Preventive Effects of Fluoro-Substituted Benzothiadiazole Derivatives and Chitosan Oligosaccharide against the Rice Seedling Blight Induced by Fusarium oxysporum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Pathogen Inoculation
2.3. Elicitor Treatment and Sampling
2.4. Efficiency of FBT and COS to Elicit Resistance against Seedling Blight
2.5. Effect of FBT and OCT on Enzymatic Activity of Root
2.5.1. Superoxide Dismutase (SOD) Assay
2.5.2. Peroxidase (POD) Assay
2.5.3. Catalase (CAT) Assay
2.5.4. Phenylalanine Ammonia-Lyase (PAL) Assay
2.6. Quantitative Analysis of Global Proteome
2.6.1. Protein Sample Preparation
2.6.2. Liquid Chromatography Tandem Mass Spectrometry (LC−MS/MS) Analysis
2.6.3. Protein Identification and Screening of Differentially Expressed Proteins (DEPs)
2.7. Determination of Momilactone in Rice Root
2.8. Confirmation of the Infection-Responsive Expression Profiles by qRT-PCR
2.9. Statistical Analysis
3. Results
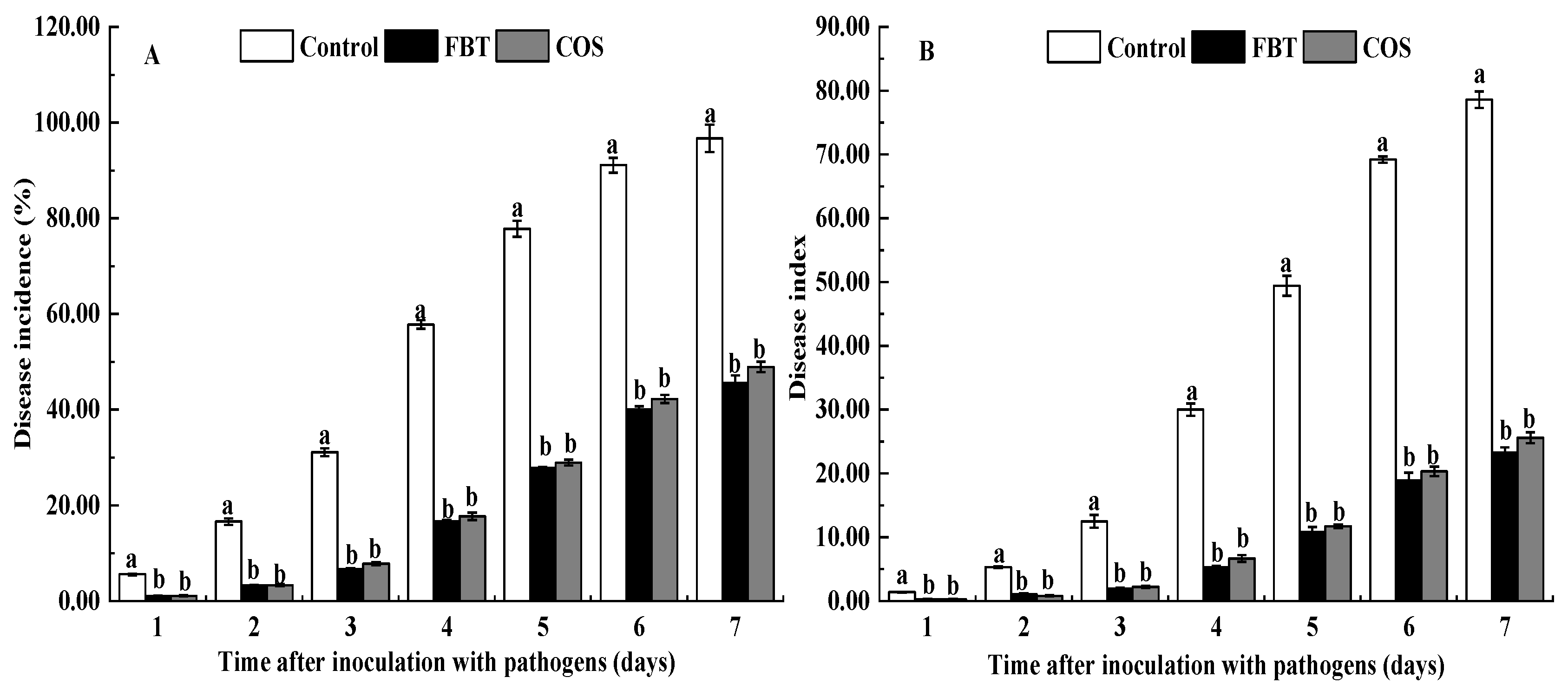
3.1. Efficacy of FBT and COS on Control of Seedling Blight
3.2. Effect of FBT and COS on Growth Status of Root
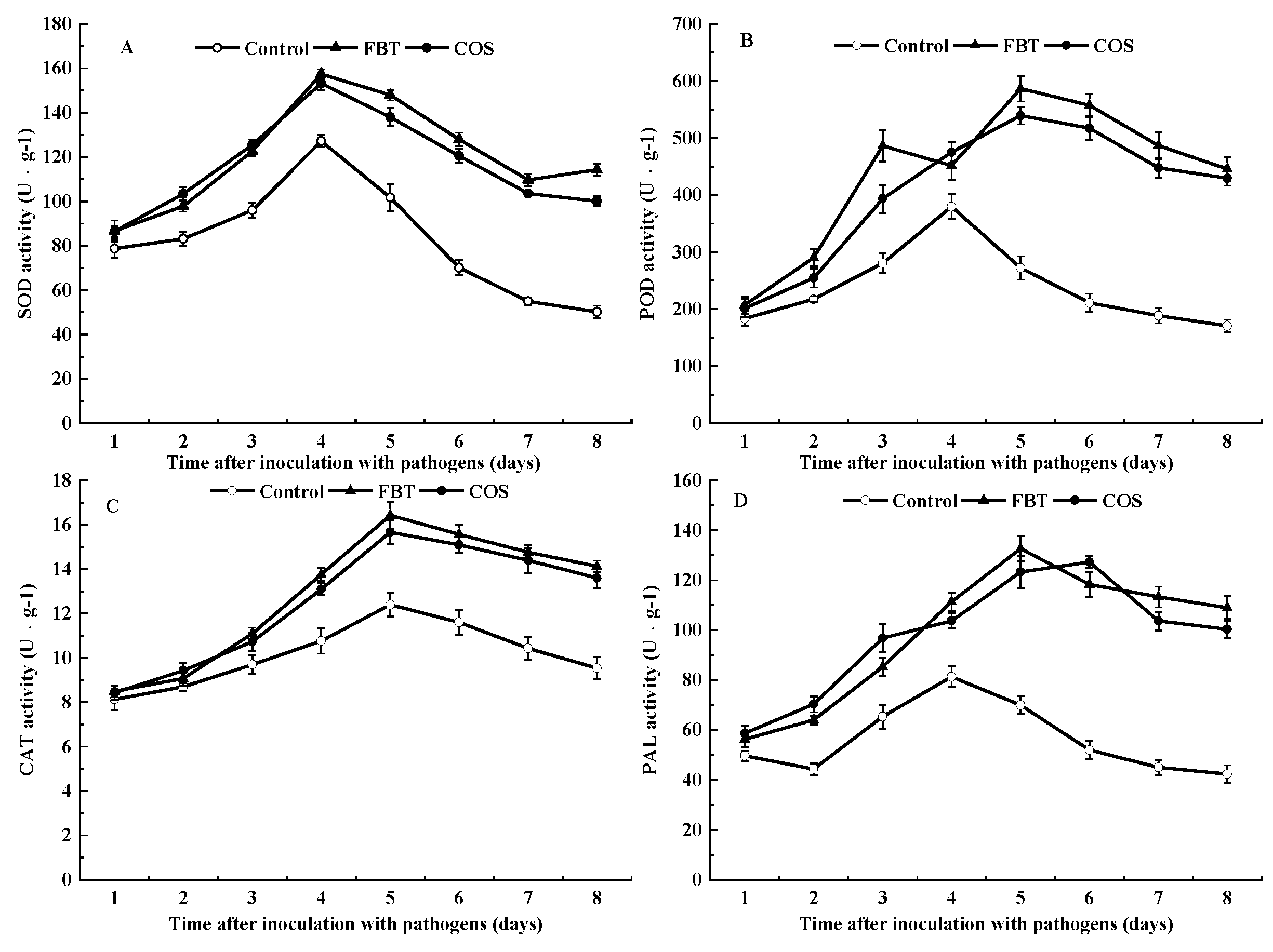
3.3. Effect of FBT and COS on Enzymatic Activities of Root
3.3.1. Superoxide Dismutase (SOD) Assay
3.3.2. Peroxidase (POD) Assay
3.3.3. Catalase (CAT) Assay
3.3.4. Phenylalanine Ammonia-Lyase (PAL) Assay
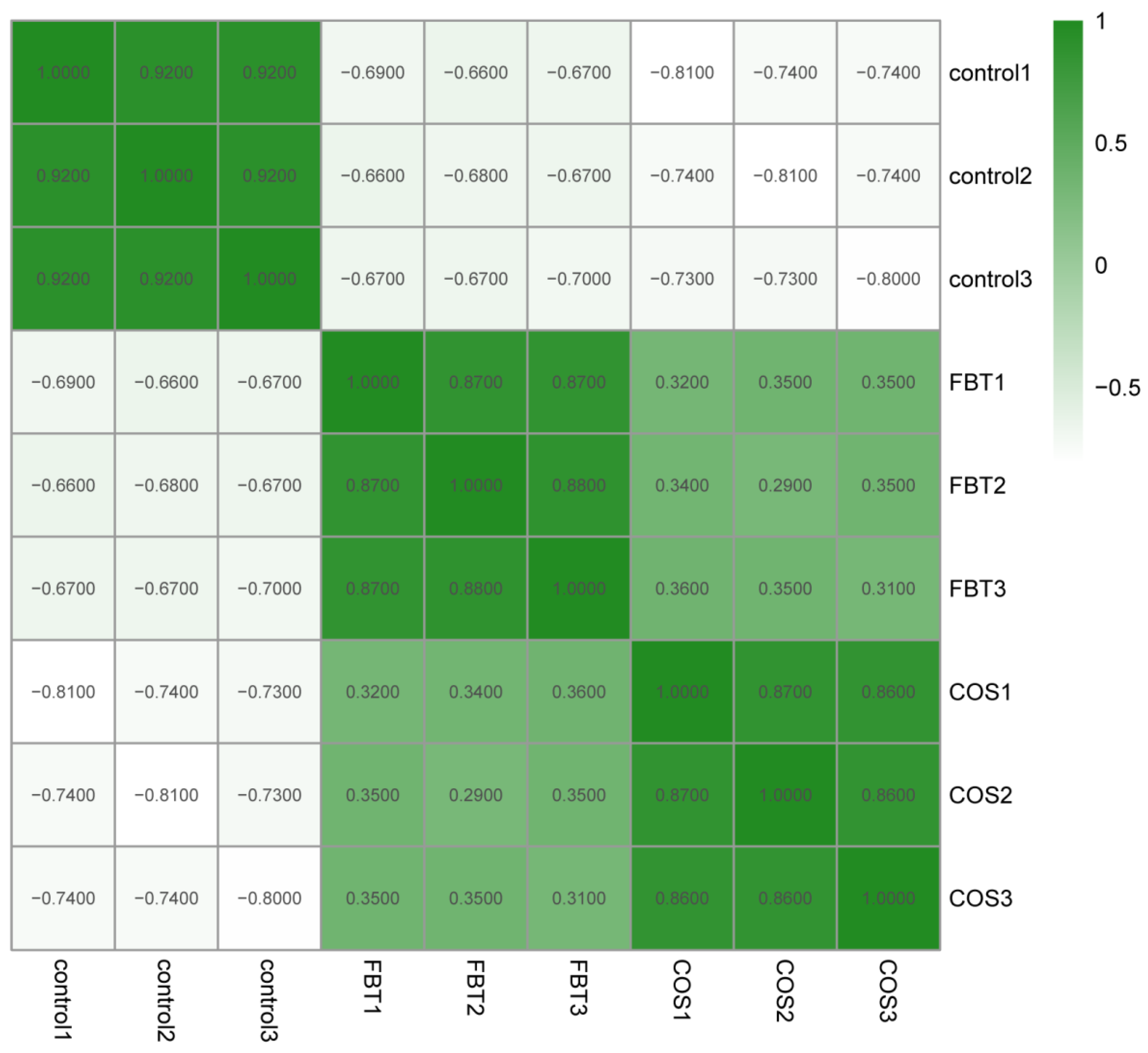
3.4. Proteomic Characteristics of All Samples
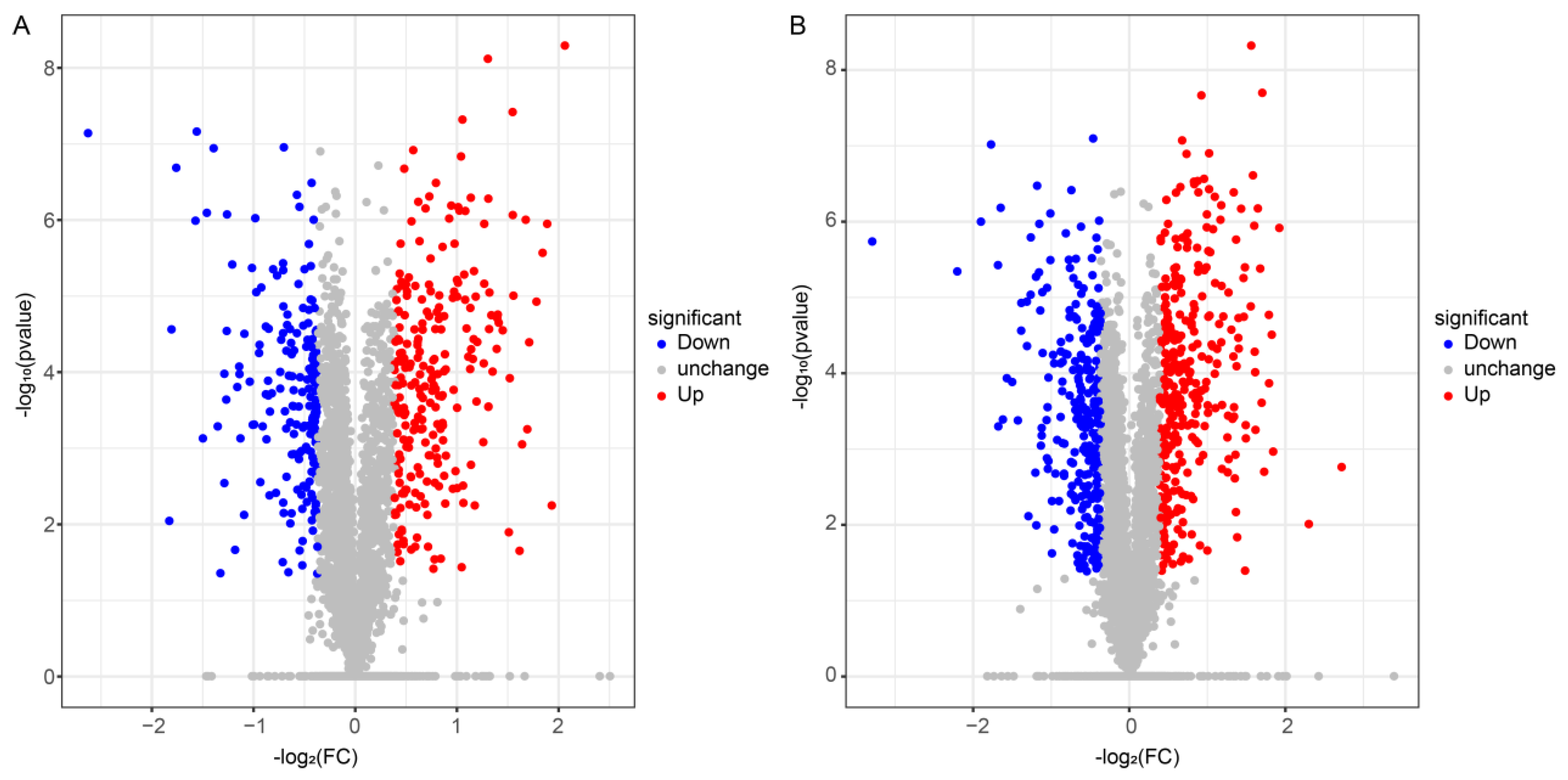
3.5. Differential Expression and Biological Pathway Enrichment Analysis
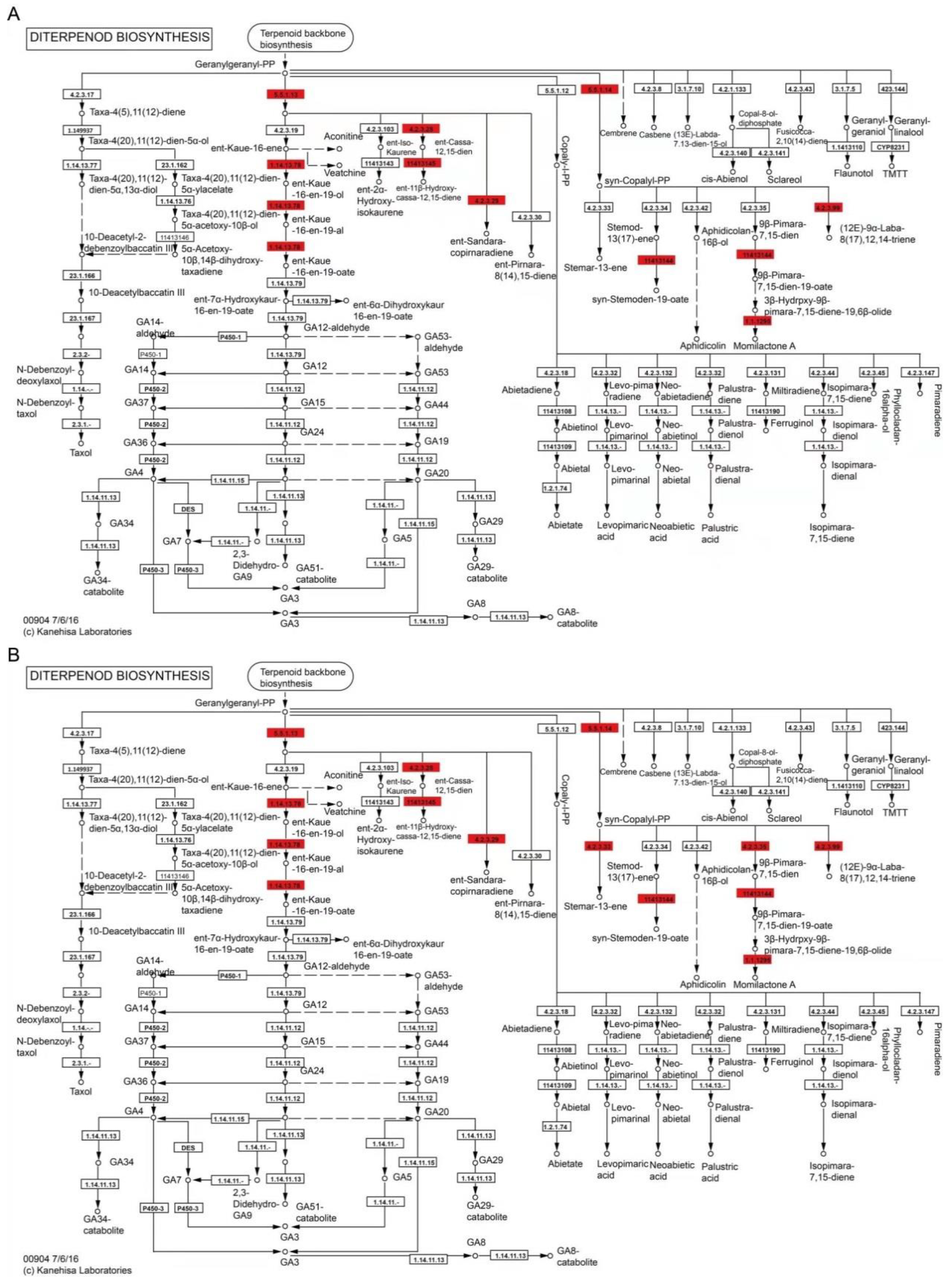
