Asparagine Synthesis during Tobacco Leaf Curing
Abstract
1. Introduction
2. Results
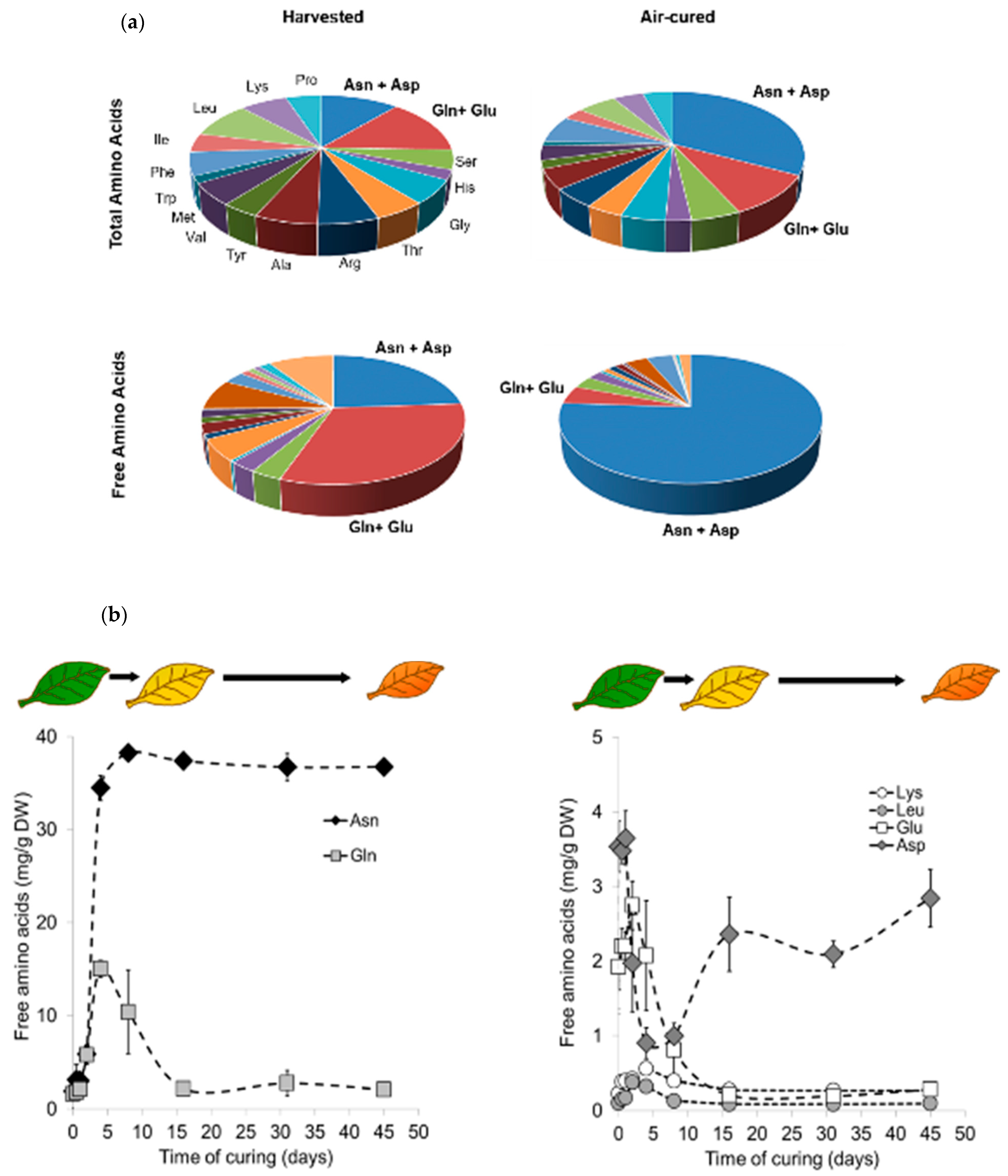
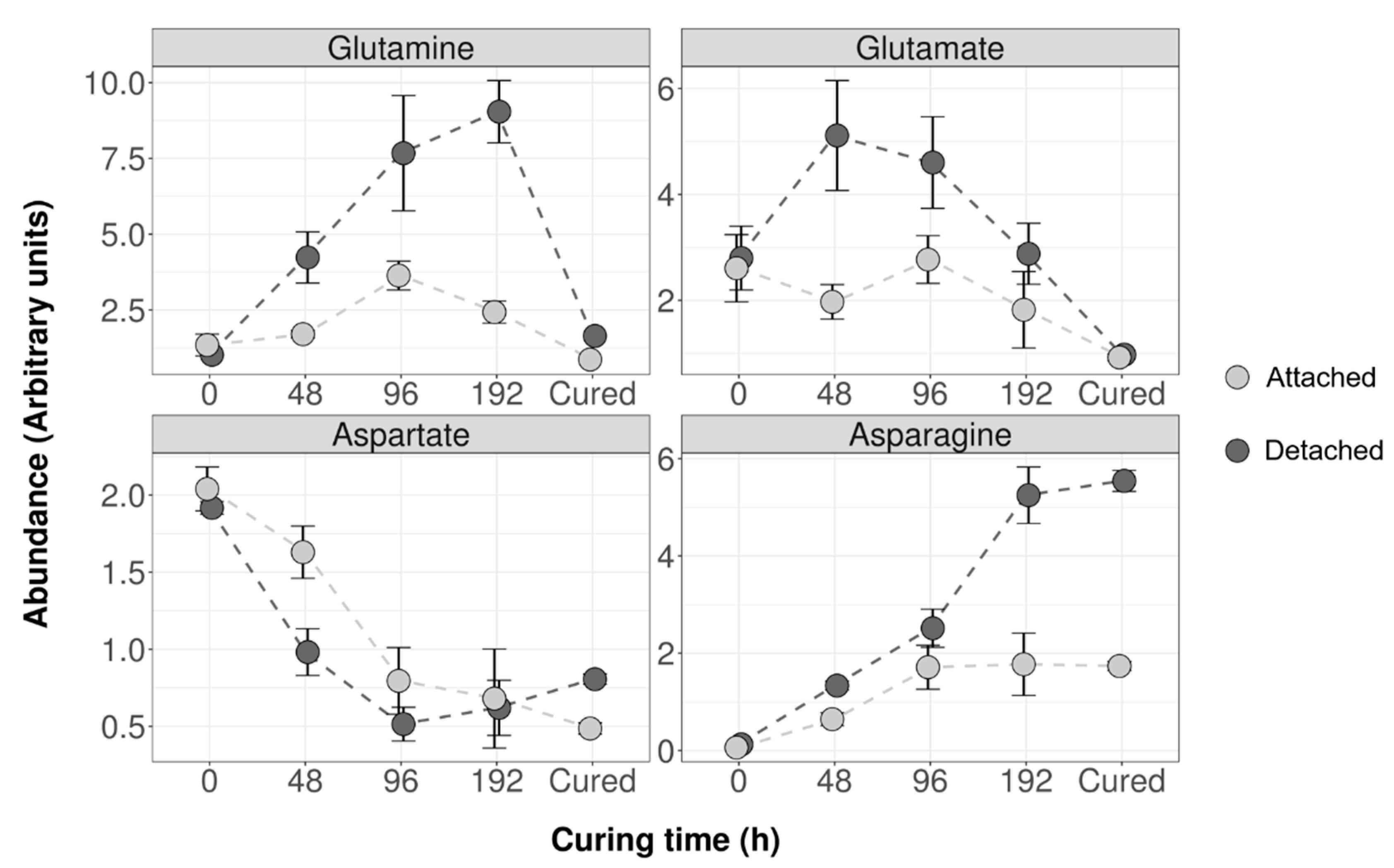
2.1. Air Curing Modifies the Asparagine, Aspartate, Glutamine, and Glutamic Acid Content in Burley Tobacco Leaves
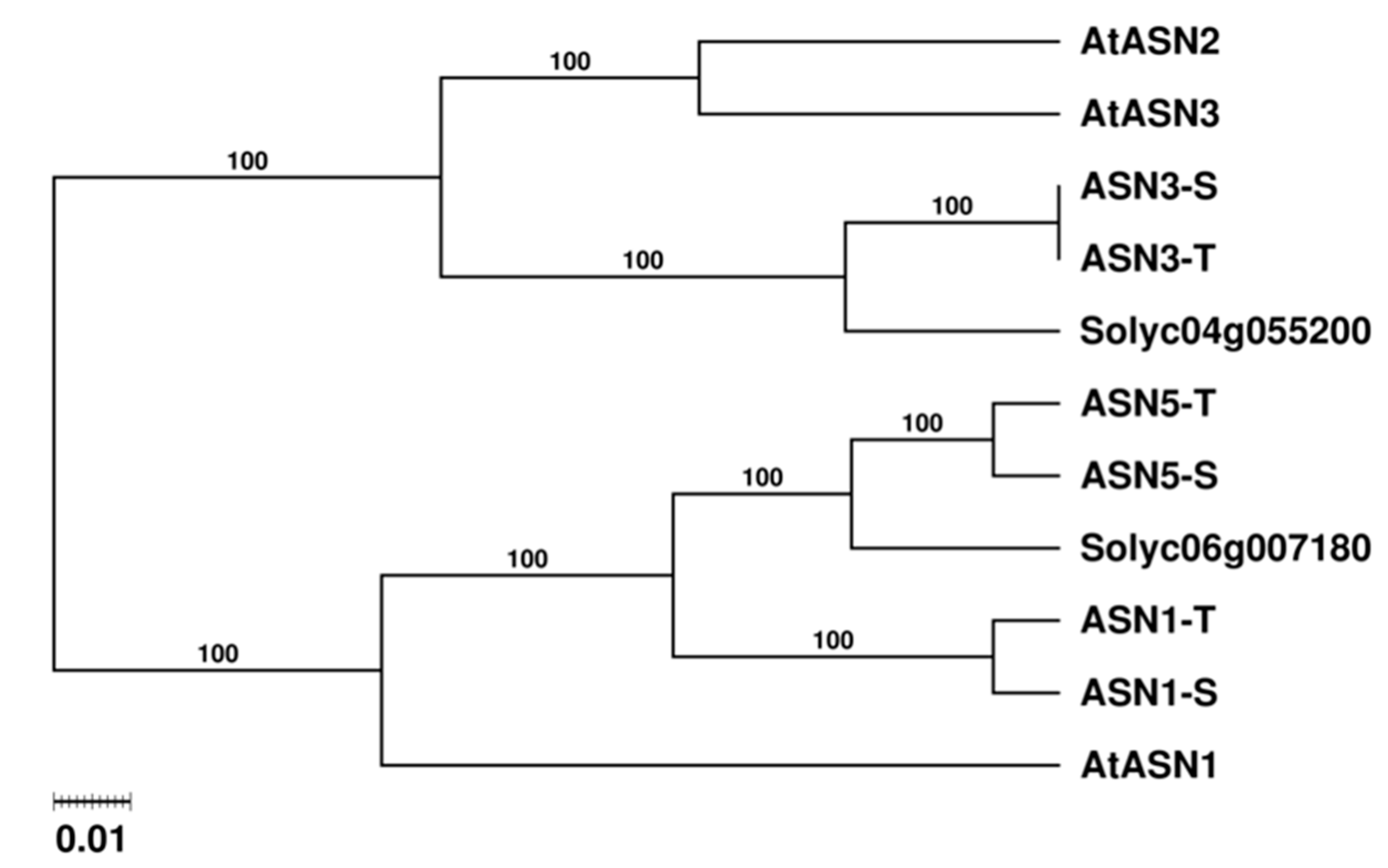
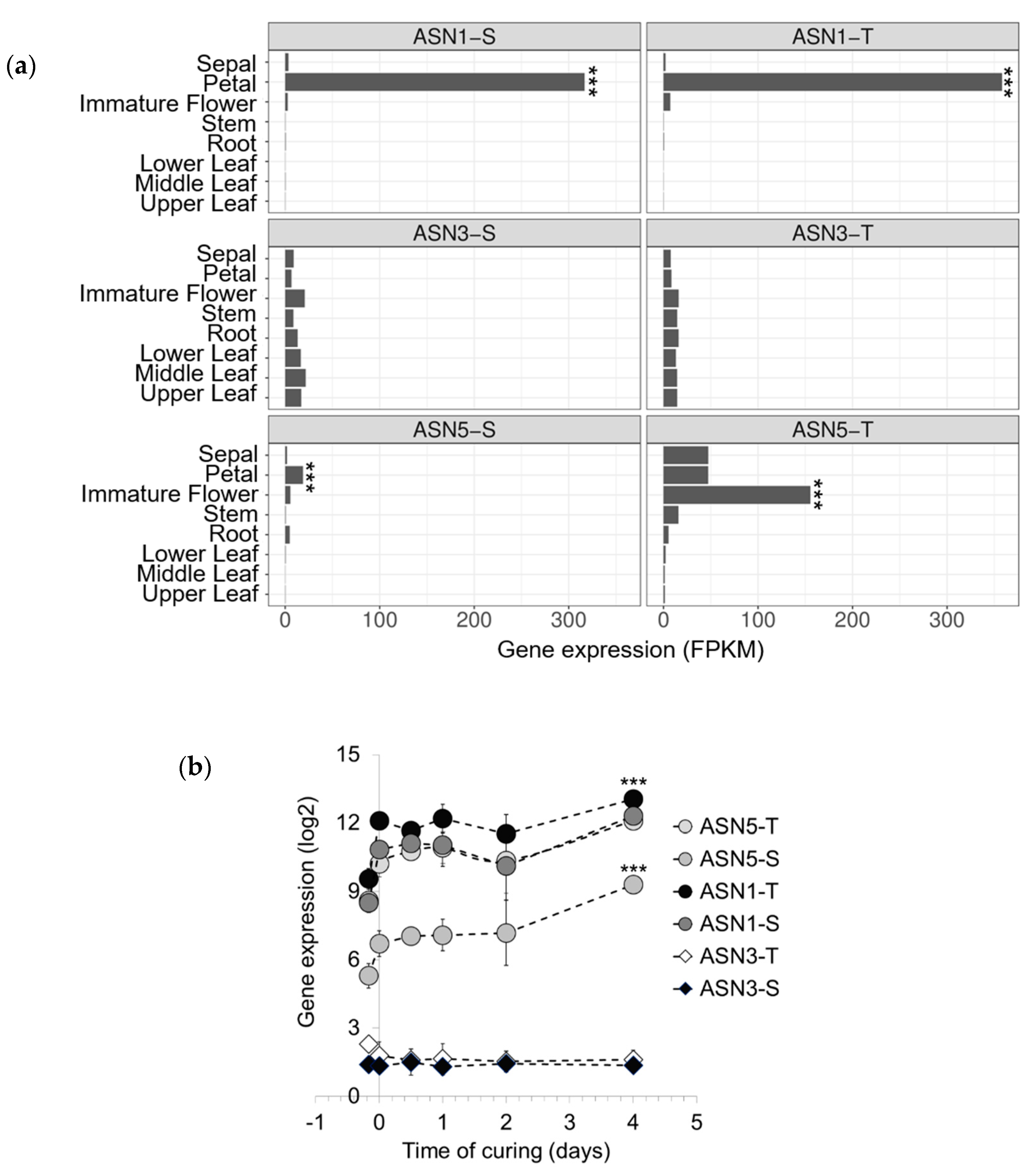
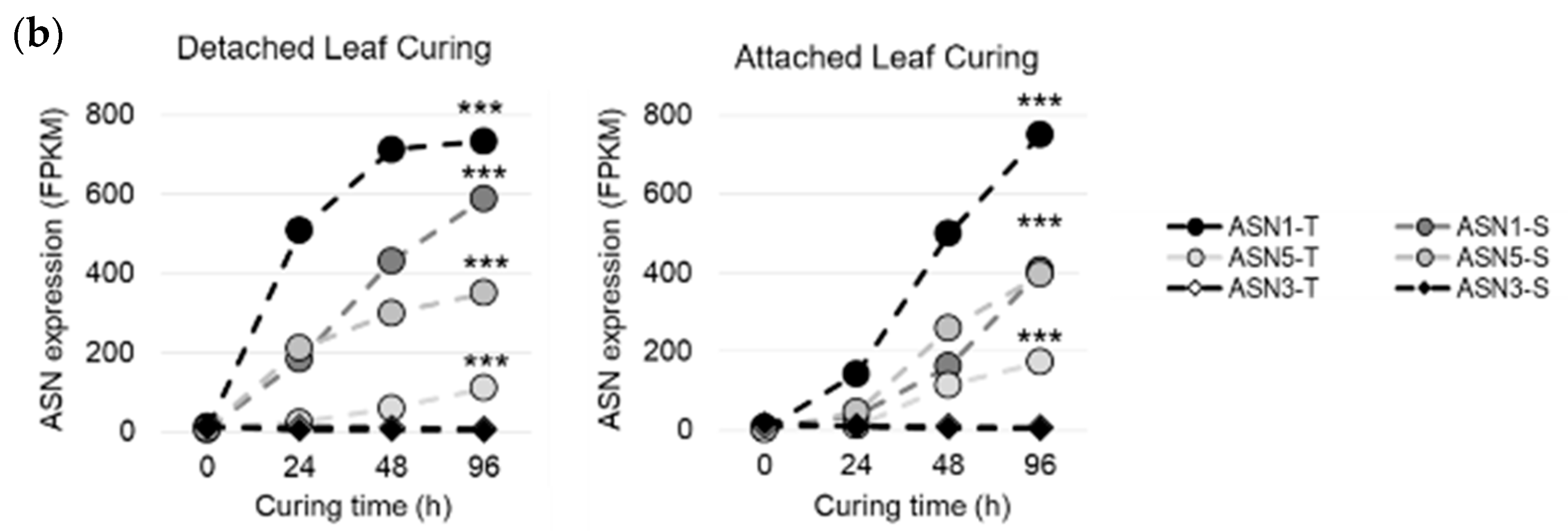
2.2. Asparagine Synthetases are Expressed Differently in Tobacco Organs and During Air Curing
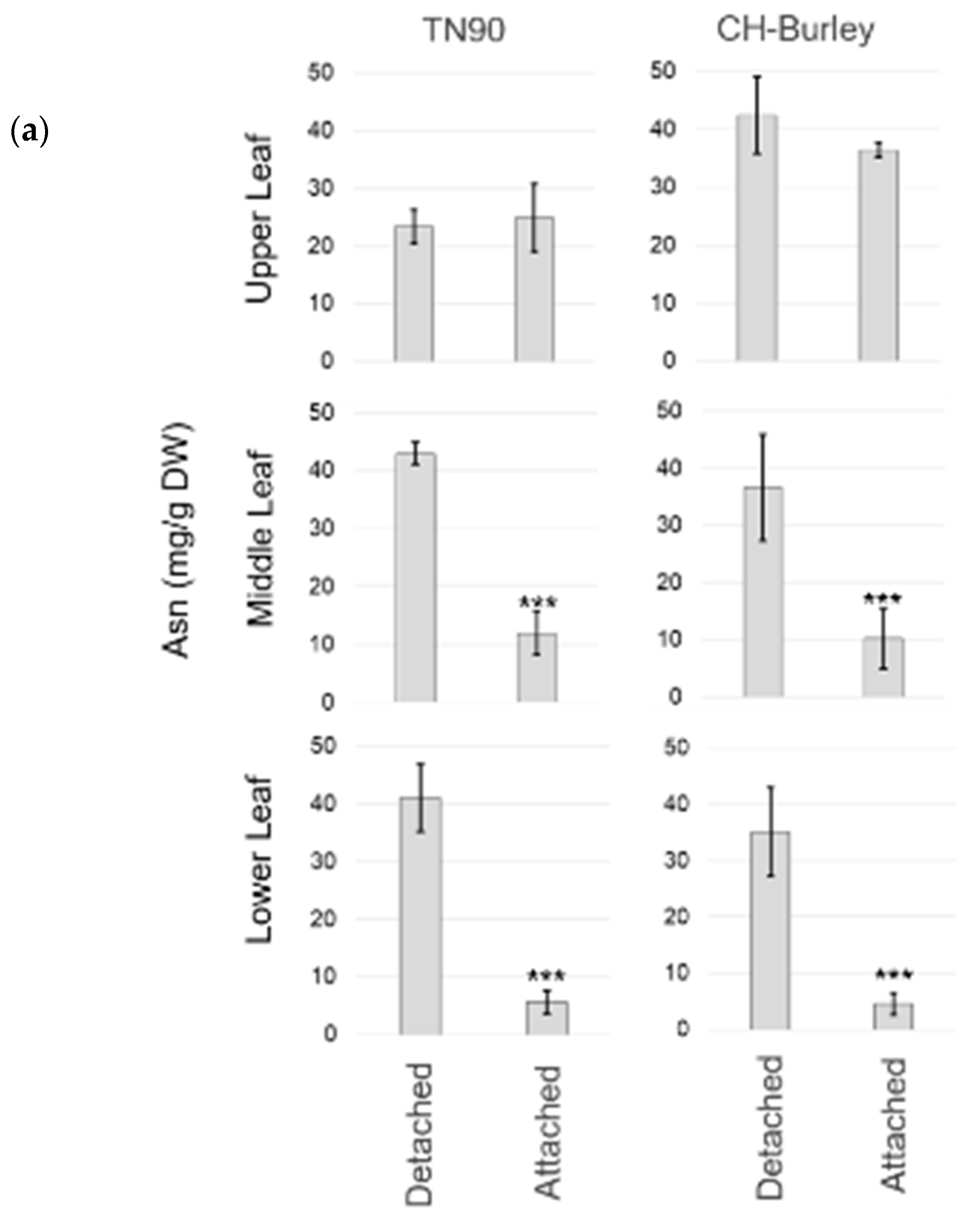
2.3. Different Air Curing Methods Significantly Impact the ASN Gene Expression and Aspartate, Glutamine, Glutamic Acid, and Asparagine Production
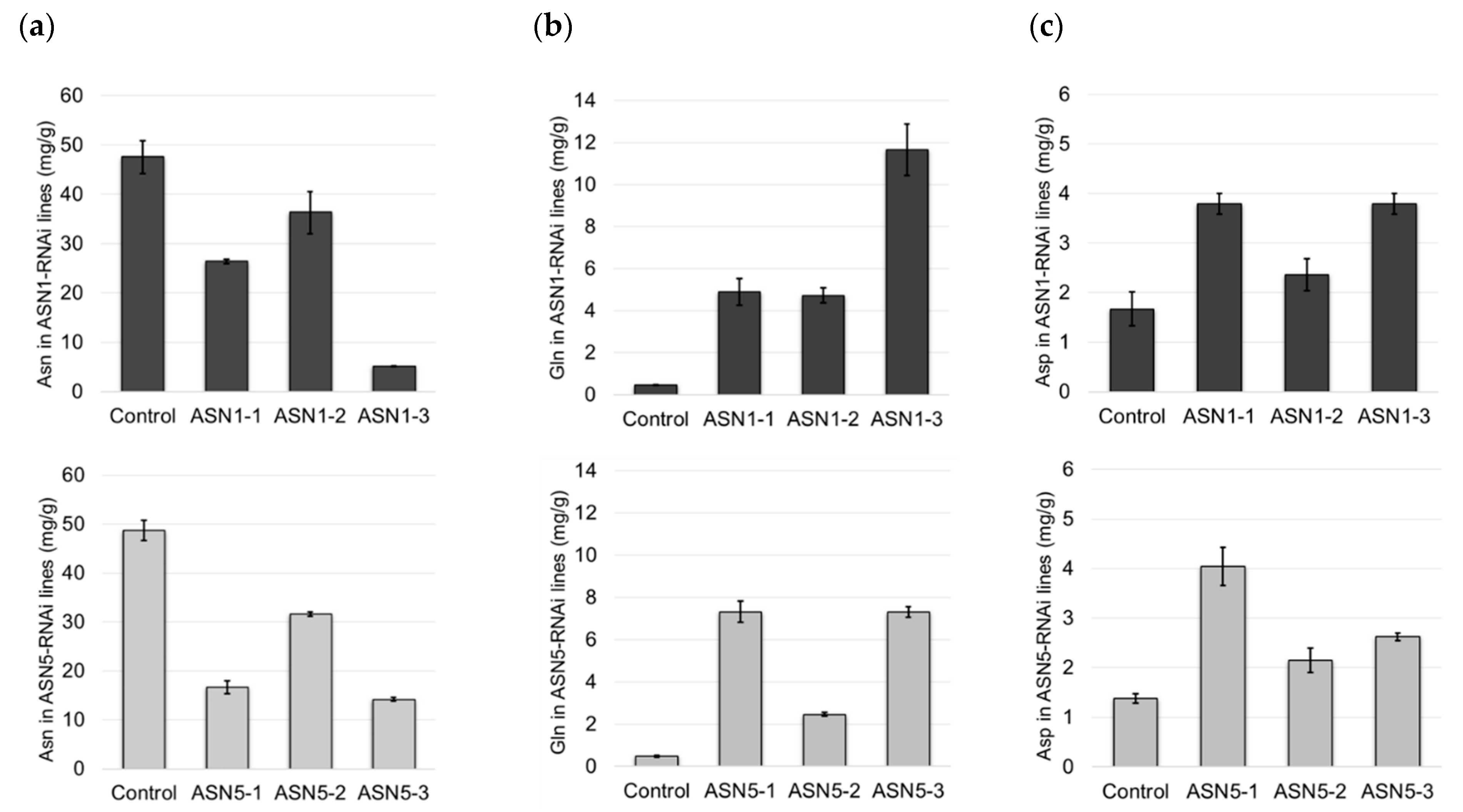
2.4. Silencing of ASN1 and ASN5 Affects the Production of Asp, Asn, and Glu
3. Discussion
4. Materials and Methods
4.1. Tobacco, Field Growth and Curing Conditions
4.2. ASN, SAG12, and RBCS Genes
4.3. Generation of ASN1-RNAi and ASN5-RNAi Tobacco Plants
4.4. Analysis of Total and Free Amino Acids
4.5. Analysis of Amino Acids, Nicotine, and Nitrates by HPLC-MS
4.6. Metabolomic Analysis: Dipeptides, Asn, Gln, Asp, Glu, and Abscisate Measurement
4.7. qPCR Experiments
4.8. RNA-seq and Gene Expression Analysis from Affymetrix Tobacco Exon Array
4.9. Statistical Analyses
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell 1990, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lothier, J.; Gaufichon, L.; Sormani, R.; Lemaître, T.; Azzopardi, M.; Morin, H.; Chardon, F.; Reisdorf-Cren, M.; Avice, J.-C.; Masclaux-Daubresse, C. The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot. 2011, 62, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M. Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J. Exp. Bot. 2014, 65, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [PubMed]
