Mechanisms and Adaptation Strategies to Improve Heat Tolerance in Rice. A Review
Abstract
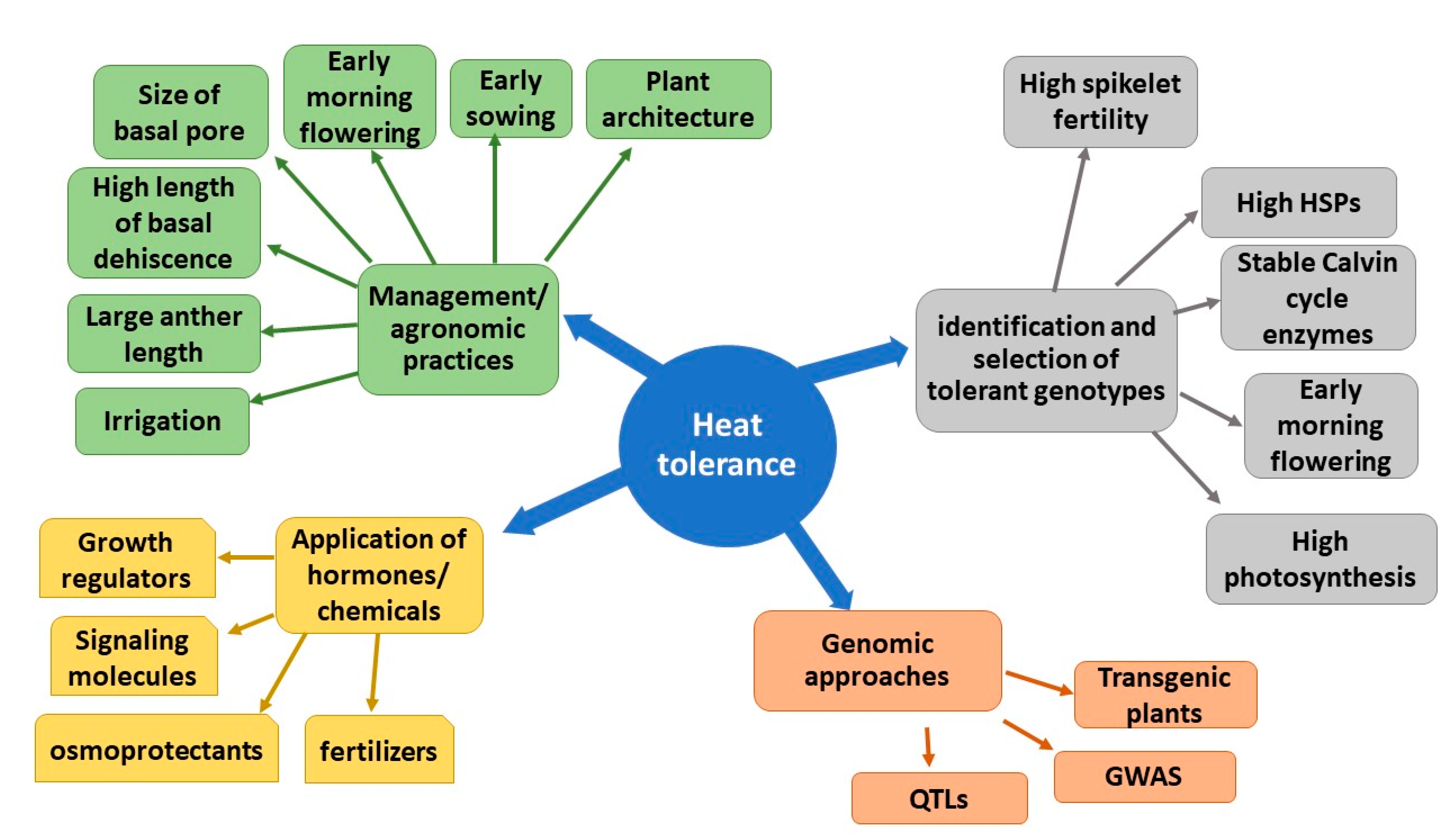
1. Introduction
2. Crop Management Practices for Heat Stress Avoidance
2.1. Agronomic Management
2.2. Heat Avoidance through Early Morning Flowering
2.3. Size of Basal Pore
2.4. Anther Size
2.5. Length of Basal Dehiscence of Anther
2.6. Plant Architecture
3. Induction of Acclimation by Using Growth Regulators/Protectants/Chemicals
3.1. Growth Regulators
3.2. Use of Organic Elicitors, Fertilizers, or Signaling Molecules
3.3. Use of Osmoprotectants
4. Breeding Approaches by Identification and Selection of Heat-Tolerant Genotypes
4.1. Low Leaf Temperature and Panicle Temperature and Well Exerted Panicle
4.2. High Carbohydrate Availability and Photosynthetic Rate
4.3. Protection from Thermal Degradation of Calvin Cycle Enzymes
4.4. High Production of Heat Shock Proteins
4.5. Higher Cell Membrane Thermostability and Chlorophyll Fluorescence
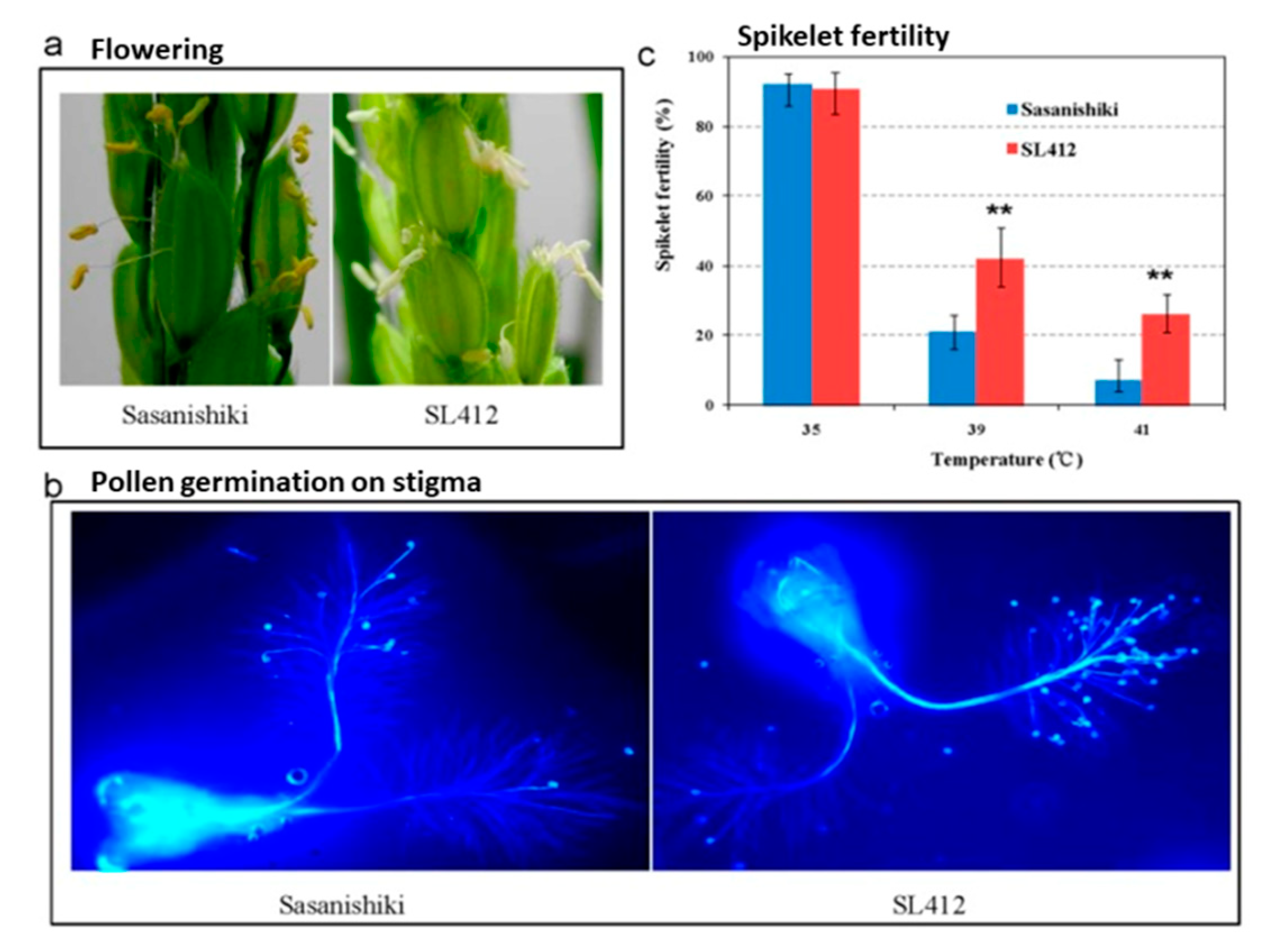
4.6. Anther Dehiscence, Spikelet Fertility, and Yield Attributes
4.7. Breeding
5. Genetic Manipulations for Heat Tolerant Transgenic Rice
6. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.; Van Rie, J.; Fabre, D.; Jagadish, K.S.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V.R. High-temperature effects on rice growth, yield, and grain quality. In Advances in Agronomy; Academic Press: London, UK, 2011; Volume 111, pp. 87–206. [Google Scholar]
- Shi, W.; Xiao, G.; Struik, P.C.; Jagadish, K.S.; Yin, X. Quantifying source-sink relationships of rice under high night-time temperature combined with two nitrogen levels. Field Crops Res. 2017, 202, 36–46. [Google Scholar] [CrossRef]
- Govindaraj, M.; Pattanashetti, S.K.; Patne, N.; Kanatti, A. Breeding Cultivars for Heat Stress Tolerance in Staple Food Crops. Next Gener. Plant Breed. 2018, 45. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering anther characteristics. Ann. Bot. 2002, 89, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K.; Cairns, J.; Lafitte, R.; Wheeler, T.W.; Price, A.H.; Craufurd, P.Q. Genetic Analysis of heat tolerance at anthesis in rice. Crop Sci. 2010, 50, 1633–1641. [Google Scholar] [CrossRef]
- Kim, J.; Shon, J.; Lee, C.K.; Yang, W.; Yoon, Y.; Yang, W.H.; Kim, Y.G.; Lee, B.W. Relationship between grain filling duration and leaf senescence of temperate rice under high temperature. Field Crops Res. 2011, 122, 207–213. [Google Scholar] [CrossRef]
- Huang, M.; Zou, Y. Comparison of grain filling characteristics between two super rice cultivars with remarkable difference in grain weight. World Appl. Sci. J. 2009, 6, 674–679. [Google Scholar]
- Chaturvedi, A.K.; Bahuguna, R.N.; Shah, D.; Pal, M.; Jagadish, S.K. High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink-strength in rice. Sci. Rep. 2017, 7, 8227. [Google Scholar] [CrossRef]
