Responses of Rice Growth to Day and Night Temperature and Relative Air Humidity—Dry Matter, Leaf Area, and Partitioning
Abstract
:1. Introduction
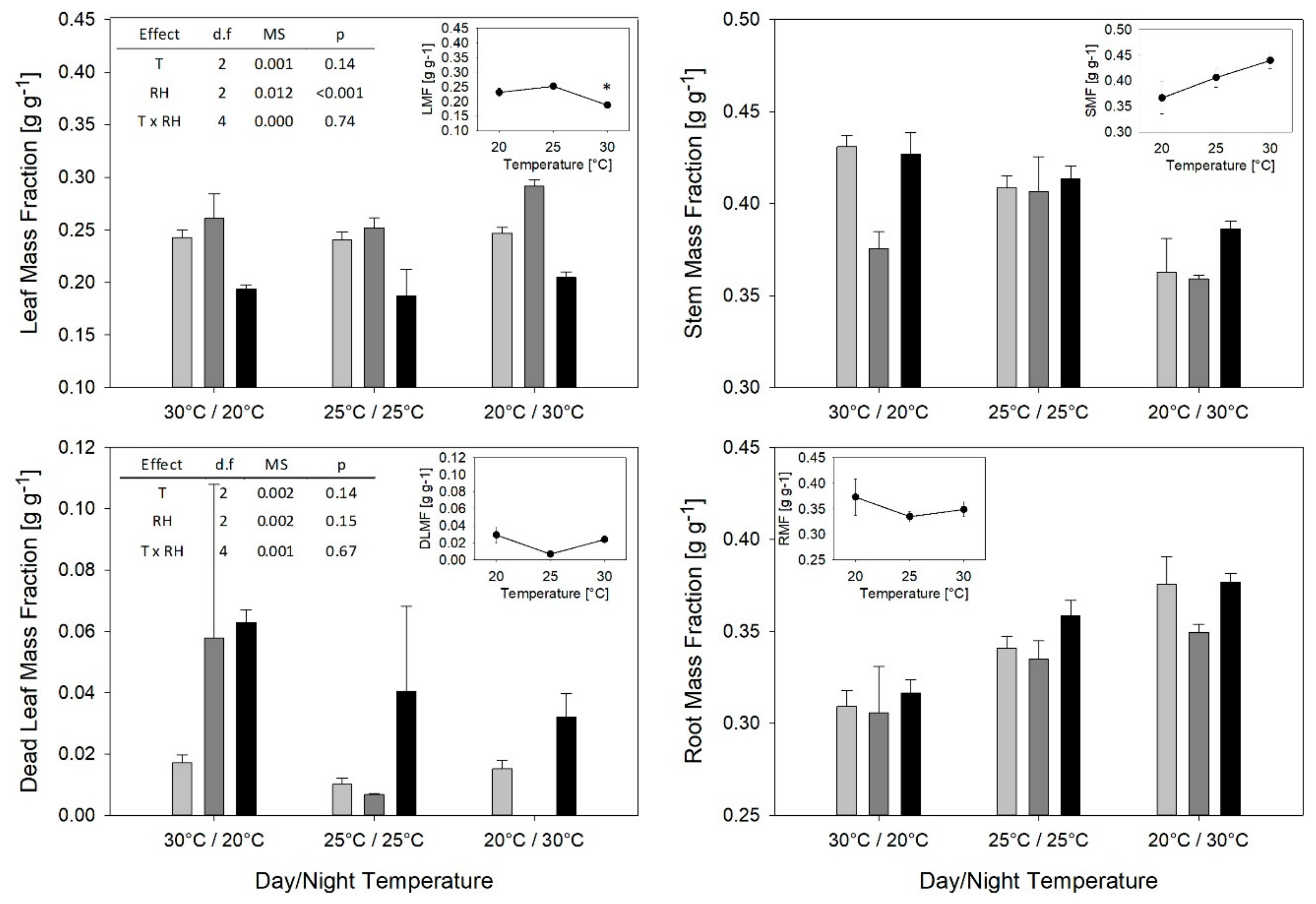
2. Results
3. Discussion
3.1. T and RH Effects on Plant Dry Matter and Leaf Area
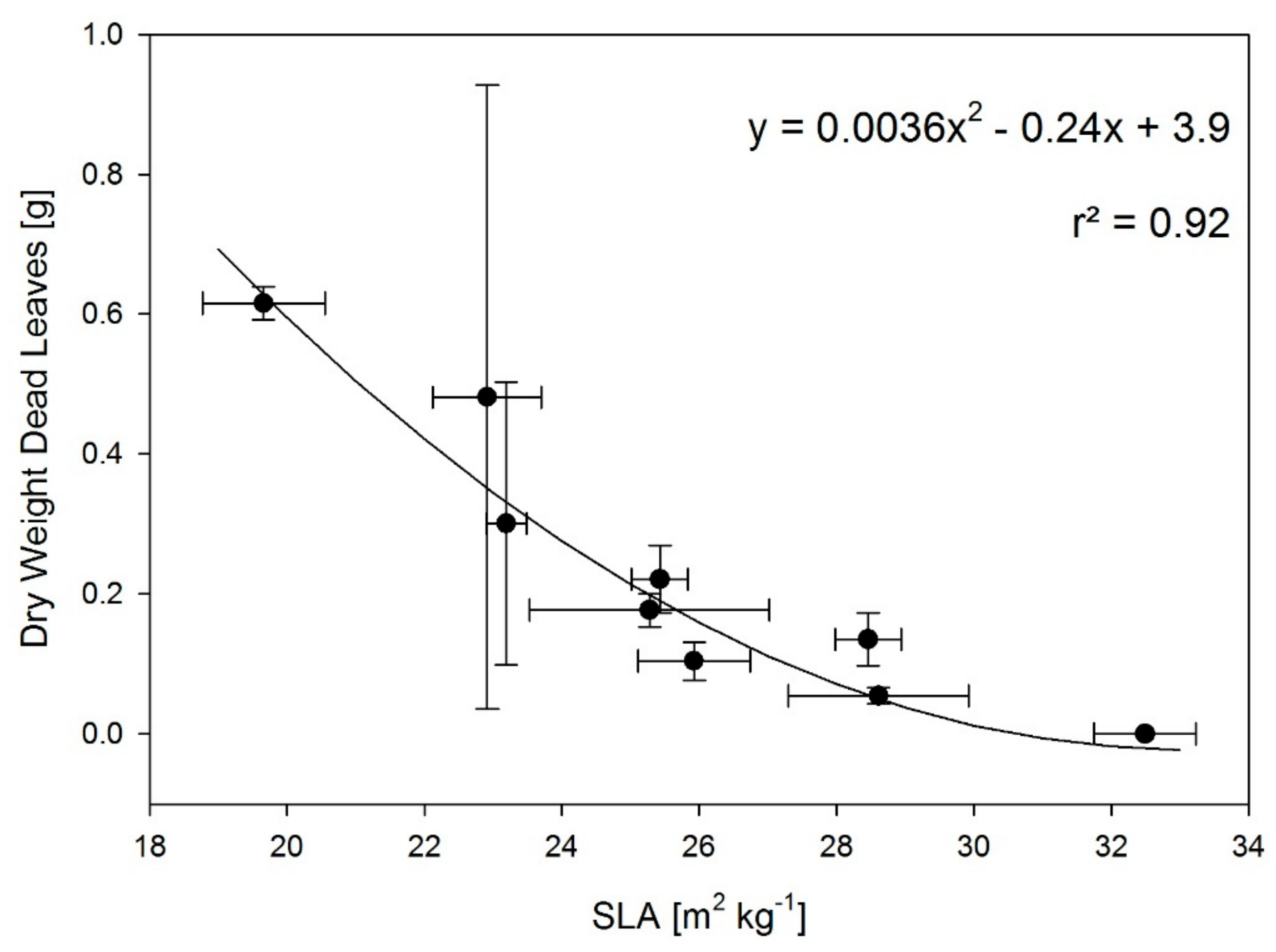
3.2. T and RH Effects on Partitioning
3.3. Limitations of the Study
4. Materials and Methods
4.1. Plant Cultivation
4.2. Measurements
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartmann, D.L.; Tank, A.M.G.K.; Rusticucci, M.; Alexander, L.V.; Bronnnimann, S.; Abdul-Rahman Charabi, Y.; Dentener, F.J.; Dlugkencky, E.J.; Easterling, D.R.; Kaplan, A.; et al. Climate Change 2013—The Physical Science Basis- Observations: Atmosphere and Surface; Cambridge University Press: Cambridge, UK, 2013; ISBN 9781107415324. [Google Scholar]
- Masutomi, Y.; Takahashi, K.; Harasawa, H.; Matsuoka, Y. Impact assessment of climate change on rice production in Asia in comprehensive consideration of process/parameter uncertainty in general circulation models. Agric. Ecosyst. Environ. 2009, 131, 281–291. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Lobell, D.B. Changes in diurnal temperature range and national cereal yields. Agric. For. Meteorol. 2007, 145, 229–238. [Google Scholar] [CrossRef]
- Vose, R.S.; Easterling, D.R.; Gleason, B. Maximum and minimum temperature trends for the globe: An update through 2004. Geophys. Res. Lett. 2005, 32, 1–5. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Peraudeau, S.; Roques, S.; Quiñones, C.O.; Fabre, D.; Van Rie, J.; Ouwerkerk, P.B.; Jagadish, K.S.; Dingkuhn, M.; Lafarge, T. Increase in night temperature in rice enhances respiration rate without significant impact on biomass accumulation. Field Crops Res. 2015, 171, 67–78. [Google Scholar] [CrossRef]
- Ohsumi, A.; Hamasaki, A.; Nakagawa, H.; Homma, K.; Horie, T.; Shiraiwa, T. Response of Leaf Photosynthesis to Vapor Pressure Difference in Rice (Oryza sativa L.) Varieties in Relation to Stomatal and Leaf Internal Conductance. Plant Prod. Sci. 2008, 11, 184–191. [Google Scholar] [CrossRef]
