Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth
Abstract
1. Introduction
2. Results
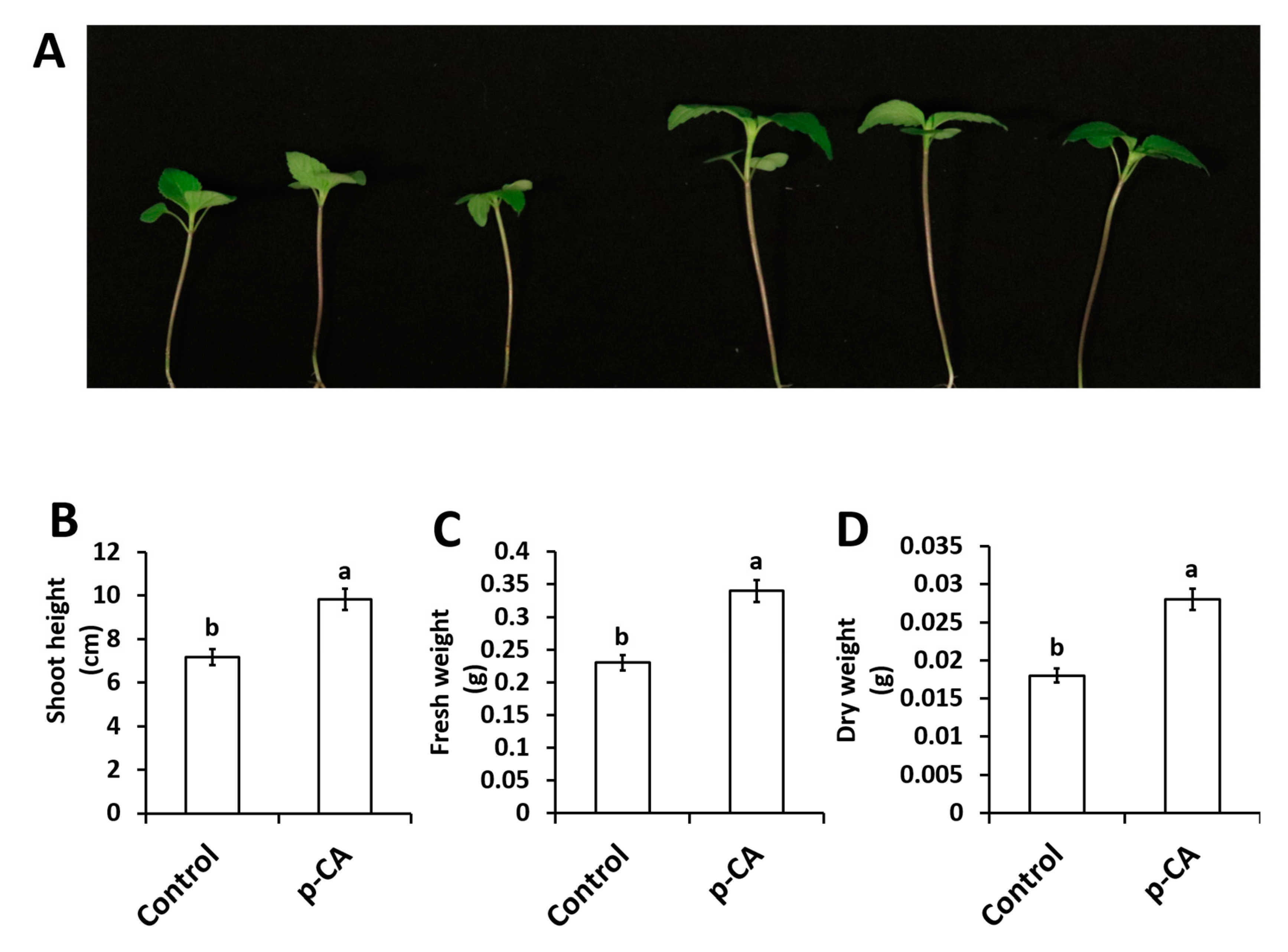
2.1. p-CA Improves Chia Seedling Growth
2.2. The Effect of Exogenous p-CA on Chlorophyll Metabolism and Osmolyte Content
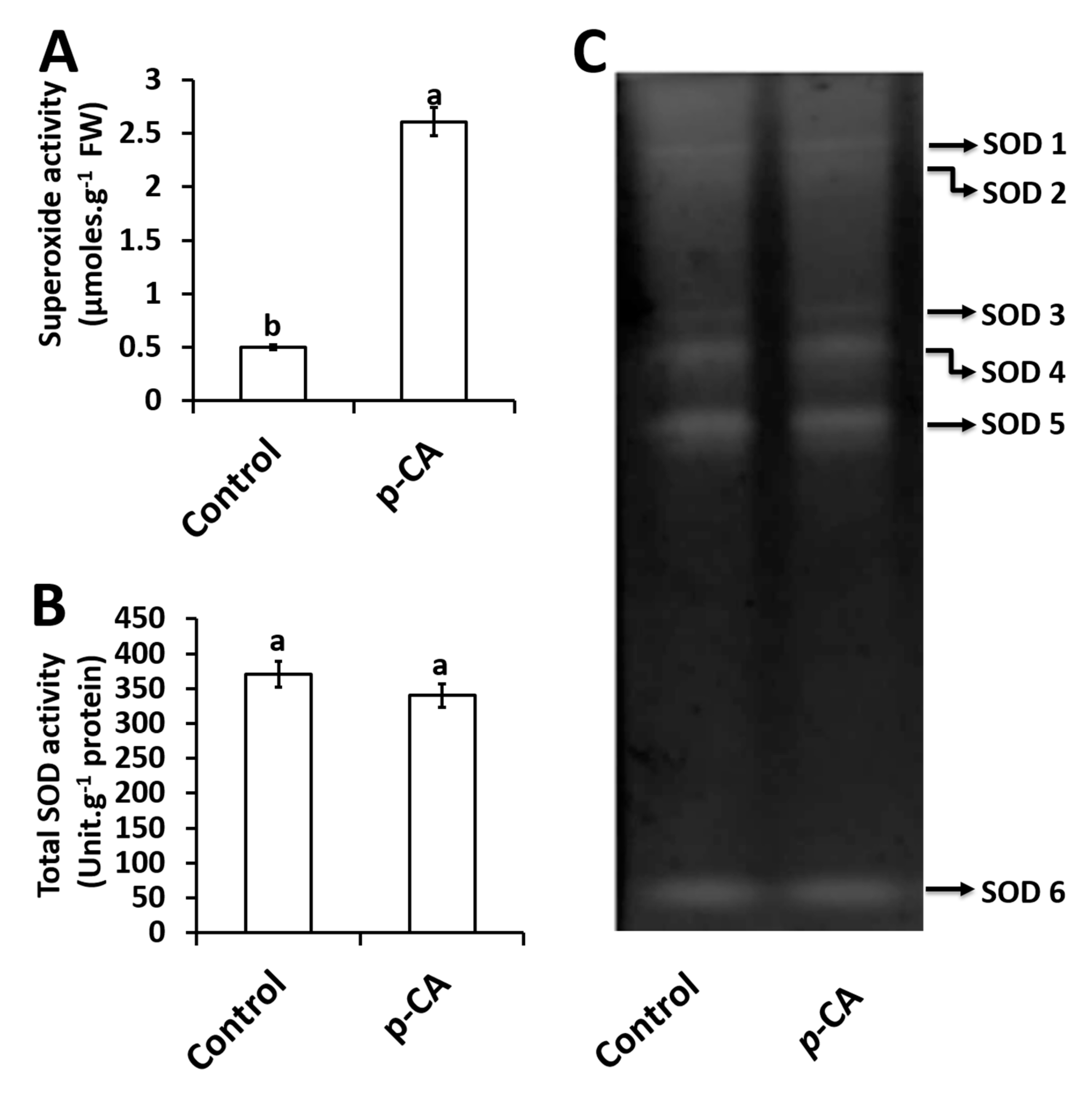
2.3. Effects of p-CA on Superoxide Radical and Superoxide Dismutase Activity
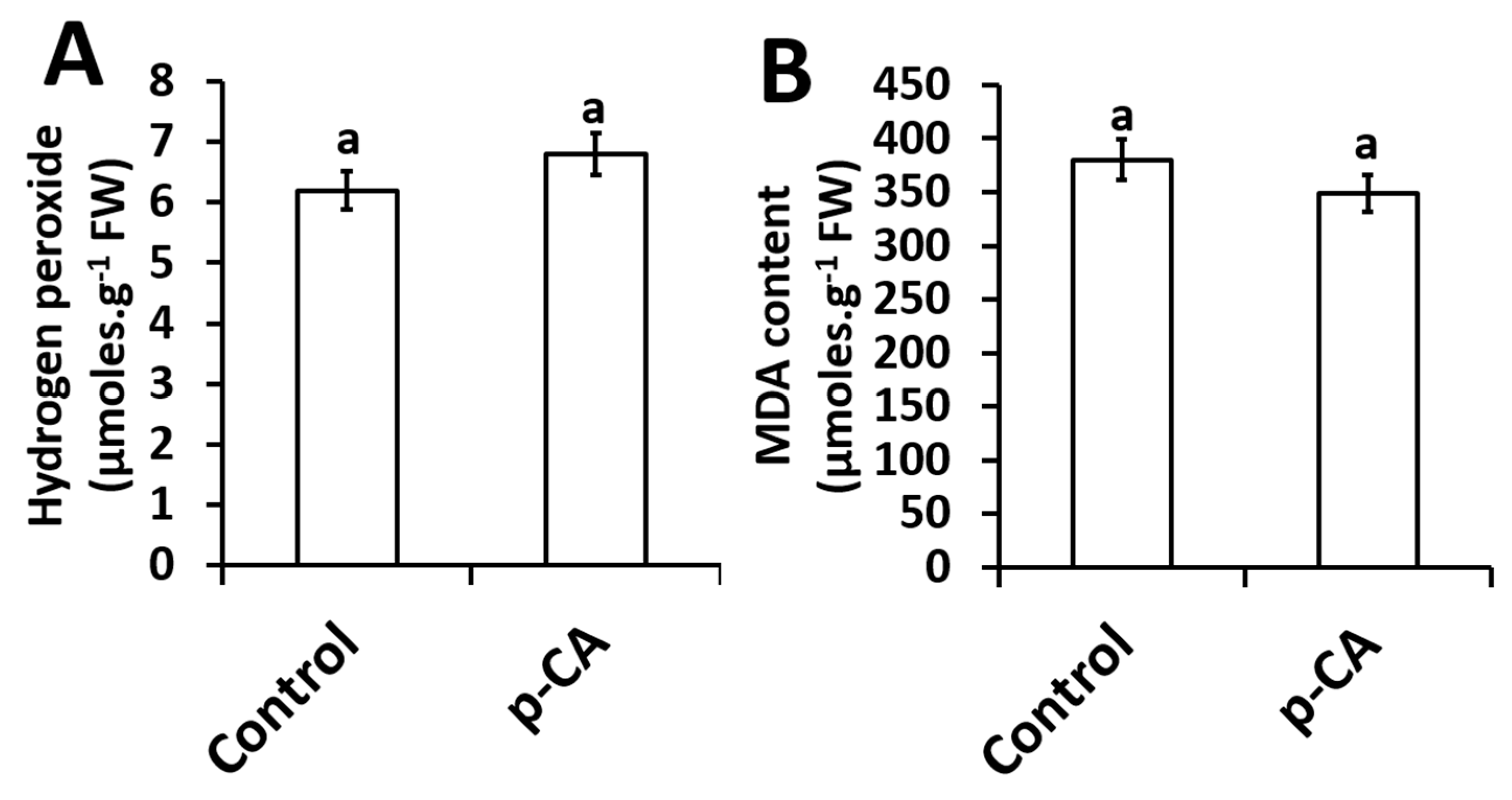
2.4. Effects of Exogenous Application of p-CA on H2O2 Content and the Extent of Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Measurement of Plant Growth
4.3. Chlorophyll Estimation
4.4. Protein Extraction for Biochemical Analysis
4.5. Assays for ROS Content
4.6. Determination of MDA Content
4.7. Quantification of SOD Activity
4.8. Determination of Proline Content
4.9. Determination of Glycine Betaine
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| p-CA | p-coumaric acid |
| DW | dry weights |
| FW | fresh weights |
| GB | glycine betaine |
| KI | potassium iodide |
| MDA | malondialdehyde |
| PUFA | polyunsaturated fatty acids |
| PVP | polyvinylpyrrolidone |
| ROS | reactive oxygen species |
| SH | shoot height |
| SOD | superoxide dismutase |
| TBA | thiobarbituric acid |
| TCA | trichloroacetic acid |
References
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Dermatol. Res. 2015, 307, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Jeong, D.; Yi, Y.; Park, J.G.; Seo, H.; Moh, S.H.; Hong, S.; Cho, J.Y. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediat. Inflamm. 2013, 2013, 518183. [Google Scholar] [CrossRef]
- Bubna, G.A.; Lima, R.B.; Zanardo, D.Y.L.; Dos Santos, W.D.; Ferrarese, M.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef]