- Yamanouchi, U.; Yano, M.; Lin, H.; Ashikari, M.; Yamada, K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7530–7535. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, H.Y.; Wang, Z.H.; Wei, L.X.; Fu, P.; Song, J.B.; Fu, D.H.; Huang, Y.J.; Liao, J.L. High nighttime temperature induces antioxidant molecule perturbations in heat-sensitive and heat-tolerant coisogenic rice (Oryza sativa) strains. J. Agric. Food Chem. 2018, 66, 12131–12140. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Klueva, N.; Perrota, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Dingkuhn, M. Variation in time of day of anthesis in rice in different climatic environments. Eur. J. Agron. 2012, 43, 166–174. [Google Scholar] [CrossRef]
- Julia, C.; Dingkuhn, M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Eur. J. Agron. 2013, 49, 50–60. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, W.M.W.; Maruyama, A.; Ohba, K. Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L.). J. Agron. Crop Sci. 2008, 194, 135–140. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, T.; Ling, X.; Fahad, S.; Peng, S.; Li, Y.; Huang, J. Sufficient leaf transpiration and nonstructural carbohydrates are beneficial for high-temperature tolerance in three rice (Oryza sativa) cultivars and two nitrogen treatments. Funct. Plant Biol. 2014, 42, 347–356. [Google Scholar] [CrossRef]
- Ohe, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Barbieri, M.; Prasadini, P.; Maunahan, A.; Gatti, L. Spatial Assessment of Heat Stress Impact on Rice Production in Two Districts of Andhra Pradesh, India. World J. Agric. Res. 2018, 6, 10–14. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.; Shah, F.; Nie, L.; Cui, K.; Shah, T.; Wu, W.; Chen, Y.; Chen, C.; Wang, K.; Wang, Q.; et al. Efficacy of sowing date adjustment as a management strategy to cope with rice (‘Oryza sativa’ L.) seed quality deterioration due to elevated temperature. Aust. J. Crop. Sci. 2013, 7, 543. [Google Scholar]
- Yu, K.; Chen, G.; Patrick, W.H., Jr. Reduction of global warming potential contribution from a rice field by irrigation, organic matter, and fertilizer management. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hirabayashi, H.; Ida, M.; Takai, T.; San-Oh, Y.A.; Yoshinaga, S.; Ando, I.; Ogawa, T.; Kondo, M. A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann. Bot. 2010, 106, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Satake, T.; Yoshida, S. High temperature induced sterility in indica rices at fl owering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. The difference in sterility due to high temperatures during the flowering period among japonica rice varieties. Plant Prod. Sci. 2001, 4, 90–93. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Elmido, A.; Mitchell, P. Are there time-of-day clock genes for flowering? In Proceedings of the Annual Meeting of the American Society of Agronomy, Charlotte, NC, USA, 21–25 October 2001; ASA: Madison, WI, USA, 2001; p. 56. [Google Scholar]
- Nishiyama, I.; Satake, T. High temperature damage in the rice plant. Jpn. J. Trop. Agric. 1981, 26, 19–25. [Google Scholar]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 2008, 48, 1140–1146. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hirabayashi, H.; Kuwagata, T.; Ogawa, T.; Kondo, M. The early-morning flowering trait of rice reduces spikelet sterility under windy and elevated temperature conditions at anthesis. Plant Prod. Sci. 2012, 15, 19–22. [Google Scholar] [CrossRef]
