Effect of Hexavalent Chromium [Cr(VI)] on Phytoremediation Potential and Biochemical Response of Hybrid Napier Grass with and without EDTA Application
Abstract
1. Introduction
2. Results
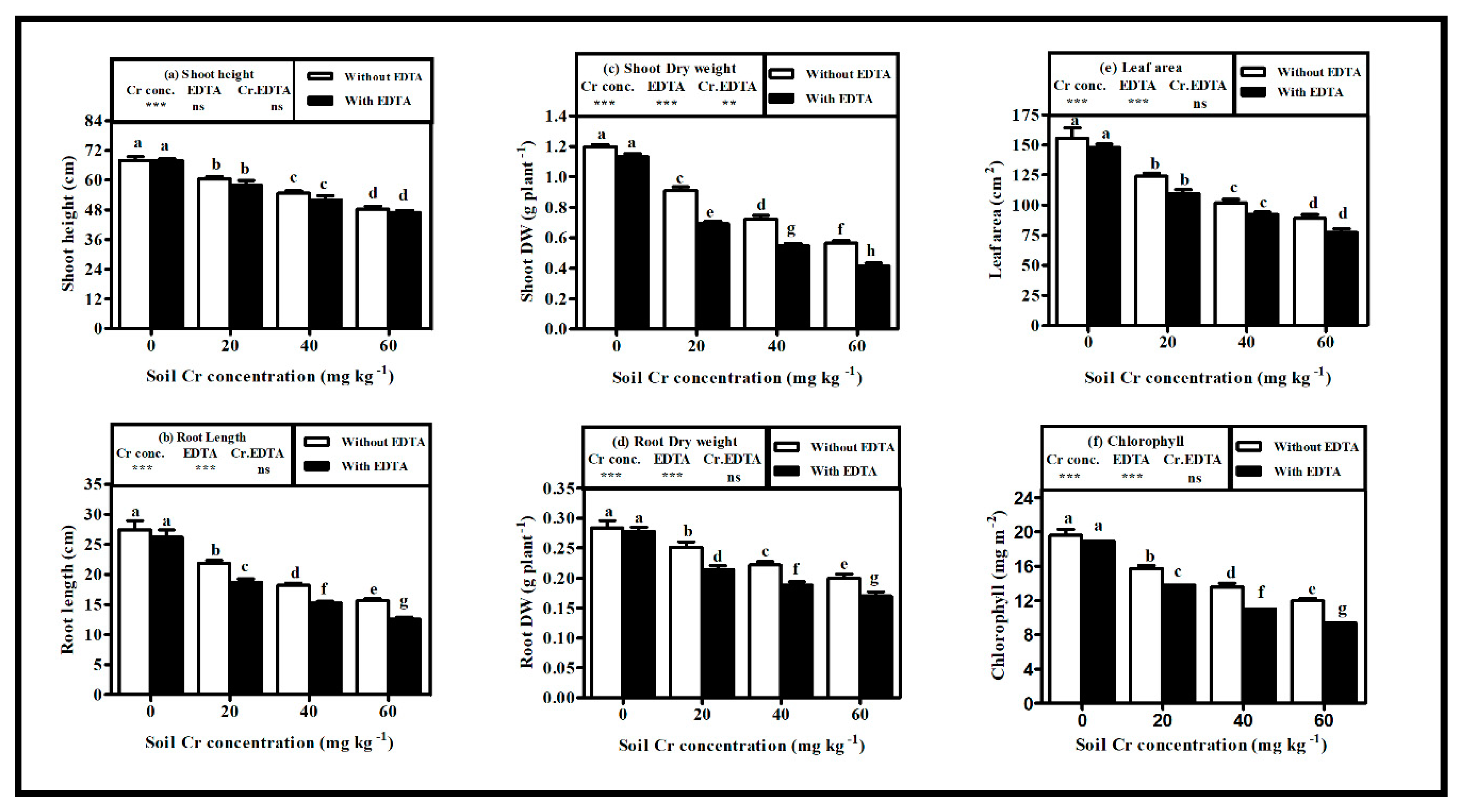
2.1. Growth Characteristics of Hybrid Napier Grass
2.2. Cr Accumulation and Phytoremediation Potential
2.3. Tolerance Index
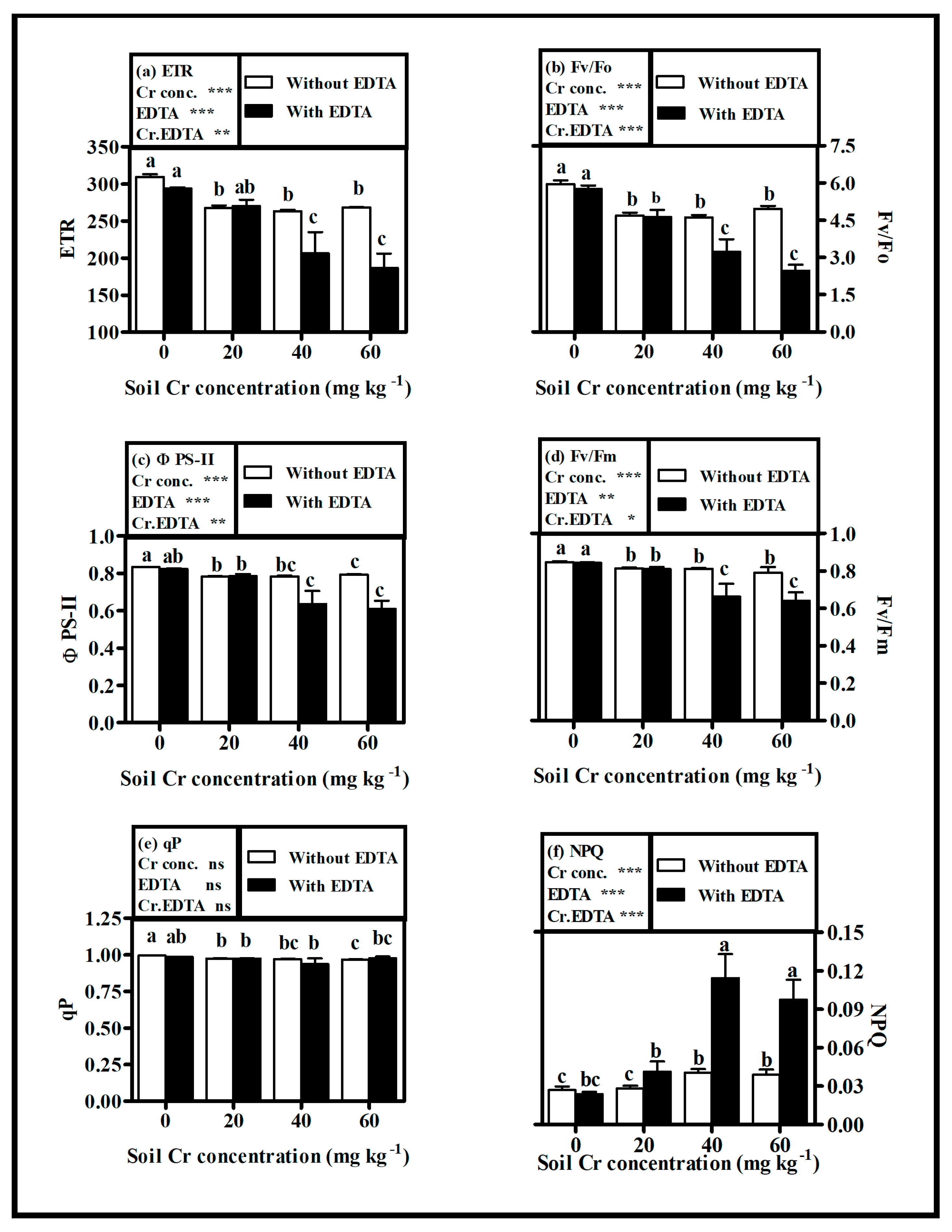
2.4. Chlorophyll Content and Photosynthetic Efficiency
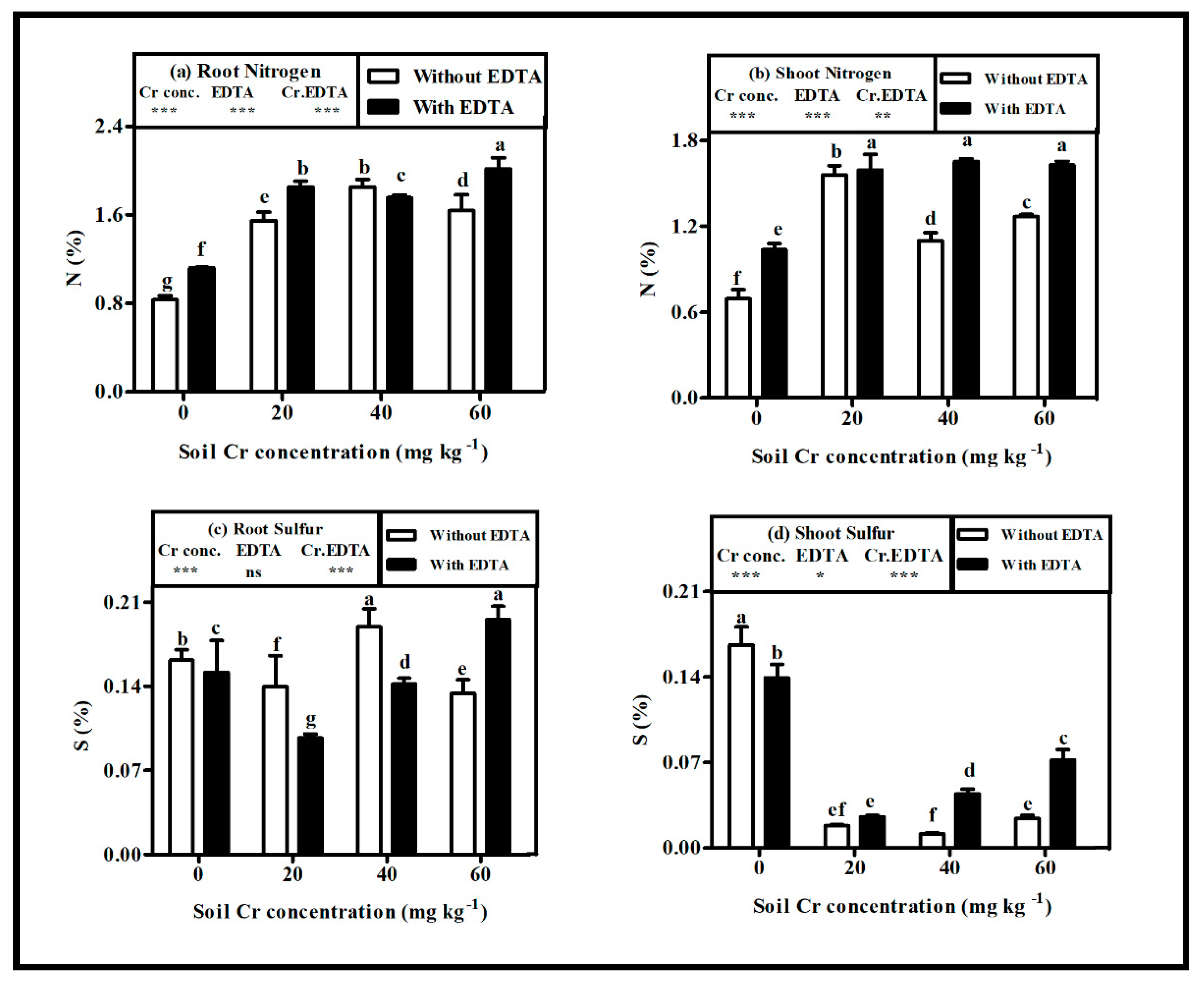
2.5. Nitrogen (N) and Sulfur (S) Status in Hybrid Napier Grass
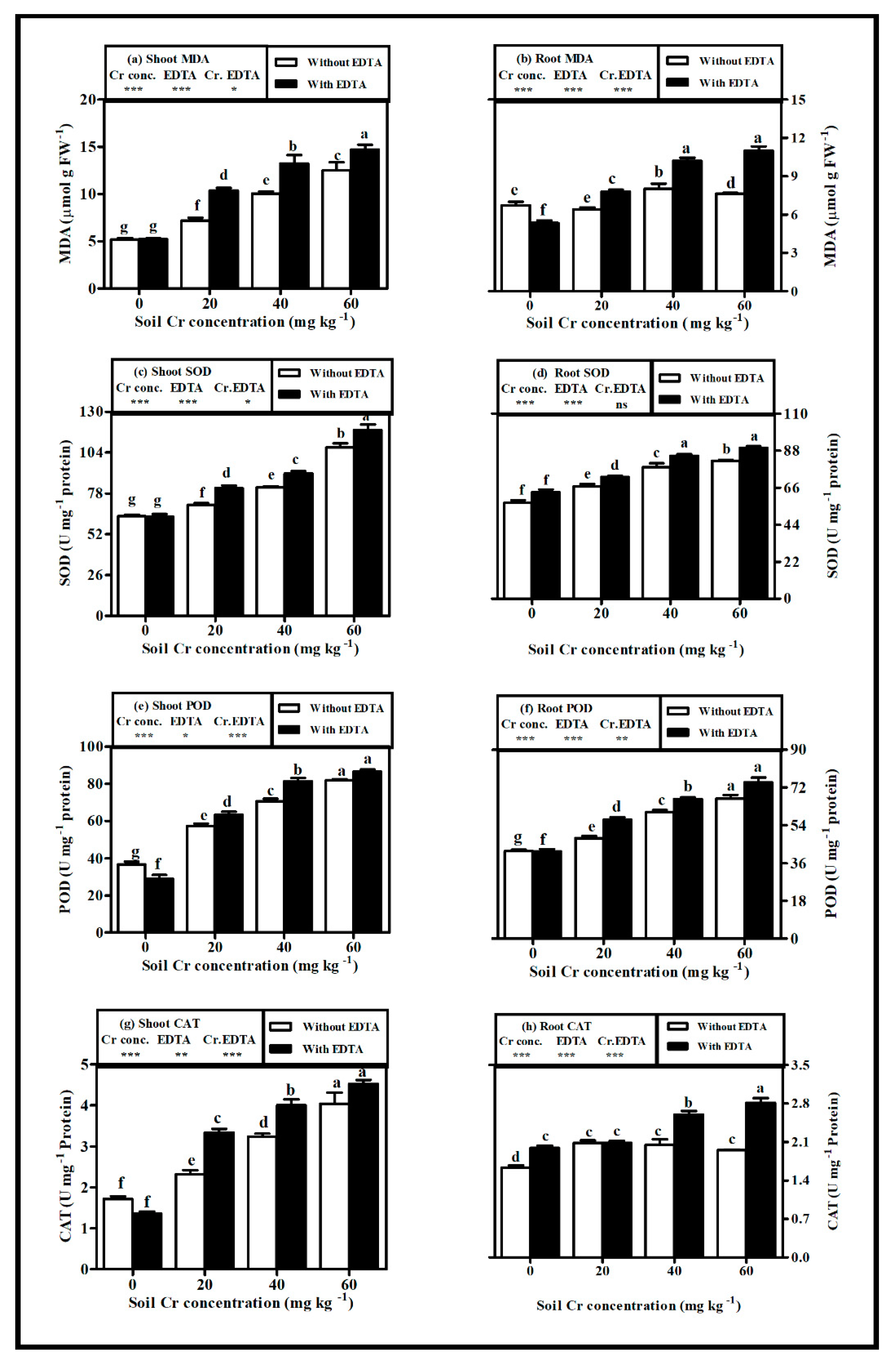
2.6. Oxidative Stress
3. Discussion
3.1. Plant Growth and Phytotoxicity
3.2. Cr Accumulation and Phytoremediation Potential
3.3. Chlorophyll and Photosynthetic Efficiency
3.4. Elemental Status
3.5. Oxidative Stress
4. Materials and Methods
4.1. Seed Collection and Cr(VI) Stock Solution Preparation
4.2. Soil Collection and Seedling Growth
4.3. Experimental Design
4.4. Growth Measurements
4.5. Cr Analysis
4.6. Phytoremediation Potential
4.7. Tolerance Index (TI)
4.8. Determination of Chlorophyll Content, Chlorophyll-α Fluorescence and Elemental Contents
4.9. Measurement of Oxidative Stress Parameters
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.; Zhou, T.; Li, Q.; Lu, L.; Lin, C. Anthropogenic chromium emissions in China from 1990 to 2009. PLoS ONE 2014, 9, 87753. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- da Conceição Gomes, M.A.; Hauser-Davis, R.A.; Suzuki, M.S.; Vitória, A.P. Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol. Environ. Saf. 2017, 140, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Han, F.X.; Sridhar, B.B.M.; Monts, D.L.; Su, Y. Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica Juncea. New Phytol. 2004, 162, 489–499. [Google Scholar] [CrossRef]
- Adiloglu, S.; Süme, M.T.S.A. Chrome (Cr) pollution in agricultural areas improvement by phytoremediation method with Canola (Brassica napus L.) plant growing. J. Essent. Oil Bear. Plants JEOP 2015, 18, 1180–1186. [Google Scholar] [CrossRef]