- Li, L.; Zhao, J.; Zhao, Y.; Lu, X.; Zhou, Z.; Zhao, C.; Xu, G. Comprehensive investigation of tobacco leaves during natural early senescence via multi-platform metabolomics analyses. Sci. Rep. 2016, 6, 37976. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.; Moison, M.; Soulay, F.; Reisdorf-Cren, M.; Masclaux-Daubresse, C. Metabolomics of laminae and midvein during leaf senescence and source-sink metabolite management in Brassica napus L. leaves. J. Exp. Bot. 2018, 69, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Li, X.; Zhang, F.; Liu, C.; Du, Y.; Gao, X.; Zhang, Z.; Zhang, X.; Hou, Z.; et al. Intergrative metabolomic and transcriptomic analyses unveil nutrient remobilization events in leaf senescence of tobacco. Sci. Rep. 2017, 7, 12126. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Moschen, S.; Luoni, S.B.; Rienzo, J.A.D.; Caro, M.D.P.; Tohge, T.; Watanabe, M.; Hollmann, J.; Gonzalez, S.; Rivarola, M.; Garcia-Garcia, F.; et al. Integrating transcriptomic and metabolomic analysis to understand natural leaf senescence in sunflower. Plant Biotechnol. J. 2016, 14, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, T.; Gaufichon, L.; Boutet-Mercey, S.; Christ, A.; Masclaux-Daubresse, C. Enzymatic and metabolic diagnostic of nitrogen deficiency in Arabidopsis thaliana Wassileskija accession. Plant Cell Physiol. 2008, 49, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Pilot, G. Amino acid export in plants: A missing link in nitrogen cycling. Mol. Plant 2011, 4, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Purdy, S.; Christ, A.; Morot-Gaudry, J.F.; Wingler, A.; Masclaux-Daubresse, C. Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Hareven, D.; Gutfinger, T.; Ken-Dror, S.; Lifschitz, E. Biosynthetic threonine deaminase gene of tomato: Isolation, structure, and upregulation in floral organs. Proc. Natl. Acad. Sci. USA 1991, 88, 2678–2682. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Pageau, K.; Lelandais, M.; Grandjean, O.; Kronenberger, L.; Valadier, M.H.; Feraud, M.; Jouglet, T.; Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006, 140, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Gan, S. Concepts and Types of Senescence in Plants. Methods Mol. Biol. 2018, 1744, 3–8. [Google Scholar] [PubMed]
- Zhao, L.; Zhang, H.; Zhang, B.; Bai, X.; Zhou, C. Physiological and molecular changes of detached wheat leaves in responding to various treatments. J. Integr. Plant Biol. 2012, 54, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Soudry, E.; Ulitzur, S.; Gepstein, S. Accumulation and remobilization of amino acids during senescence of detached and attached leaves: In planta analysis of tryptophan levels by recombinant luminescent bacteria. J. Exp. Bot. 2005, 56, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.R.; Chung, K.M.; Park, J.H.; Oh, S.A.; Ahn, T.; Hong, S.H.; Jang, S.K.; Nam, H.G. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 2001, 13, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Woo, H.R.; Kim, J.; Lim, P.O.; Lee, I.C.; Choi, S.H.; Hwang, D.; Nam, H.G. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 2009, 323, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Vickery, H.B.; Pucher, G.W. The Chemical Changes That Occur during the Curing of Tobacco Leaves. Science 1931, 73, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Frankenburg, W.G. Chemical changes in the harvested tobacco leaf. II. Chemical and enzymic conversions during fermentation and aging. Adv. Enzymol. Relat. Subj. Biochem. 1950, 10, 325–441. [Google Scholar] [PubMed]