- Macias, F. Allelopathy in the search for natural herbicides model. In Allelopathy, Current Status and Future Goals; Dakshini, K.M.M., Einhellig, F.A., Eds.; ACS Publications: Washington, DC, USA, 1995; pp. 310–329. [Google Scholar]
- Baleroni, C.R.S.; Ferrarese, M.L.; Braccini, A.L.; Scapim, C.A.; Ferrarese-Filho, O. Effects of ferulic and p-coumaric acids on canola (Brassica napus L. cv. Hyola 401) seed germination. Seed Sci. Technol. 2000, 28, 201–207. [Google Scholar]
- Janovicek, K.J.; Vyn, T.J.; Voroney, R.P.; Allen, O.B. Early corn seedling growth response to phenolic acids. Can. J. Plant Sci. 1997, 77, 391–393. [Google Scholar] [CrossRef]
- Patterson, D.T. Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max L.). Weed Sci. 1981, 29, 53–59. [Google Scholar] [CrossRef]
- Politycka, B.; Mielcarz, B. Involvement of ethylene in growth inhibition of cucumber roots by ferulic and p-coumaric acids. Allelopath. J. 2007, 19, 451–460. [Google Scholar]
- Reigosa, M.J.; Souto, X.C.; Gonzálezm, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Orcaray, L.; Igal, M.; Zabalza, A.; Royuela, M. Role of exogenously supplied ferulic and p-coumaric acids in mimicking the mode of action of acetolactate synthase inhibiting herbicides. J. Agric. Food Chem. 2011, 59, 10162–10168. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, D.I.L.; Lima, R.B.; Ferrarese, M.L.L.; Bubna, G.A.; Ferrarese-Filho, O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ. Exp. Bot. 2009, 66, 25–30. [Google Scholar] [CrossRef]
- Jones, S.; Keyster, M.; Klein, A. Exogenous caffeic acid alters physiological and molecular responses in chia (Salvia hispanica L.). S. Afr. J. Bot. 2017, 100, 339–340. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 2008, 165, 297–305. [Google Scholar] [CrossRef]
- Klein, A.; Keyster, M.; Ludidi, N. Response of soybean nodules to exogenously applied caffeic acid during NaCl-induced salinity. S. Afr. J. Bot. 2015, 96, 13–18. [Google Scholar] [CrossRef]