- Kuwagata, T.; Ishikawa-Sakurai, J.; Hayashi, H.; Nagasuga, K.; Fukushi, K.; Ahamed, A.; Takasugi, K.; Katsuhara, M.; Murai-Hatano, M. Influence of Low Air Humidity and Low Root Temperature on Water Uptake, Growth and Aquaporin Expression in Rice Plants. Plant Cell Physiol. 2012, 53, 1418–1431. [Google Scholar] [CrossRef]
- Weerakoon, W.M.W.; Maruyama, A.; Ohba, K. Impact of Humidity on Temperature-Induced Grain Sterility in Rice (Oryza sativa L.). J. Agron. Crop Sci. 2008, 194, 135–140. [Google Scholar] [CrossRef]
- Shi, W.; Raveendran, M.; Rahman, H.; Selvam, J.; Peng, S.; Zou, Y.; Jagadish, K.S.V. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Wang, D.; Zhu, C.; Chen, J. Plant Physiological, Morphological and Yield-Related Responses to Night Temperature Changes across Different Species and Plant Functional Types. Front. Plant Sci. 2016, 7, 835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated [CO2] and night temperature on rice growth and yield. Agric. For. Meteorol. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Ziska, L.H.; Namuco, O.; Moya, T.; Quilang, J. Growth and Yield Response of Field-Grown Tropical Rice to Increasing Carbon Dioxide and Air Temperature. Agron. J. 1997, 89, 45. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Graf, A.; Schlereth, A.; Stitt, M.; Smith, A.M. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. USA 2010, 107, 9458–9463. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, A. Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol. 2009, 50, 744–755. [Google Scholar] [CrossRef]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef]
- Stuerz, S.; Sow, A.; Muller, B.; Manneh, B.; Asch, F. Canopy microclimate and gas-exchange in response to irrigation system in lowland rice in the Sahel. Field Crops Res. 2014, 163, 64–73. [Google Scholar] [CrossRef]
- Stuerz, S.; Sow, A.; Muller, B.; Manneh, B.; Asch, F. Leaf area development in response to meristem temperature and irrigation system in lowland rice. Field Crops Res. 2014, 163, 74–80. [Google Scholar] [CrossRef]
- Hirai, G.I.; Okmura, T.; Takeuchi, S.; Tanaka, O.; Chujo, H. Studies on the effect of the relative humidity of the atmosphere on the growth and physiology of rice plants: Effects of relative humidity during the light and dark periods on the growth. Plant Prod. Sci. 2000, 3, 129–133. [Google Scholar] [CrossRef]