- Yoshida, S.; Satake, T.; Mackill, D.S. The Philippines: IRRI.; Fundamentals of Rice Crop Science. In High Temperature Stress in Rice; IRRI Research Paper Series 67; IRRI: Los Baños, Philippines, 1981. [Google Scholar]
- Sheehy, J.E.; Elmido, A.; Centeno, G.; Pablico, P. Searching for new plants for climate change. J. Agric. Meteorol. 2005, 60, 463–468. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sathishraj, R.; Manoharan, M.; Sumanth, H.N.; Muthurajan, R.; Ishimaru, T.; Krishna, J.S.V. Is early morning flowering an effective trait to minimize heat stress damage during flowering in rice? Field Crops Res. 2017, 203, 238–242. [Google Scholar] [CrossRef]
- Hirabayashi, H.; Sasaki, K.; Kambe, T.; Gannaban, R.B.; Miras, M.A.; Mendioro, M.S.; Simon, E.V.; Lumanglas, P.D.; Fujita, D.; Takemoto-Kuno, Y.; et al. qEMF3, a novel QTL for the early-morning flowering trait from wild rice Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa L. J. Exp. Bot. 2015, 66, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Kobayasi, K.; Masui, H.; Atsuta, Y.; Matsui, T.; Yoshimoto, M.; Hasegawa, T. Flower opening time in rice–Cultivar difference and effect of weather factors–. In Proceedings of the MARCO Symposium, Tsukuba, Japan, 5–7 October 2009. [Google Scholar]
- Nishiyama, I.; Blanco, L. Artificial control of flower opening time during the day in rice plants. Jpn. J. Crop Sci. 1981, 1, 59–66. [Google Scholar] [CrossRef][Green Version]
- Matsui, T. Function of long basal dehiscence of the theca in rice (Oryza sativa L.) pollination under hot and humid condition. Phyton 2005, 45, 401–407. [Google Scholar]
- Matsui, T.; Kagata, H. Characteristics of floral organs related to reliable self pollination in rice (Oryza sativa L.). Ann. Bot. 2003, 91, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Omasa, K.; Horie, T. Mechanism of anther dehiscence in rice (Oryza sativa L.). Ann. Bot. 1999, 84, 501–506. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. High temperatures at flowering inhibit swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 430–434. [Google Scholar] [CrossRef]
- Shah, F.; Huang, J.; Cui, K.; Nie, L.; Shah, T.; Chen, C.; Wang, K. Impact of high-temperature stress on rice plant and its traits related to tolerance. J. Agric. Sci. 2011, 149, 545–556. [Google Scholar] [CrossRef]
- Poli, Y.; Basava, R.K.; Panigrahy, M.; Vinukonda, V.P.; Dokula, N.R.; Voleti, S.R.; Desiraju, S.; Neelamraju, S. Characterization of a Nagina22 rice mutant for heat tolerance and mapping of yield traits. Rice 2013, 6, 36. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Impact of high nighttime temperature on respiration, membrane stability, antioxidant capacity, and yield of rice plants. Crop Sci. 2009, 49, 313–322. [Google Scholar] [CrossRef]
- Chandrakala, J.U.; Chaturvedi, A.K.; Ramesh, K.V.; Rai, P.; Khetarpal, S.; Pal, M. Acclimation response of signalling molecules for high temperature stress on photosynthetic characteristics in rice genotypes. Indian J. Plant Physiol. 2013, 18, 142–150. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Chang, P.F.L.; Jinn, T.L.; Huang, W.K.; Chen, Y.; Chang, H.M.; Wang, C.W. Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. Plant Sci. 2007, 172, 64–75. [Google Scholar] [CrossRef]