- Kalyvas, G.; Tsitselis, G.; Gasparatos, D.; Massas, I. Efficacy of EDTA and olive mill wastewater to enhance As, Pb, and Zn phytoextraction by Pteris vittata L. from a soil heavily polluted by mining activities. Sustainability 2018, 10, 1962. [Google Scholar] [CrossRef]
- Ebrahimi, M. Effect of EDTA treatment method on leaching of Pb and Cr by Phragmites australis (Cav.) Trin. Ex Steudel (common reed). Caspian J. Environ. Sci. 2015, 13, 153–166. [Google Scholar]
- Ma, Y.; Hooda, P.S. Trace Elements in Soils, 1st ed.; John Wiley & Sons: Chichester, West Sussex, UK, 2010; pp. 461–480. [Google Scholar]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Isıklı, B.; Demir, T.; Ürer, S.; Berber, A.; Akar, T.; Kalyoncu, C. Effects of chromium exposure from a cement factory. Environ. Res. 2003, 91, 113–118. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer: New York, NY, USA, 1986. [Google Scholar]
- Mandal, A.; Voutchkov, M. Heavy metals in soils around the cement factory in Rockfort, Kingston, Jamaica. Int. J. Geosci. 2011, 2, 48. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Eriksson, J. Concentrations of 61 Trace Elements in Sewage Sludge, Farmyard Manure, Mineral Fertiliser, Precipitation and in Oil and Crops; Swedish Environmental Protection Agency: Stockholm, Sweden, 2001.
- Takeda, A.; Kimura, K.; Yamasaki, S. Analysis of 57 elements in Japanese soils, with special reference to soil group and agricultural use. Geoderma 2004, 119, 291–307. [Google Scholar] [CrossRef]
- Burt, R.; Wilson, M.; Mays, M.; Lee, C. Major and trace elements of selected pedons in the USA. J. Environ. Qual. 2003, 32, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Salminen, R.; Batista, M.; Bidovec, M. Part 1. Background Information, Methodology, and Maps. In FOREGS Geochemical Atlas of Europe; Geological Survey of Finland: Espoo, Finland, 2005. [Google Scholar]