- Sakurai-Ishikawa, J.; Murai-Hatano, M.; Hayashi, H.; Ahamed, A.; Fukushi, K.; Matsumoto, T.; Kitagawa, Y.; Sakurai-Ishikawa, J.; Murai-Hatano, M. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant Cell Environ. 2011, 34, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Sunoj, V.J.; Shroyer, K.J.; Jagadish, S.K.; Prasad, P.V. Diurnal temperature amplitude alters physiological and growth response of maize (Zea mays L.) during the vegetative stage. Environ. Exp. Bot. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Tardieu, F.; Granier, C.; Muller, B. Modelling leaf expansion in a fluctuating environment: Are changes in specific leaf area a consequence of changes in expansion rate? New Phytol. 1999, 143, 33–43. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review Hendrik. Aust. J. Plant Physiol. 2000, 27, 1191. [Google Scholar]
- Rawson, H.; Gardner, P.; Long, M. Sources of Variation in Specific Leaf Area in Wheat Grown at High Temperature. Funct. Plant Boil. 1987, 14, 287–298. [Google Scholar] [CrossRef]
- Morita, S.; Yonemaru, J.I.; Takanashi, J.I. Grain Growth and Endosperm Cell Size Under High Night Temperatures in Rice (Oryza sativa L.). Ann. Bot. 2005, 95, 695–701. [Google Scholar] [CrossRef]
- Glaubitz, U.; Li, X.; Köhl, K.I.; Van Dongen, J.T.; Hincha, D.K.; Zuther, E. Differential physiological responses of different rice (Oryza sativa) cultivars to elevated night temperature during vegetative growth. Funct. Plant Boil. 2014, 41, 437–448. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Dell Inc. Dell Statistica (Data Analysis Software System); Dell Inc.: Landrock, TX, USA, 2016. [Google Scholar]


| RH Day/Night [%] | T Day/Night [°C] | Total Dry Matter [g] | Leaf Area [cm2] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40/90 | 30/20 | 10.4 | ± | 0.8 | A | 632 | ± | 14 | A | |
| 25/25 | 10.2 | ± | 0.8 | 629 | ± | 20 | A | |||
| 20/30 | 8.6 | ± | 0.9 | 601 | ± | 36 | B | |||
| 65/65 | 30/20 | 6.0 | ± | 1.4 | B | 341 | ± | 29 | c | B |
| 25/25 | 7.9 | ± | 1.3 | 558 | ± | 39 | b | A | ||
| 20/30 | 9.4 | ± | 0.5 | 890 | ± | 11 | a | A | ||
| 90/40 | 30/20 | 9.8 | ± | 0.3 | AB | 372 | ± | 5 | B | |
| 25/25 | 7.6 | ± | 0.1 | 333 | ± | 51 | B | |||
| 20/30 | 7.0 | ± | 0.5 | 363 | ± | 22 | C | |||
| Parameter | Effect | d.f | MS | p |
|---|---|---|---|---|