- Kranner, I.; Roach, T.; Beckett, R.C.; Whitaker, C. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. Plant Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Gokul, A.; Roode, E.; Klein, A.; Keyster, M. Exogenous 3, 3′-diindolylmethane increases Brassica napus L. seedling shoot growth through modulation of superoxide and hydrogen peroxide content. J. Plant Physiol. 2016, 196, 93–98. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. J. 2005, 25, 995–1003. [Google Scholar] [CrossRef]
- Bushway, A.A.; Belyea, P.R.; Bushway, R.J. Chia seed as a source of oil, polysaccharide, and protein. J. Food Sci. 1981, 46, 1349–1350. [Google Scholar] [CrossRef]
- Capitani, M.I.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- da Silva Marineli, R.; Moraes, E.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R., Jr. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- da Silva Marineli, R.; Moura, C.S.; Moraes, E.A.; Lenquiste, S.A.; Lollo, P.C.B.; Morato, P.N.; Maróstica, M.R., Jr. Chia (Salvia hispanica L.) enhances HSP, PGC-1α expressions and improves glucose tolerance in diet-induced obese rats. Nutr. J. 2015, 31, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef]
- Porras-Loaiza, P.; Jiménez-Munguía, M.T.; Sosa-Morales, M.E.; Palou, E.; López-Malo, A. Physical properties, chemical characterization and fatty acid composition of Mexican chia (Salvia hispanica L.) seeds. Int. J. Food Sci. Technol. 2014, 49, 571–577. [Google Scholar] [CrossRef]
- Ng, P.L.L.; Ferrarese, M.L.L.; Huber, D.A.; Ravagnani, A.L.S.; Ferrarese-Filho, O. Canola (Brassica napus L.) seed germination influenced by cinnamic and benzoic acids and derivatives: Effects on peroxidase. Seed Sci. Technol. 2003, 31, 39–46. [Google Scholar] [CrossRef]