- Suseela, M.R.; Sinha, S.; Singh, S.; Saxena, R. Accumulation of chromium and scanning electron microscopic studies in Scirpus lacustris L. treated with metal and tannery effluent. Bull. Environ. Contam. Toxicol. 2002, 68, 540–548. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv: Azad) root mitochondria. Plant Cell Environ. 2002, 25, 687–693. [Google Scholar] [CrossRef]
- Shanker, A.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.; Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (L.) R. Wilczek. cv CO4) roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. Biomed. Res. Int. 2014, 2014, 934182. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lv, C.; Xu, M.; Chen, G.; Lv, C.; Gao, Z. Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ. Sci. Pollut. Res. 2015, 23, 1768–1778. [Google Scholar] [CrossRef]
- Bareen, E.F.; Tahira, S.A. Efficiency of seven different cultivated plant species for phytoextraction of toxic metals from tannery effluent contaminated soil using EDTA. Soil Sediment Contam. 2010, 19, 160–173. [Google Scholar] [CrossRef]
- Premaratne, S.; Premalal, G.G.C. Hybrid Napier (P. purpureum × P. americanum) var. CO-3: A resourceful fodder grass for dairy development in Sri Lanka. J. Agric. Sci. 2006, 2, 22–33. [Google Scholar]
- Jampeetong, A.; Muenrew, J. Interactive effects of NH4+ concentration and O2 availability on growth, morphology, and mineral allocation of hybrid Napier grass (Pennisetum purpureum × P. americanum cv. Pakchong1). Ecol. Eng. 2016, 91, 409–418. [Google Scholar] [CrossRef]
- Amin, H.; Arain, B.A.; Abbasi, M.S.; Amin, F.; Jahangir, T.M.; Soomro, N.U. Evaluation of chromium phyto-toxicity, phyto-tolerance, and phyto-accumulation using biofuel plants for effective phytoremediation. Int. J. Phytoremediation 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 185–192. [Google Scholar] [CrossRef]