- Kobayasi, K.; Atsuta, Y. Sterility and poor pollination due to early flower opening induced by methyl jasmonate. Plant Prod. Sci. 2010, 13, 29–36. [Google Scholar] [CrossRef]
- Zeng, X.C.; Zhou, X.; Zhang, W.; Murofushi, N.; Kitahara, T.; Kamuro, Y. Opening of rice floret in rapid response to methyl jasmonate. J. Plant Growth Regul. 1999, 18, 153–158. [Google Scholar] [CrossRef]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. 2007, 99, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Wei, Y.X.; Peng, C.L. Effects of endogenous ascorbic acid on resistance to high-temperature stress in excised rice leaves. Photosynthetica 2018, 56, 1453–1458. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Characterization of Rice (Oryza sativa L.) Physiological responses to a-tocopherol, glycine betaine or salicylic acid application. J. Agric. Sci. 2011, 3, 3–13. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef]
- Dhaubhadel, S.; Browning, K.S.; Gallie, D.R.; Krishna, P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002, 29, 681–691. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Thussagunpanit, J.; Jutamanee, K.; Khamsuk, O.; Suksamrarn, A. Exposure brassinosteroid and brassinosteroid mimics continually improve photosynthesis in rice subject to heat stress. Agrotechnology 2017, 6, 4. [Google Scholar]
- Tang, R.S.; Zheng, J.C.; Jin, Z.Q.; Zhang, D.D.; Huang, Y.H.; Chen, L.G. Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, Gas and ABA in rice (Oryza sativa L.). Plant Growth Regul. 2008, 54, 37–43. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Shah, A.N.; Wu, C.; Yousaf, M.; Nasim, W.; Alharby, H.; et al. Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Nayak, A.K.; Tripathi, R.; Katara, J.L.; Bihari, P.; Lal, B.; Gautam, P. Boron application improves yield of rice cultivars under high temperature stress during vegetative and reproductive stages. Int. J. Biometeorol. 2018, 62, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J. Exp. Bot. 2000, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Mäkelä, P.; Karkkainen, J.; Somersalo, S. Effect of glycine betaine on chloroplast ultrastructure, chlorophyll and protein content, and RUBPCO activities in tomato grown under drought or salinity. Biol. Plant. 2000, 3, 471–475. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Shono, M.; Tobita, S. Effects of proline and betaine on heat inactivation of ribulose-1, 5-bisphosphate carboxylase/oxygenase in crude extracts of rice seedlings. Photosynthetica 2000, 36, 557–563. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, H.; Li, L.; Liu, X.; Chen, L.; Chen, W.; Ding, Y. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Q.; Dong, Q.; Zhang, Z.; Lin, C.; Hu, W.; Pan, R.; Guan, Y.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Sheehy, J.E.; Thomas, J.M.G. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Masuduzzaman, A.S.M.; Ahmad, H.U.; Haque, M.; Ahmed, M.M.E. Evaluation of rice lines tolerant to heat during flowering stage. Rice Res. Open Access 2016, 4, 170. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chai, K.H.; Ko, S.S.; Kuang, L.Y.; Lur, H.S.; Charng, Y.Y. A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties. Plant Physiol. 2014, 164, 2045–2053. [Google Scholar] [CrossRef]