- Andersen, R.N.; Fleming, P.D.; Burton, H.R.; Hamilton-Kemp, T.R.; Sutton, T.G. N′-Acyl and N′-nitroso pyridine alkaloids in alkaloid lines of burley tobacco during growth and air-curing. J. Agric. Food Chem. 1989, 37, 44–50. [Google Scholar] [CrossRef]
- Leffingwell, J.C. Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types; Chapter 8; Blackwell Science: Boston, MA, USA, 1999. [Google Scholar]
- Breeze, E.; Harrison, E.; McHattie, S.; Hugues, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, Z.; Gan, S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ. 2004, 27, 521–549. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 292, 19952–19958. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Gao, X.; Guo, Y. Initiation, Progression, and Genetic Manipulation of Leaf Senescence. Methods Mol. Biol. 2018, 1744, 9–31. [Google Scholar] [PubMed]
- The Arabidopsis Information Resource. Available online: https://www.arabidopsis.org (accessed on 15 October 2019).
- Solanaceae Genomics Network. Available online: https://solgenomics.net (accessed on 15 October 2019).
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.J.; Chardon, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Science. 2010, 179, 141–153. [Google Scholar] [CrossRef]
- Wu, F.; Eannetta, N.T.; Xu, Y.; Plieske, J.; Ganal, M.; Pozzi, C.; Bakaher, N.; Tanksley, S.D. COSII genetic maps of two diploid Nicotiana species provide a detailed picture of synteny with tomato and insights into chromosome evolution in tetraploid N. tabacum. Theor. Appl. Genet. 2010, 120, 809–827. [Google Scholar] [CrossRef] [PubMed]