- Raptis, S.; Gasparatos, D.; Economou-Eliopoulos, M.; Petridis, A. Chromium uptake by lettuce as affected by the application of organic matter and Cr(VI)-irrigation water: Implications to the land use and water management. Chemosphere 2018, 210, 597–606. [Google Scholar] [CrossRef]
- Dey, U.; Mondal, N.K. Ultrastructural deformation of plant cell under heavy metal stress in Gram seedlings. Cogent Environ. Sci. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Bareen, F.; Khadija, R.; Muhammad, S.; Aisha, N. Uptake and leaching of Cu, Cd, and Cr after EDTA application in sand columns using sorghum and pearl millet. Pol. J. Environ. Stud. 2019, 28, 2065–2077. [Google Scholar] [CrossRef]
- Ali, S.Y.; Chaudhury, S. EDTA-enhanced phytoextraction by tagetes sp. and effect on bioconcentration and translocation of heavy metals. Environ. Proc. 2016, 3, 735. [Google Scholar] [CrossRef]
- Naseem, S.; Yasin, M.; Ahmed, A.; Faisal, M. Chromium accumulation and toxicity in corn (Zea mays L.) seedlings. Pol. J. Environ. Stud. 2015, 24, 899–904. [Google Scholar]
- Nagarajan, M.; Ganes, K.S. Effect of chromium on growth, biochemicals and nutrient accumulation of paddy (Oryza sativa L.). Int. Lett. Nat. Sci. 2014, 23, 63–71. [Google Scholar] [CrossRef]
- Huffman, E.W., Jr.; Allaway, H.W. Chromium in plants: Distribution in tissues, organelles, and extracts and availability of bean leaf chromium to animals. J. Agric. Food Chem. 1973, 21, 982–986. [Google Scholar] [CrossRef]
- Diwan, H.; Ahmad, A.; Iqbal, M. Chromium-induced alterations in photosynthesis and associated attributes in Indian mustard. J. Environ. Biol. 2012, 33, 239–244. [Google Scholar] [PubMed]
- Saravanan, A.; Jayasree, R.; Hemavathy, R.V.; Jeevanantham, S.; Hamsini, S.; Senthil, K.P.; Yuvaraj, D. Phytoremediation of Cr (VI) ion contaminated soil using Black gram (Vigna mungo): Assessment of removal capacity. J. Environ. Chem. Eng. 2019, 7, 103052. [Google Scholar]