- Zhang, C.X.; Fu, G.F.; Yang, X.Q.; Yang, Y.J.; Zhao, X.; Chen, T.T.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J. Agron. Crop Sci. 2015, 202, 394–408. [Google Scholar] [CrossRef]
- Sathishraj, R.; Bheemanahalli, R.; Ramachandran, M.; Dingkuhn, M.; Muthurajan, R.; Jagadish, S.V.K. Capturing heat stress induced variability in spikelet sterility using panicle, leaf and air temperature under field conditions. Field Crops Res. 2016, 190, 10–17. [Google Scholar] [CrossRef]
- Jumiatun; Junaedi, A.; Lubis, I.; Chozin, M.A.; Miyazaki, A. Morphological, physiological and yield responses of some rice varieties (Oryza sativa L.) as exposed under high temperature in Indonesia. Am. J. Plant Physiol. 2016, 11, 33–41. [Google Scholar]
- Egeh, A.O. High Temperature Effects on Crop and Grain Growth of Four Rice Cultivars. Ph.D. Thesis, University of the Philippines at Low Banos, Laguna, Philippines, 1991. [Google Scholar]
- Gesch, R.W.; Kang, I.H.; Gallo-Meagher, M.; Vu, J.C.V.; Boote, K.J.; Allen, L.H.; Bowes, G. Rubsico expression in rice leaves is related to genotypic variation of photosynthesis under elevated growth CO2 and temperature. Plant Cell Environ. 2003, 26, 1941–1950. [Google Scholar] [CrossRef]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef] [PubMed]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Yamori, W.; Carmo-Silva, A.E.; Salvucci, M.E.; Caemmerer, S.; Atwell, B.J. Rubisco activity is associated with photosynthetic thermotolerance in a wild rice (Oryza meridionalis). Physiol. Plant. 2012, 146, 99–109. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Jing, Y.; Kuang, T. Over-and anti-sense expressions of the large isoform of ribulose-1,5-bisphosphate carboxylase/oxygenase activase gene in Oryza sativa affect the photosynthetic capacity. Photosynthetica 2007, 45, 194–201. [Google Scholar] [CrossRef]
- Makino, A.; Sage, R.F. Temperature response of photosynthesis in transgenic rice transformed with ‘sense’ or ‘antisense’rbc S. Plant Cell Physiol. 2007, 48, 1472–1483. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Atwell, B.J.; Muylaert, S.; Van Reusel, B.; Ruiz, G.A.; Van Rie, J.; Gallé, A. A thermotolerant variant of Rubisco activase from a wild relative improves growth and seed yield in rice under heat stress. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Panigrahy, M.; Neelamraju, S.; Nageswarara Rao, D.; Ramanan, R. Heat tolerance in rice mutants is associated with reduced accumulation of reactive oxygen species. Biol. Plant. 2011, 55, 721–724. [Google Scholar] [CrossRef]
- Liu, J.G.; Qin, Q.L.; Zhang, Z.; Peng, R.H.; Xiong, A.S.; Chen, J.M.; Yao, Q.H. OsHSF7 gene in rice, Oryza sativa L., encodes a transcription factor that functions as a high temperature receptive and responsive factor. Biochem. Mol. Biol. Rep. 2009, 42, 16–21. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice. J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Chandel, G.; Dubey, M.; Meena, R. Differential expression of heat shock proteins and heat stress transcription factor genes in rice exposed to different levels of heat stress. J. Plant Biochem. Biotechnol. 2013, 22, 277–285. [Google Scholar] [CrossRef]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Rao, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef] [PubMed]