3.6. Specific Pathway of Diterpenoid Biosynthesis Analysis
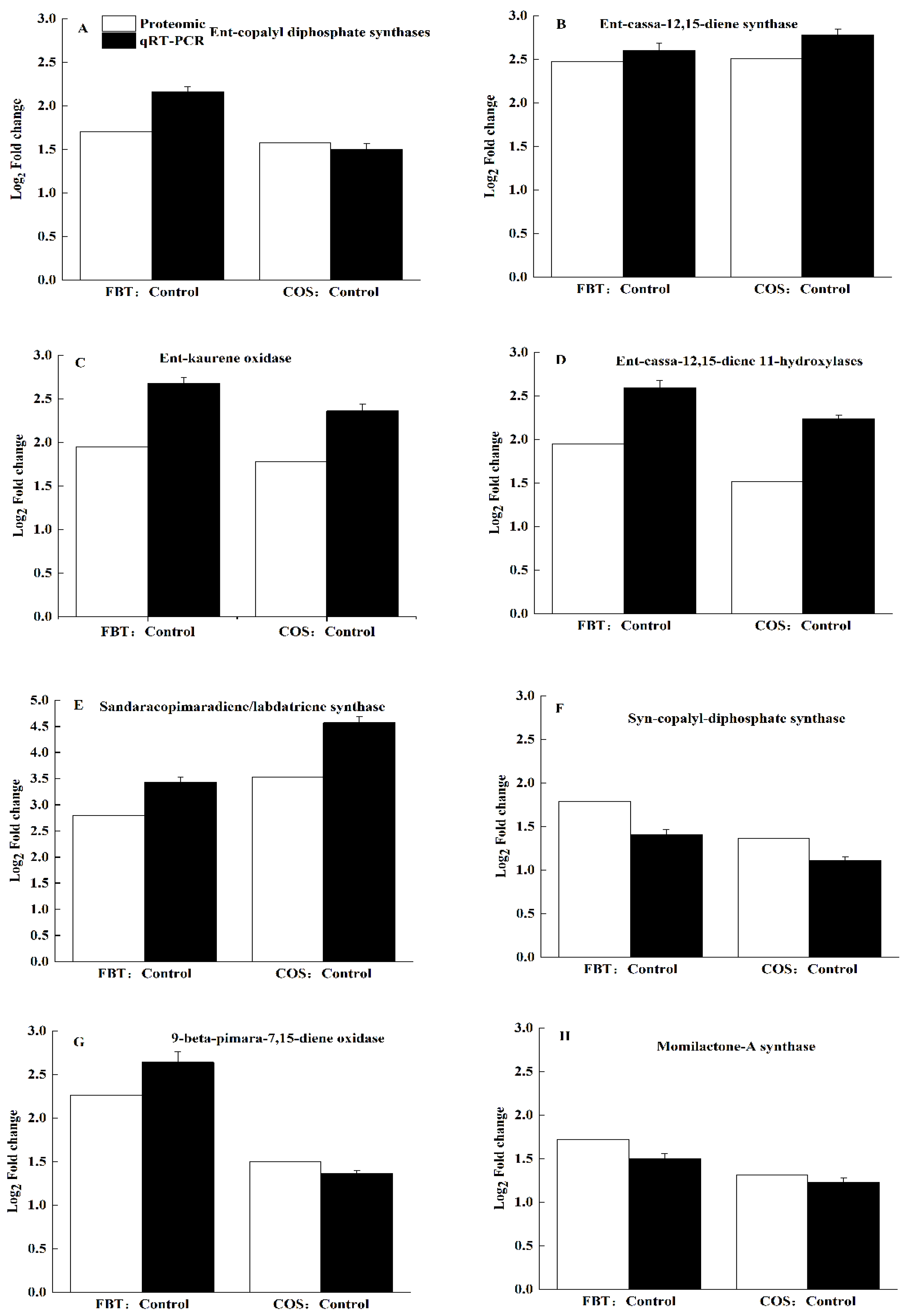
3.7. Confirm Unigenes Expression Using Real-Time Quantitative Reverse Transcription PCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mir, S.; Bhat, M.A.; Bashir, A.; Rashid, R.; Bano, H.; Mir, S.A. Present status and future prospects of marker assisted selection for sheath blight resistance breeding in rice (Oryza sativa L.). Skuast J. Res. 2015, 17, 72–90. [Google Scholar]
- Marchetti, M.; Bollich, C. Quantification of the relationship between sheath blight severity and yield loss in rice. Plant Dis. 1991, 8, 773–775. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- Ou, S.H. Rice Diseases; CAB: London, UK, 1985; pp. 61–68. [Google Scholar]
- Niehs, S.P.; Dose, B.; Scherlach, K.; Roth, M.; Hertweck, C. Genomics-driven discovery of a symbiont-specific cyclopeptide from bacteria residing in the rice seedling blight Fungus. ChemBioChem 2018, 19, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Larran, S.; Siurana, M.P.S.; Caselles, J.R.; Simón, M.R.; Perelló, A. Fusarium sudanense, endophytic fungus causing typical symptoms of seedling blight and seed rot on wheat. J. King Saud Univ.-Sci. 2018, 14, 299–315. [Google Scholar] [CrossRef]
- Nagamine, T.; Ozaki, K. Effects of Pythium spp. and temperature on the outbreak of damping, so-called murenae, of rice seedlings. Bull. Natl. Inst. Agrobiol. Resour. (Jpn.) 1987, 3, 89–104. [Google Scholar]