- Bindler, G.; Plieske, J.; Bakaher, N.; Gunduz, I.; Ivanov, N.; der Hoeven, R.V.; Ganal, M.; Donini, P. A high density genetic map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development. Theor. Appl. Genet. 2011, 123, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Willig, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 2014, 5, 3833. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Bovet, L.; Cordier, A.; Stanke, M.; Gunduz, I.; Peitsch, M.C.; Ivanov, N.V. Design of a tobacco exon array with application to investigate the differential cadmium accumulation property in two tobacco varieties. BMC Genom. 2012, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.A.; Walton, L.R. Curing Burley Tobacco; Cooperative Extension Service; College of Agriculture, University of Kentucky: Lexington, KY, USA, 1986. [Google Scholar]
- Burton, H.R.; Kasperbauer, M.J. Chemical changes during air curing of Burley tobacco. In CORESTA Congress; University of Kentucky: Lexington, KY, USA, 1982; p. 82. [Google Scholar]
- Jiang, C.Z.; Rodermel, S.R. Rodermel, Regulation of Photosynthesis during Leaf Development in RbcS Antisense DNA Mutants of Tobacco. Plant Physiol. 1995, 107, 215–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lohman, K.N.; Gan, S.; John, M.C.; Amasino, R.M. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol. Plant. 1994, 92, 322–328. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y. Hormone Treatments in Studying Leaf Senescence. Methods Mol. Biol. 2018, 1744, 125–132. [Google Scholar] [PubMed]