- Katiyar-Agarwal, S.; Agarwal, M.; Grover, A. Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol. Biol. 2003, 51, 677–686. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, K.S. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Jiao, Y.; Qin, Y.; Liu, X.; He, K.; Chen, C.; Ma, L.; Wang, J.; Xiong, L.; et al. Global genome expression analysis of rice in response to drought and high-salinity stresses in shoot, flag leaf, and panicle. Plant Mol. Biol. 2007, 63, 591–608. [Google Scholar] [CrossRef]
- Lian, G.L.; Chen, L.Y.; Lei, D.Y.; Zhzng, S.T. Progresses in research on heat tolerance in rice. Hybrid Rice 2005, 20, 1–5, (In Chinese with English Abstract). [Google Scholar]
- Maavimani, M.; Jebaraj, S.; Raveendran, M.; Vanniarajan, C.; Balakrishnan, K.; Muthamilan, M. Cellular membrane thermostability is related to rice (Oryza sativa L) yield under heat stress. Int. J. Trop. Agric. 2014, 32, 201–208. [Google Scholar]
- Prasanth, V.V.; Basava, K.R.; Babu, M.S.; VGN, V.T.; Devi, S.R.; Mangrauthia, S.K.; Voleti, S.R.; Sarla, N. Field level evaluation of rice introgression lines for heat tolerance and validation of markers linked to spikelet fertility. Physiol. Mol. Biol. Plants 2016, 22, 179–192. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, L.; Jang, K.F. QTL mapping for teat tolerance of the tassel period of rice. Mol. Plant Breed. 2008, 6, 867–873. [Google Scholar]
- Chen, Q.Q.; Yu, S.B.; Li, C.H. Identification of QTLs for heat tolerance at flowering stage in rice. Sci. Agric. Sin. 2008, 41, 315–321. [Google Scholar]
- Howarth, C.J. Genetic improvements of tolerance to high temperature. In Abiotic Stresses: Plant Resistance Through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Howarth Press Inc.: New York, NY, USA, 2005. [Google Scholar]
- Bohnert, H.J.; Gong, Q.; Li, P.; Ma, S. Unraveling abiotic stress tolerance mechanisms getting genomics going. Curr. Opin. Plant Biol. 2006, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Y.; Zhao, J.G.; Zhan, X.D. Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice. Chin. J. Rice Sci. 2003, 17, 223–227. [Google Scholar]
- Zhang, G.L.; Chen, L.Y.; Xiao, G.Y.; Xiao, Y.H.; Chen, X.B.; Zhang, S.T. Bulked segregant analysis to detect QTL related to heat tolerance in rice (Oryza sativa L.) using SSR markers. Agric. Sci. China 2009, 8, 482–487. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, Y.; Luo, L.; Deng, H.; Zhang, G.; Tang, W.; Chen, L. Quantitative trait loci associated with pollen fertility under high temperature stress at flowering stage in rice (Oryza sativa). Rice Sci. 2011, 18, 204–209. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Jiang, D. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J. Plant Physiol. 2011, 168, 585–593. [Google Scholar] [CrossRef]
- Cao, L.Y.; Zhu, J.; Zhao, S.T.; He, L.; Yan, Q. Mapping QTLs for heat tolerance in a DH population from indica-japonica cross of rice (Oryza sativa). J. Agric. Biotechnol. 2002, 10, 210–214. [Google Scholar]