- Li, Y.; Zhang, X.; Zhang, R.; Liu, J.; Ali, E.; Ji, P.; Pan, H. Occurrence of seedling blight caused by Fusarium tricinctum on rice in China. Plant Dis. 2019, 103, 1789. [Google Scholar] [CrossRef]
- Wang, M.; Wei, P.; Cao, M.; Zhu, L.; Lu, Y. First report of rice seedling blight caused by Burkholderia plantarii in North and Southeast China. Plant Dis. 2016, 100, 645. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Cheng, W.; Song, X.S.; Li, H.P.; Cao, L.H.; Sun, K.; Qiu, X.L.; Xu, Y.B.; Yang, P.; Huang, T.; Zhang, J.B. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2016, 13, 1335–1345. [Google Scholar] [CrossRef]
- Shaheen, R.; Sharif, M.Z.; Amrao, L.; Zheng, A.; Manzoor, M.; Majeed, D.; Kiran, H.; Jafir, M.; Ali, A. Investigation of bacterial leaf blight of rice through various detection tools and its impact on crop yield in punjab. Pak. J. Bot. 2019, 51, 307–312. [Google Scholar]
- Nasir, M.; Iqbal, B.; Hussain, M.; Mustafa, A.; Ayub, M. Chemical management of bacterial leaf blight disease in rice. J. Agric. Res. 2019, 57, 99–103. [Google Scholar]
- Singh, U.B.; Malviya, D.; Singh, S.; Pradhan, J.K.; Singh, B.P.; Roy, M.; Imram, M.; Pathak, N.; Baisyal, B.; Rai, J.P. Bio-protective microbial agents from rhizosphere eco-systems trigger plant defense responses provide protection against sheath blight disease in rice (Oryza sativa L.). Microbiol. Res. 2016, 192, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Rasul, M.; Yasmin, S.; Zubair, M.; Mahreen, N.; Yousaf, S.; Arif, M.; Sajid, Z.I.; Mirza, M.S. Phosphate solubilizers as antagonists for bacterial leaf blight with improved rice growth in phosphorus deficit soil. Biol. Control 2019, 136, 103997. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef]