- Herrera-Rodríguez, M.B.; Maldonado, J.M.; Pérez-Vicente, R. Role of asparagine and asparagine synthetase genes in sunflower (Helianthus annuus) germination and natural senescence. J. Plant Physiol. 2006, 163, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Marmagne, A.; Talbotec, J.; Krupinska, K.; Masclaux-Daubresse, C. The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. J. Exp. Bot. 2015, 66, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Noodén, L.D. Defining Senescence and Death in Photosynthetic Tissues. In Plastid Development in Leaves during Growth and Senescence; Biswal, B., Krupinska, K., Biswal, U., Eds.; Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes); Volume 36, Springer Link: Berlin, Germany, 2013; pp. 283–306. [Google Scholar]
- Warman, T.W.; Solomos, T. Ethylene Production and Action during Foliage Senescence in Hedera helix L. J. Exp. Bot. 1988, 39, 685–694. [Google Scholar] [CrossRef]
- Keskitalo, J.; Bergquist, G.; Gardestrom, P.; Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Keech, O.; Pesquet, E.; Ahad, A.; Askne, A.; Nordvall, D.; Vodnala, S.M.; Tuominen, H.; Hurry, V.; Dizengremel, P.; Gardestrom, P. The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant Cell Environ. 2007, 30, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.L. Carbon and nitrogen nutrient balance signaling in plants. Plant Signal. Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce gamma-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Winge, P.; Tran, D.H.T.; Jørstad, T.S.; Størseth, T.R.; Christensen, T.; Bones, A.M. Transcriptional profiling of an Fd-GOGAT1/GLU1 mutant in Arabidopsis thaliana reveals a multiple stress response and extensive reprogramming of the transcriptome. BMC Genom. 2010, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Wong, P.; Chen, L.; Chow, C.M.; Coruzzi, G.M. Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol. 2003, 132, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gong, H.; He, Q.; Zeng, Z.; Busse, J.S.; Jin, W.; Bethke, P.C.; Jiang, J. Silencing of vacuolar invertase and asparagine synthetase genes and its impact on acrylamide formation of fried potato products. Plant Biotechnol. J. 2016, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Gaufichon, L.; Masclaux-Daubresse, C.; Tcherkez, G.; Reisdorf-Cren, M.; Sakakibara, Y.; Hase, T.; Clement, G.; Avice, J.-C.; Grandjean, O.; Marmagne, A.; et al. Arabidopsis thaliana ASN2 encoding asparagine synthetase is involved in the control of nitrogen assimilation and export during vegetative growth. Plant Cell Environ. 2013, 36, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.M.; Amasino, R.M. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiol. 2001, 127, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nishi, Y.; Watanabe, A.; Terashima, I. Possible Mechanisms of Adaptive Leaf Senescence. Plant Biol. 2001, 3, 234–243. [Google Scholar] [CrossRef]
- Agüera, E.; Cabello, P.; De La Haba, P. Induction of leaf senescence by low nitrogen nutrition in sunflower (Helianthus annuus) plants. Physiol. Plant. 2010, 138, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Burton, H.R.; Fleming, P.D.; Hamilto-Kemp, T.R.; Gay, S.L. Effects of air-curing environment on alkaloid-derived nitrosamines in burley tobacco. IARC Sci. Publ. 1987, 84, 451–455. [Google Scholar]