- Gerig, T.M.; Blum, U. Effects of mixtures of 4 phenolic acids on leaf-area expansion of cucumber seedlings grown in Portsmouth B1 soil materials. J. Chem. Ecol. 1991, 17, 29–40. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Sheng, H.; Wang, Y.; Kang, H.; Zeng, J. Salicylic acid and nitric oxide increase photosynthesis and antioxidant defense in wheat under UV-B stress. Biol. Plant. 2016, 60, 686–694. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–823. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Kovrizhnikh, V.A.; Timofeev, K.N. Superoxide mediated chlorophyll allomerization in a dimethyl sulphoxide-water mixture. Free Radic. Res. Commun. 1991, 15, 197–201. [Google Scholar] [CrossRef]
- Klein, A.; Keyster, M.; Ludidi, N. Caffeic acid decreases salinity-induced root nodule superoxide radical accumulation and limits salinity-induced biomass reduction in soybean. Acta Physiol. Plant. 2013, 35, 3059–3066. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Sheokand, S.; Bhankar, V.; Sawhney, V. Ameliorative effect of exogenous nitric oxide on oxidative metabolism in NaCl treated chickpea plants. Braz. J. Plant Physiol. 2009, 22, 81–90. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Ion relations of plants under drought and salinity. Aust. J. Plant Physiol. 1986, 13, 75–91. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 477–522. [Google Scholar]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Schwabe, F.; Erban, A.; Udvardi, M.K.; Kopka, J. Comparative metabolomics of drought acclimation in model and forage legumes. Plant Cell Environ. 2012, 35, 136–149. [Google Scholar] [CrossRef]