- Van, D.E.; Koornneef, A.; Ton, J.; Pieterse, C.M. Induced resistance-orchestrating defence mechanisms through crosstalk and priming. Annu. Plant Rev. 2018, 34, 334–370. [Google Scholar]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Tan, B.A.; Daim, L.D.J.; Ithnin, N.; Ooi, T.E.K.; Md-Noh, N.; Mohamed, M.; Mohd-Yusof, H.; Appleton, D.R.; Kulaveerasingam, H. Expression of phenylpropanoid and flavonoid pathway genes in oil palm roots during infection by Ganoderma boninense. Plant Gene 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Zhang, L.; Qingsong, W.; Limei, W.; Zhiming, Z.; Saijin, W. Effects of agro-antibiotic 211 on related substances inducing rice resistance to sheath blight. Plant Dis. Pests 2019, 10, 28–30. [Google Scholar] [CrossRef]
- Kano, A.; Gomi, K.; Yamasaki-Kokudo, Y.; Satoh, M.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; Ishida, Y.A. A rare sugar, D-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa. Phytopathology 2010, 100, 85–90. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, D.S.; You, P.D.W. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7% efficiency. Angew. Chem. Int. Ed. 2011, 50, 2995–2998. [Google Scholar] [CrossRef]
- Han, Z.; Xie, X.; Jin, Z.; Song, J.; Chai, A.; Shi, Y.; Li, B. Systemic Resistance Induced by fluoro-substituted benzothiadiazole derivatives against Plasmodiophora brassicae in Chinese cabbage. Acta Hortic. Sin. 2015, 42, 697–705. [Google Scholar]