- Ramana, S.; Biswas, A.K.; Singh, A.B.; Ahirwar, N.K.; Subba Rao, A. Tolerance of ornamental succulent plant crown of thorns (Euphorbia milli) to chromium and its remediation. Int. J. Phytoremediation 2014, 17, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Mganga, N.; Manoko, M.L.K.; Rulangaranga, Z.K. Classification of plants according to their heavy metal content around north mara gold mine, Tanzania: Implication for phytoremediation. Tanzan. J. Sci. 2011, 37, 109–119. [Google Scholar]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.B.; de Oliveira, J.G.; Azevedo, R.A.; Ribeiro, D.R.; da Silva, M.G.; Vitória, A.P. Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. Environ. Exp. Bot. 2009, 65, 403–409. [Google Scholar] [CrossRef]
- Subrahmanyam, D. Effects of chromium toxicity on leaf photosynthetic characteristics and oxidative changes in wheat (Triticum aestivum L.). Photosynthetica 2008, 46, 339–345. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Radziemska, M. Influence of chromium (III) and (VI) on the concentration of mineral elements in oat (Avena sativa L.). Fresenius Environ. Bull. 2013, 22, 979–986. [Google Scholar]
- Wyszkowski, M.; Radziemska, M. Effects of chromium (III and VI) on spring barley and maize biomass yield and content of nitrogenous compounds. J. Toxicol. Environ. Health A 2010, 73, 1274–1282. [Google Scholar] [CrossRef]
- Dube, B.; Tewari, K.; Chatterjee, J.; Chatterjee, C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 2003, 53, 1147–1153. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H.; Wirtz, M.; Hell, R.; Malagoli, M. Interactions between chromium and sulfur metabolism in Brassica juncea. J. Environ. Qual. 2008, 37, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.J.; Liu, X.M.; Lütz-Meind, U.; Peer, T. Effects of lead and EDTA-Assisted lead on biomass, lead uptake and mineral nutrients in Lespedeza chinensis and Lespedeza davidii. Water Air Soil Pollut. 2011, 220, 57–68. [Google Scholar] [CrossRef]