| Dry Matter | T | 2 | 0.35 | 0.84 |
| RH | 2 | 9.76 | 0.02 | |
| T × RH | 4 | 9.01 | 0.01 | |
| Leaf Area | T | 2 | 6.7 × 104 | <0.001 |
| RH | 2 | 1.9 × 105 | <0.001 | |
| T × RH | 4 | 8.2 × 104 | <0.001 |
| RH Day/Night [%] | T Day/Night [°C] | Total Dry Matter [g] | Leaf Area [cm−2] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 65/65 | 20/20 | 2.9 | ± | 1.4 | b | 162 | ± | 67 | b |
| 25/25 | 7.9 | ± | 1.3 | a | 558 | ± | 39 | a | |
| 30/30 | 9.2 | ± | 0.4 | a | 439 | ± | 13 | a | |
| Growing Conditions | SLA | LMF | SMF | RMF | DLMF |
|---|---|---|---|---|---|
| T day | −0.57 | −0.30 | 0.76 ** | −0.84 ** | 0.47 |
| RH day | −0.43 | −0.55 | 0.11 | 0.13 | 0.59 |
| T night | 0.65 * | 0.01 | −0.20 | 0.63 * | −0.53 |
| RH night | 0.43 | 0.55 | −0.11 | −0.13 | −0.59 |
| Growth Rates | SLA | LMF | SMF | RMF | DLMF |
|---|---|---|---|---|---|
| LA day−1 | 0.88 ** | 0.85 ** | −0.37 | 0.02 | −0.86 ** |
| DW day−1 | 0.39 | 0.39 | 0.30 | −0.24 | −0.66 |
| RH Day/Night [%] | T Day/Night [°C] | Treatment | |
|---|---|---|---|
| 40/90 | 30/20 | Tnat | RHnat |
| 25/25 | Tcon | RHnat | |
| 20/30 | Tinv | RHnat | |
| 65/65 | 30/20 | Tnat | RHcon |
| 25/25 | Tcon | RHcon | |
| 20/30 | Tinv | RHcon | |
| 20/20 | Tcon-l | RHcon | |
| 30/30 | Tcon-h | RHcon | |
| 90/40 | 30/20 | Tnat | RHinv |
| 25/25 | Tcon | RHinv | |
| 20/30 | Tinv | RHinv | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuerz, S.; Asch, F. Responses of Rice Growth to Day and Night Temperature and Relative Air Humidity—Dry Matter, Leaf Area, and Partitioning. Plants 2019, 8, 521. https://doi.org/10.3390/plants8110521
Stuerz S, Asch F. Responses of Rice Growth to Day and Night Temperature and Relative Air Humidity—Dry Matter, Leaf Area, and Partitioning. Plants. 2019; 8(11):521. https://doi.org/10.3390/plants8110521
Chicago/Turabian StyleStuerz, Sabine, and Folkard Asch. 2019. "Responses of Rice Growth to Day and Night Temperature and Relative Air Humidity—Dry Matter, Leaf Area, and Partitioning" Plants 8, no. 11: 521. https://doi.org/10.3390/plants8110521
APA StyleStuerz, S., & Asch, F. (2019). Responses of Rice Growth to Day and Night Temperature and Relative Air Humidity—Dry Matter, Leaf Area, and Partitioning. Plants, 8(11), 521. https://doi.org/10.3390/plants8110521