- Zhou, Y.; Ma, J.; Xie, J.; Deng, L.; Yao, S.; Zeng, K. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, X.; Du, Y. Oligochitosan: A plant diseases vaccine—A review. Carbohydr. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–76. [Google Scholar] [CrossRef]
- Bailly, C.; Leymarie, J.; Lehner, A.; Rousseau, S.; Come, D.; Corbineau, F. Catalase activity and expression in developing sunflower seeds as related to drying. J. Exp. Bot. 2004, 55, 475–483. [Google Scholar] [CrossRef]
- Beaudoin-Eagan, L.D.; Thorpe, T.A. Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985, 78, 438–441. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F. iProX: An integrated proteome resource. Nucleic Acids Res. 2018, 47, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T.; Sata, N.; Yamamura, S. Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 2002, 115, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vanloon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263. [Google Scholar] [CrossRef]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 264. [Google Scholar] [CrossRef]
- Ram, R.M.; Keswani, C.; Bisen, K.; Tripathi, R.; Singh, S.P.; Singh, H.B. Biocontrol technology: Eco-friendly approaches for sustainable agriculture. Omics Technol. Bio-Eng. Acad. 2018, 2, 177–190. [Google Scholar]
- Wojtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A.; Stone, J.M.; Asai, T.; Plotnikov, J.; Denoux, C.; Hayes, T.; Gerrish, C.; Davies, D.R. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Marjamaa, K.; Kukkola, E.M.; Fagerstedt, K.V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009, 60, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Turpaev, K. Reactive oxygen species and regulation of gene expression. Biochemistry (Moscow) 2002, 67, 281–292. [Google Scholar] [CrossRef]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant. 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Y.; Bi, Y.; Li, C.; Deng, H.; Hu, L.; Dong, B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014, 168, 113–119. [Google Scholar] [CrossRef]
- Panina, Y.S.; Vasyukova, N.I.; Chalenko, G.I.; Ozeretskovskaya, O.L. Changes in Catalase Activity in Potato Tubers, Induced by Immunoregulators. Dokl. Biol. Sci. 2004, 395, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Quirino, B.; Candido, E.; Campos, P.; Franco, O.; Krüger, R. Proteomic approaches to study plant–pathogen interactions. Phytochemistry 2010, 71, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 14, 3861–3864. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, J.; Guo, B.; Duan, J.; Che, C.-T. Momilactone and related diterpenoids as potential agricultural chemicals. J. Agric. Food Chem. 2018, 66, 7859–7872. [Google Scholar] [CrossRef]
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defence mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Nozaki, H.; Hayashi, K.I.; Nishimura, N.; Kawaide, H.; Takaoka, D. Momilactone A and B as allelochemicals from moss Hypnum plumaeforme: First occurrence in Bryophytes. J. Agric. Chem. Soc. Jpn. 2007, 71, 3127–3130. [Google Scholar]