- Singh, M.; Kushwaha, B.K.; Singh, S.; Kumar, V.; Singh, V.P.; Prasad, S.M. Sulphur alters chromium (VI) toxicity in Solanum melongena seedlings: Role of Sulphur assimilation and Sulphur-containing antioxidants. Plant Physiol. Biochem. 2017, 112, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Khan, I.; Tanveer, M.; Ali, M.; Hussain, I.; Wang, L.C. Chromium and aluminum phytotoxicity in maize: Morpho-physiological responses and metal uptake. CLEAN-Soil Air Water 2016, 44, 1075–1084. [Google Scholar] [CrossRef]
- Upadhyay, R.; Panda, S.K. Influence of chromium salts on increased lipid peroxidation and differential pattern in antioxidant metabolism in Pistia stratiotes L. Braz. Arch. Biol. Technol. 2010, 53, 1137–1144. [Google Scholar] [CrossRef]
- Daud, M.K.; Mei, L.; Variath, M.T.; Ali, S.; Li, C.; Rafiq, M.T.; Zhu, S.J. Chromium (VI) uptake and tolerance potential in cotton cultivars: Effect on their root physiology, ultramorphology, and oxidative metabolism. Biomed. Res. Int. 2014, 2014, 975946. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Butt, T.A.; Mirza, C.R.; Yousaf, S.; Nawaz, I.; Iqbal, M. Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci. Rep. 2019, 9, 5658–5669. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- González, A. Application of the portable chlorophyll meter in wheat and barley improvement programs. Agroecología 2009, 4, 111–116. [Google Scholar]
| Cr Concentration (mg kg−1) | Parameter | Region | Reference |
|---|---|---|---|
| 50–600 | Back ground concentration | - | Ma and Hooda [8] |
| 5–3000 | Back ground concentration | India | Shanker et al. [9] |
| 2–60 | Natural concentration | Turkey | Isıklı et al. [10] |
| 10–50 | Natural concentration | - | Adriano [11] |
| 100 | Average concentration | West Indies | Mandal and Voutchkov [12] |
