 495–504. [Google Scholar] [CrossRef]
- Pressman, E.; Schaffer, A.A.; Compton, D.; Zamski, E. Carbohydrate Content of Young Asparagus Plants as Affected by Temperature Regimes. Plant Physiol. 1994, 143, 621–624. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K. Growth regulators and mowing heights enhance the morphological and physiological performance of Seaspray turfgrass during drought conditions. Acta Physiol. Plant. 2015, 37, 232. [Google Scholar] [CrossRef]
- Elansary, H.O.; Skalicka-Wozniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Radyukina, N.L.; Shashukova, A.V.; Makarova, S.S.; Kuznetsov, V.V. Exogenous proline modifies differential expression of superoxide dismutase genes in UV-B-irradiated Salvia officinalis plants. Russ. J. Plant Physiol. 2011, 58, 51–59. [Google Scholar] [CrossRef]
| Soil Type | ALA (ppm) | 1,8-cineole RI = 1040 | Linalool RI = 1117 | Camphor RI = 1139 | Borneol RI = 1188 | |
|---|---|---|---|---|---|---|
| (control) | A | 0 | 13.51c * | 5.32d | 11.02d | 10.68d |
| B | 0 | 14.12bc | 5.49d | 11.52cd | 10.98cd | |
| A | 3 | 14.0bc | 5.94c | 11.53cd | 11.21c | |
| B | 3 | 14.25b | 6.23b | 11.89c | 11.43c | |
| A | 5 | 15.34ab | 6.12b | 12.23b | 12.11b | |
| B | 5 | 15.71a | 6.35b | 12.61b | 12.59ab | |
| A | 10 | 15.82a | 7.12a | 13.19a | 12.86a | |
| B | 10 | 15.91a | 7.35a | 13.76a | 12.98a | |
| Alternaria dauci | A | 0 | 14.53c | 6.24d | 13.14d | 12.14b |
| B | 0 | 14.92c | 6.35d | 13.35cd | 12.26b | |
| A | 3 | 15.04bc | 7.46c | 13.59c | 13.34ab | |
| B | 3 | 15.61b | 7.51c | 13.75c | 13.45a | |
| A | 5 | 16.11ab | 8.24b | 14.22b | 13.61a | |
| B | 5 | 16.61a | 8.41ab | 14.54a | 13.75a | |
| A | 10 | 16.91a | 8.56a | 14.86a | 13.88a | |
| B | 10 | 16.85a | 8.74a | 14.94a | 13.91a | |
| Rhizoctonia solani | A | 0 | 13.23d | 5.84e | 12.02c | 11.01c |
| B | 0 | 13.62c | 6.03e | 12.66c | 11.32c | |
| A | 3 | 14.08c | 6.54d | 13.43b | 12.32b | |
| B | 3 | 14.52bc | 6.68d | 13.79b | 12.45b | |
| A | 5 | 15.04b | 7.11c | 14.31a | 12.85b | |
| B | 5 | 15.64a | 7.46b | 14.46a | 13.34a | |
| A | 10 | 15.81a | 7.71a | 14.64a | 13.81a | |
| B | 10 | 16.14a | 8.01a | 14.84a | 13.85a |
| Composition | Loam + 30% Sand | Loam | LSD p = 0.05 |
|---|---|---|---|
| pH EC (mmho cm−1) Water holding capacity (%) Soil organic carbon (SOC, %) | 8.1 0.954 33.5 0.41 | 8 0.986 45.1 0.52 | 0.12 0.31 2.2 0.02 |
| Nitrogen (N, g ha−1) | 162 × 103 | 190 × 103 | 3.5 |
| Phosphorus (P, g ha−1) | 15.69 × 103 | 18.4 × 103 | 1.2 |
| Potassium (K, g ha−1) | 146.2 × 103 | 199.5 × 103 | 3.2 |
| Iron (Fe, mg kg−1) | 4.1 | 4.6 | 0.1 |
| Copper (Cu, mg kg−1) | 0.35 | 0.42 | 0.05 |
| Zink (Zn, mg kg−1) | 3.5 | 5.1 | 0.06 |
| Manganese (Mn, mg kg−1) | 2.8 | 2.9 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. 5-Aminolevulinic Acid and Soil Fertility Enhance the Resistance of Rosemary to Alternaria dauci and Rhizoctonia solani and Modulate Plant Biochemistry. Plants 2019, 8, 585. https://doi.org/10.3390/plants8120585
Elansary HO, El-Ansary DO, Al-Mana FA. 5-Aminolevulinic Acid and Soil Fertility Enhance the Resistance of Rosemary to Alternaria dauci and Rhizoctonia solani and Modulate Plant Biochemistry. Plants. 2019; 8(12):585. https://doi.org/10.3390/plants8120585
Chicago/Turabian StyleElansary, Hosam O., Diaa O. El-Ansary, and Fahed A. Al-Mana. 2019. "5-Aminolevulinic Acid and Soil Fertility Enhance the Resistance of Rosemary to Alternaria dauci and Rhizoctonia solani and Modulate Plant Biochemistry" Plants 8, no. 12: 585. https://doi.org/10.3390/plants8120585
APA StyleElansary, H. O., El-Ansary, D. O., & Al-Mana, F. A. (2019). 5-Aminolevulinic Acid and Soil Fertility Enhance the Resistance of Rosemary to Alternaria dauci and Rhizoctonia solani and Modulate Plant Biochemistry. Plants, 8(12), 585. https://doi.org/10.3390/plants8120585