- Kato-Noguchi, H.; Kobayashi, K. Jasmonic acid, protein phosphatase inhibitor, metals and UV-irradiation increased momilactone A and B concentrations in the moss Hypnum plumaeforme. J. Plant Physiol. 2009, 166, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Secretion of momilactone A and B by the moss Hypnum plumaeforme. Plant Signal. Behav. 2009, 4, 737–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato-Noguchi, H.; Kobayashi, K.; Shigemori, H. Allelopathy of the moss Hypnum plumaeforme by the production of momilactone A and B. Weed Res. 2010, 49, 621–627. [Google Scholar] [CrossRef]
- Fukuta, M.; Dang Xuan, T.; Deba, F.; Tawata, S.; Dang Khanh, T.; Min Chung, I. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Tsunakawa, M.; Ohba, A.; Sasaki, N.; Kabuto, C.; Kato, T.; Kitahara, Y.; Takahashi, N. Momilactone-C, a minor constituent of growth inhibitors in rice husk. Chem. Lett. 1976, 5, 1157–1158. [Google Scholar] [CrossRef] [Green Version]
- Na, L.; Wang, S.; Lou, H. A new pimarane-type diterpenoid from moss Pseudoleskeella papillosa (Lindb.) Kindb. Acta Pharm. Sin. B 2012, 2, 256–259. [Google Scholar]
- Li, G.; Xu, Q.-L.; He, C.-M.; Zeng, L.; Wang, H.-F. Two new anti-fungal diterpenoids from the husks of Oryza sativa. Phytochem. Lett. 2014, 10, 309–312. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Koga, J.; Toshima, H.; Hasegawa, M. Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane f, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015, 63, 4050–4059. [Google Scholar] [CrossRef]
- Cho, J.G.; Cha, B.J.; Min, L.S.; Shrestha, S.; Jeong, R.H.; Sung, L.D.; Kim, Y.C.; Lee, D.G.; Kang, H.C.; Kim, J. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on no production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef]