| 59.5 | Average concentration | Poland | Kabata-Pendias [13] |
| 22 | Average concentration | Sweden | Eriksson [14] |
| 58 | Average concentration | Japan | Takeda et al. [15] |
| 54 | Average concentration | USA | Burt et al. [16] |
| 94.8 | Average concentration | Finland | Salminen et al. [17] |
| Soil Cr(VI) Conc. (mg kg−1) | Cr Concentration (mg kg−1) | Cr Accumulation (µg Plant−1) | TF | BAF | |||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | ||||
| 0 | Without EDTA | 2.45 ± 0.89 g | 1.48 ± 0.45 f | 0.69 ± 0.34 g | 1.77 ± 0.6 f | - | - |
| With EDTA | 1.22 ± 0.02 g | 2.38 ± 1.52 f | 0.34 ± 0.08 g | 2.69 ± 1.4 f | - | - | |
| 20 | Without EDTA | 374.6 ± 2.4 d | 22.2 ± 1.41 g | 94.37 ± 7.75 c | 20.29 ± 1.6 g | 0.06 ± 0.005 e | 1.11 ± 0.07 f |
| With EDTA | 233.7 ± 0.6 f | 46.97 ± 3.15 e | 50.01 ± 1.55 e | 32.66 ± 2.8 e | 0.2 ± 0.014 e | 2.35 ± 0.16 c | |
| 40 | Without EDTA | 473.6 ± 1.7 b | 64.6 ± 0.84 d | 105 ± 2.49 a | 46.66 ± 1.9 c | 0.14 ± 0.002 g | 1.61 ± 0.02 d |
| With EDTA | 261 ± 0.92 e | 114.5 ± 5.6 b | 49.05 ± 1.51 f | 62.71 ± 4.3 b | 0.44 ± 0.022 c | 2.86 ± 0.14 a | |
| 60 | Without EDTA | 504.7 ± 0.7 a | 73.35 ± 4.06 c | 101 ± 3.44 b | 41.4 ± 2.65 d | 0.15 ± 0.008 f | 1.22 ± 0.07 e |
| With EDTA | 467 ± 1.96 c | 157.21 ± 12 a | 79.45 ± 3.6 d | 65.9 ± 7.79 a | 0.34 ± 0.03 d | 2.62 ± 0.2 b | |
| Statistical Effect | |||||||
| Cr(VI) conc. | *** | *** | *** | *** | *** | *** | |
| EDTA | *** | *** | *** | *** | *** | *** | |
| Cr(VI) conc. × EDTA | *** | *** | *** | ns | *** | *** | |
| Soil Cr(VI) Conc. (mg kg−1) | Shoot TI | Root TI | ||
|---|---|---|---|---|
| Without EDTA | With EDTA | Without EDTA | With EDTA | |
| 0 | 1 | 1 | 1 | 1 |
| 20 | 0.76 ± 0.024 a | 0.61 ± 0.021 b | 0.89 ± 0.047 a | 0.77 ± 0.02 b |
| 40 | 0.6 ± 0.022 c | 0.48 ± 0.02 d | 0.79 ± 0.054 b,c | 0.68 ± 0.03 c |
| 60 | 0.47 ± 0.017 e | 0.37 ± 0.022 f | 0.71 ± 0.02 c,d | 0.61 ± 0.026 d |
| Statistical Effect | ||||