- Zhao, Z.G.; Jiang, L.; Xiao, Y.H.; Zhai, H.; Wan, J. Identification of QTLs for heat tolerance at the booting stage in rice (Oryza sativa L.). Acta Agron. Sin. 2006, 32, 640–644. [Google Scholar]
- Shanmugavadivel, P.S.; Sv, A.M.; Prakash, C.; Ramkumar, M.K.; Tiwari, R.; Mohapatra, T.; Singh, N.K. High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array. Rice 2017, 10, 28. [Google Scholar]
- Zhao, L.; Lei, J.; Huang, Y.; Zhu, S.; Chen, H.; Huang, R.; Peng, Z.; Tu, Q.; Shen, X.; Yan, S. Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed. Sci. 2016, 66, 358–366. [Google Scholar] [CrossRef]
- Ye, C.; Argayoso, M.A.; Redoña, E.D.; Sierra, S.N.; Laza, M.A.; Dilla, C.J.; Mo, Y.; Thomson, M.J.; Chin, J.; Delavina, C.B.; et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Ye, G.; Tenorio, F.; Argayoso, M.; Laza, M.; Koh, H.J.; Redoña, E.; Jagadish, K.S.; Gregorio, G.B. Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet. 2015, 16, 41. [Google Scholar] [CrossRef]
- Huang, X.M.; Zhao, Y.; Wei, X.; Li, C.; Wang, A.; Zhao, Q.; Li, W.; Guo, Y.; Deng, L.; Zhu, C.; et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 2012, 44, 32–39. [Google Scholar] [CrossRef]
- Lafarge, T.; Bueno, C.; Frouin, J.; Jacquin, L.; Courtois, B.; Ahmadi, N. Genome-wide association analysis for heat tolerance at flowering detected a large set of genes involved in adaptation to thermal and other stresses. PLoS ONE 2017, 12, e0171254. [Google Scholar] [CrossRef]
- Nakamoto, H.; Hiyama, T. Heat-shock proteins and temperature stress. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 399–416. [Google Scholar]
- Liu, N.; Ko, S.; Yeh, K.C.; Charng, Y. Isolation and characterization of tomato Hsa32 encoding a novel heat-shock protein. Plant Sci. 2006, 170, 976–985. [Google Scholar] [CrossRef]
- Zou, J.; Liu, C.; Chen, X. Proteomics of rice in response to heat stress and advances in genetic engineering for heat tolerance in rice. Plant Cell Rep. 2011, 30, 2155–2165. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17. 7. Plant Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Zou, Y.; Liu, C.; Liu, Y.; Wang, Y.; Zhang, W. Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett. 2011, 585, 231–239. [Google Scholar] [CrossRef]
- Murakami, T.; Matsuba, S.; Funatsuki, H.; Kawaguchi, K.; Saruyama, H.; Tanida, M.; Sato, Y. Over-expression of a small heat shock protein, sHSP17. 7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed 2004, 13, 165–175. [Google Scholar] [CrossRef]
- Sohn, S.; Back, K. Transgenic rice tolerant to high temperature with elevated contents of dienoic fatty acids. Biol. Plant. 2007, 51, 340–342. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Li, Y.; Tan, Y.; Kong, J.; Li, H.; Li, Y.; Zhu, Y. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 2007, 26, 1635–1646. [Google Scholar] [CrossRef]
- Koh, S.; Lee, S.; Kim, M.; Koh, J.; Lee, S.; An, G.; Choe, S.; Kim, S. T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 2007, 65, 453–466. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Sato, H.; Todaka, D.; Kudo, M.; Mizoi, J.; Kidokoro, S.; Zhao, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. The Arabidopsis transcriptional regulator DPB 3-1 enhances heat stress tolerance without growth retardation in rice. Plant Biotech. J. 2016, 14, 1756–1767. [Google Scholar] [CrossRef]