- Chung, I.; Ateeque, A.; Kim, J. Natural Herbicidal Agent Containing Momilactones A and B Identified in and Isolated from Rice Hulls. U.S. Patent Application No. 20060160701A1, 20 July 2006. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Wang, J.; Liu, C.; Hu, J.; Tan, K.; Zhao, F.; Yuan, M.; Zhang, J.; Gai, Z. Preventive Effects of Fluoro-Substituted Benzothiadiazole Derivatives and Chitosan Oligosaccharide against the Rice Seedling Blight Induced by Fusarium oxysporum. Plants 2019, 8, 538. https://doi.org/10.3390/plants8120538
Ma B, Wang J, Liu C, Hu J, Tan K, Zhao F, Yuan M, Zhang J, Gai Z. Preventive Effects of Fluoro-Substituted Benzothiadiazole Derivatives and Chitosan Oligosaccharide against the Rice Seedling Blight Induced by Fusarium oxysporum. Plants. 2019; 8(12):538. https://doi.org/10.3390/plants8120538
Chicago/Turabian StyleMa, Bo, Junhe Wang, Chuanzeng Liu, Jifang Hu, Kefei Tan, Fuyang Zhao, Ming Yuan, Junhua Zhang, and Zhijia Gai. 2019. "Preventive Effects of Fluoro-Substituted Benzothiadiazole Derivatives and Chitosan Oligosaccharide against the Rice Seedling Blight Induced by Fusarium oxysporum" Plants 8, no. 12: 538. https://doi.org/10.3390/plants8120538
APA StyleMa, B., Wang, J., Liu, C., Hu, J., Tan, K., Zhao, F., Yuan, M., Zhang, J., & Gai, Z. (2019). Preventive Effects of Fluoro-Substituted Benzothiadiazole Derivatives and Chitosan Oligosaccharide against the Rice Seedling Blight Induced by Fusarium oxysporum. Plants, 8(12), 538. https://doi.org/10.3390/plants8120538