| Cr conc. | *** | *** | ||
| EDTA | *** | *** | ||
| Cr × EDTA | ns | ** | ||
| Properties | Determined Value |
|---|---|
| Sand (%) | 1.4 ± 0.052 |
| Silt (%) | 23.9 ± 0.56 |
| Clay (%) | 74.7 ± 0.84 |
| pH | 6.0 ± 0.06 |
| Texture class | Silty clay |
| Electrical conductivity (mS m−1) | 0.71 ± 0.28 |
| Total Carbon (C, %) | 0.132 ± 0.002 |
| Hydrogen (H, %) | 0.381 ± 0.007 |
| Nitrogen (N, %) | 0.057 ± 0.002 |
| Sulfur (S, %) | 0.003 ± 0 |
| Total chromium (Crtotal, mg kg−1) | 0.0104 ± 0 |
| Treatments | Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| NET-Plants | ET-Plants | |||||||
| Cr0 | Cr20 | Cr40 | Cr60 | Cr0 | Cr20 | Cr40 | Cr60 | |
| Soil Cr(VI) conc. (mg k−1) | 0 | 20 | 40 | 60 | 0 | 20 | 40 | 60 |
| EDTA (mM) | - | - | - | - | 4 | 4 | 4 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ram, B.K.; Han, Y.; Yang, G.; Ling, Q.; Dong, F. Effect of Hexavalent Chromium [Cr(VI)] on Phytoremediation Potential and Biochemical Response of Hybrid Napier Grass with and without EDTA Application. Plants 2019, 8, 515. https://doi.org/10.3390/plants8110515
Ram BK, Han Y, Yang G, Ling Q, Dong F. Effect of Hexavalent Chromium [Cr(VI)] on Phytoremediation Potential and Biochemical Response of Hybrid Napier Grass with and without EDTA Application. Plants. 2019; 8(11):515. https://doi.org/10.3390/plants8110515
Chicago/Turabian StyleRam, Bhagat Kanwar, Ying Han, Gang Yang, Qin Ling, and Faqin Dong. 2019. "Effect of Hexavalent Chromium [Cr(VI)] on Phytoremediation Potential and Biochemical Response of Hybrid Napier Grass with and without EDTA Application" Plants 8, no. 11: 515. https://doi.org/10.3390/plants8110515
APA StyleRam, B. K., Han, Y., Yang, G., Ling, Q., & Dong, F. (2019). Effect of Hexavalent Chromium [Cr(VI)] on Phytoremediation Potential and Biochemical Response of Hybrid Napier Grass with and without EDTA Application. Plants, 8(11), 515. https://doi.org/10.3390/plants8110515