- Matysik, J.; Alia, B.B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Egbichi, I.; Keyster, M.; Jacobs, A.; Klein, A.; Ludidi, N. Modulation of antioxidant enzyme activities and metabolites ratios by nitric oxide in short-term salt stressed soybean root nodules. S. Afr. J. Bot. 2013, 88, 326–333. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Saxena, D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Ullah, S.; Kolo, Z.; Egbichi, I.; Keyster, M.; Ludidi, N. Nitric oxide influences glycine betaine content and ascorbate peroxidase activity in maize. S. Afr. J. Bot. 2016, 105, 218–225. [Google Scholar] [CrossRef]
- IKiliç, I.; Yeşiloğlu, I.Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Rajendrakumar, C.S.; Suryanarayana, T.; Reddy, A.R. DNA helix destabilization by proline and betaine: Possible role in the salinity tolerance process. FEBS Lett. 1997, 410, 201–205. [Google Scholar] [CrossRef]
| Trait (µg.g−1FW) | Control | p-CA |
|---|---|---|
| Chlorophyll a | 15.70 ± 0.83b | 21.30 ± 0.88a |
| Chlorophyll b | 24.80 ± 1.01b | 34.40 ± 0.50a |
| Total Chlorophyll | 40.50 ± 1.80b | 55.70 ± 1.38a |
| Carotenoids | 1009.70 ± 10.17b | 1263.30 ± 8.82a |
| Glycine Betaine | 6030 ± 233.03b | 7380 ± 60.02a |
| Proline | 1.21 ± 0.02b | 3.26 ± 0.04a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth. Plants 2019, 8, 546. https://doi.org/10.3390/plants8120546
Nkomo M, Gokul A, Keyster M, Klein A. Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth. Plants. 2019; 8(12):546. https://doi.org/10.3390/plants8120546
Chicago/Turabian StyleNkomo, Mbukeni, Arun Gokul, Marshall Keyster, and Ashwil Klein. 2019. "Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth" Plants 8, no. 12: 546. https://doi.org/10.3390/plants8120546
APA StyleNkomo, M., Gokul, A., Keyster, M., & Klein, A. (2019). Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth. Plants, 8(12), 546. https://doi.org/10.3390/plants8120546