- Queitsch, S.W.; Vierling, H.E.; Lindquest, S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 2000, 12, 479–492. [Google Scholar] [CrossRef]
- Lee, D.G.; Ahsan, N.; Lee, S.H.; Kang, K.Y.; Bahk, J.D.; Lee, I.J.; Lee, B.H. A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics 2007, 7, 3369–3383. [Google Scholar] [CrossRef]
| Rice Trans Host | Gene | Encoding Protein | Source | Mechanism | Reference |
|---|---|---|---|---|---|
| Hoshinoyume | sHSP17.7 | HSP17.7 | Oryza sativa L. | CaMV 35S promotor; enhanced heat and drought stress | [115] |
| Pusa basmati | AtHsp101 | HSP101 | Arabidopsis thaliana | CaMV 35S promotor, enhanced heat tolerance | [90] |
| Nipponbare | mtHsp70 | HSP70 | Oryza sativa L. | CaMV 35S promoter; mtHsp70 over-expression suppressed programmed cell death and ROS | [116] |
| Hoshinoyume | sHsp17.7 | HSP17.7 | Oryza sativa L. | CaMV 35S promoter, enhanced heat and UV-B tolerance | [117] |
| Spl7 mutant | Spl7 | HSFA4d | Oryza sativa L. | CaMV 35S promoter | [10] |
| Oryza sativa | fad7 | Omega 3, fatty acid desaturase | Arabidopsis thaliana | Maize Ubi1 promoter; silencing of endogenous FAD genes | [118] |
| Zhonghua11 Oryza sativa L. | SBPase | SBPase | Oryza sativa L. | ubiquitin promoter, over-expressing SBPase increased tolerance | [119] |
| Oryza sativa ssp. Indica | RCA | Rubisco activase | Oryza australiensis | overexpression improved growth and yield | [84] |
| Oryza sativa L. | rbcS | Oryza sativa L. cv Notohikari | Increased rubisco and photosynthesis in rbcS-sense lines compared to wild type | [83] | |
| Dongjin | OsGSK1 | Glycogen synthase kinase3-like | Oryza sativa L. | enhanced tolerance | [120] |
| Sasanishiki | OsWRKY11 | WRKY11 | Oryza sativa L. cv. Nipponbare | HSP101 promoter, increased desiccation tolerance and survival rate of green parts | [121] |
| Oryza sativa L. | DPB3-1 | DPB3 | Arabidopsis thaliana | DPB31 overexpression, heat stress inducible genes were upregulated | [122] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Anwar, S.; Ashraf, M.Y.; Khaliq, B.; Sun, M.; Hussain, S.; Gao, Z.-q.; Noor, H.; Alam, S. Mechanisms and Adaptation Strategies to Improve Heat Tolerance in Rice. A Review. Plants 2019, 8, 508. https://doi.org/10.3390/plants8110508
Khan S, Anwar S, Ashraf MY, Khaliq B, Sun M, Hussain S, Gao Z-q, Noor H, Alam S. Mechanisms and Adaptation Strategies to Improve Heat Tolerance in Rice. A Review. Plants. 2019; 8(11):508. https://doi.org/10.3390/plants8110508
Chicago/Turabian StyleKhan, Shahbaz, Sumera Anwar, M. Yasin Ashraf, Binish Khaliq, Min Sun, Sajid Hussain, Zhi-qiang Gao, Hafeez Noor, and Sher Alam. 2019. "Mechanisms and Adaptation Strategies to Improve Heat Tolerance in Rice. A Review" Plants 8, no. 11: 508. https://doi.org/10.3390/plants8110508
APA StyleKhan, S., Anwar, S., Ashraf, M. Y., Khaliq, B., Sun, M., Hussain, S., Gao, Z.-q., Noor, H., & Alam, S. (2019). Mechanisms and Adaptation Strategies to Improve Heat Tolerance in Rice. A Review. Plants, 8(11), 508. https://doi.org/10.3390/plants8110508