- Cheng, M.; Fry, J.E.; Pang, H.; Zhou, H.; Hironaka, C.M.; Duncan, D.R.; Conner, T.W.; Wan, Y. Genetic Transformation of Wheat Mediated by Agrobacterium tumefaciens. Plant Physiol. 1997, 115, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eicholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Lewis, R.S.; Bowen, S.W.; Keogh, M.R.; Dewey, R.E. Three nicotine demethylase genes mediate nornicotine biosynthesis in Nicotiana tabacum L.: Functional characterization of the CYP82E10 gene. Phytochemistry 2010, 71, 1988–1998. [Google Scholar]
- Moldoveanu, S.C. Analysis of Protein Amino Acids in Tobacco Using Microwave Digestion of Plant Material. Beitr. Tabakforsch. Int. 2005, 21, 451–465. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42319 (accessed on 15 October 2019).
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL16290 (accessed on 15 October 2019).
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovet, L.; Cheval, C.; Hilfiker, A.; Battey, J.; Langlet, D.; Broye, H.; Schwaar, J.; Ozelley, P.; Lang, G.; Bakaher, N.; et al. Asparagine Synthesis during Tobacco Leaf Curing. Plants 2019, 8, 492. https://doi.org/10.3390/plants8110492
Bovet L, Cheval C, Hilfiker A, Battey J, Langlet D, Broye H, Schwaar J, Ozelley P, Lang G, Bakaher N, et al. Asparagine Synthesis during Tobacco Leaf Curing. Plants. 2019; 8(11):492. https://doi.org/10.3390/plants8110492
Chicago/Turabian StyleBovet, Lucien, Cecilia Cheval, Aurore Hilfiker, James Battey, Delphine Langlet, Herve Broye, Joanne Schwaar, Pierrick Ozelley, Gerhard Lang, Nicolas Bakaher, and et al. 2019. "Asparagine Synthesis during Tobacco Leaf Curing" Plants 8, no. 11: 492. https://doi.org/10.3390/plants8110492
APA StyleBovet, L., Cheval, C., Hilfiker, A., Battey, J., Langlet, D., Broye, H., Schwaar, J., Ozelley, P., Lang, G., Bakaher, N., Laparra, H., & Goepfert, S. (2019). Asparagine Synthesis during Tobacco Leaf Curing. Plants, 8(11), 492. https://doi.org/10.3390/plants8110492



