Fruit Architecture in Polyamine-Rich Tomato Germplasm Is Determined via a Medley of Cell Cycle, Cell Expansion, and Fruit Shape Genes
Abstract
1. Introduction
2. Results
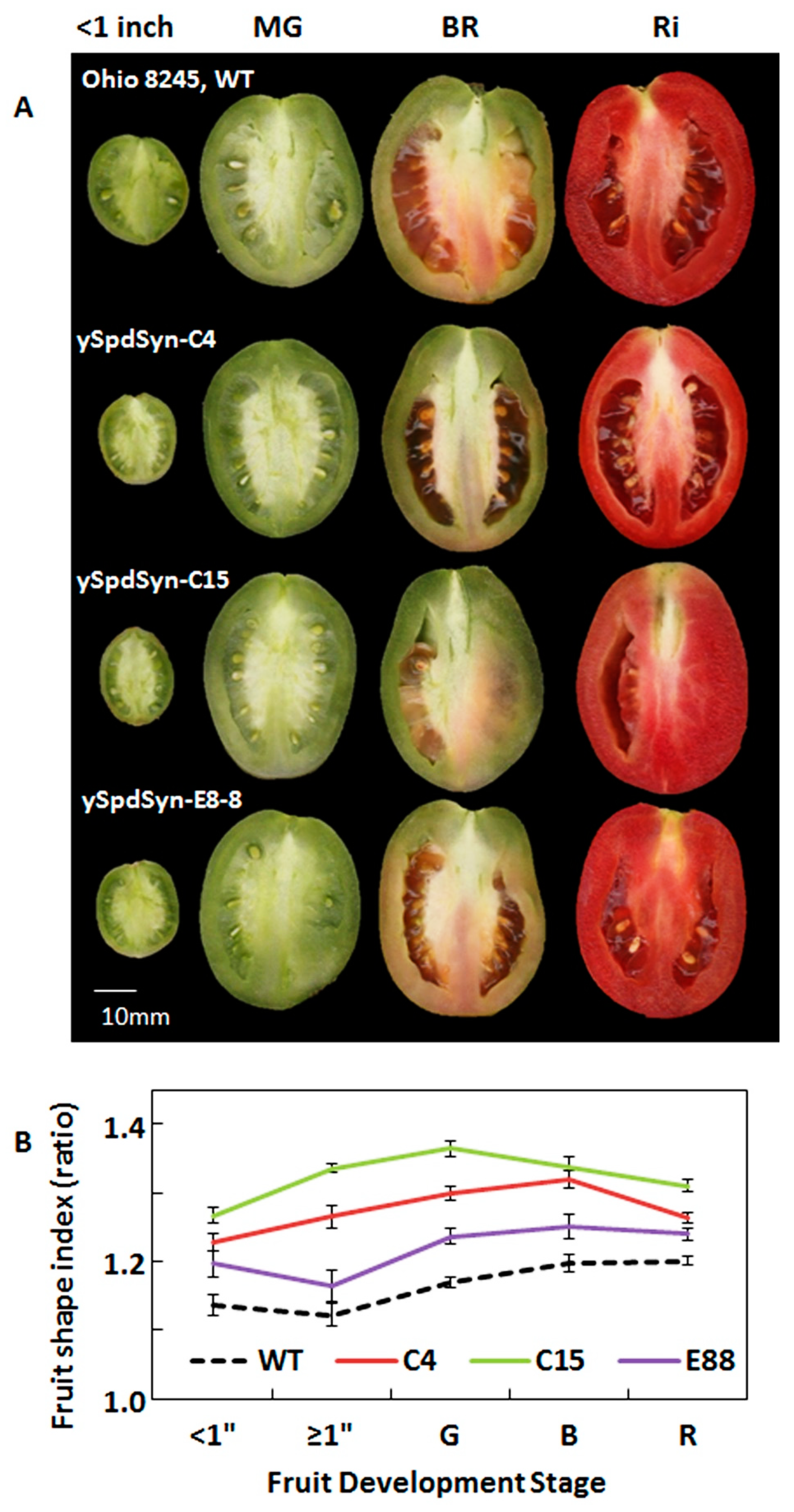
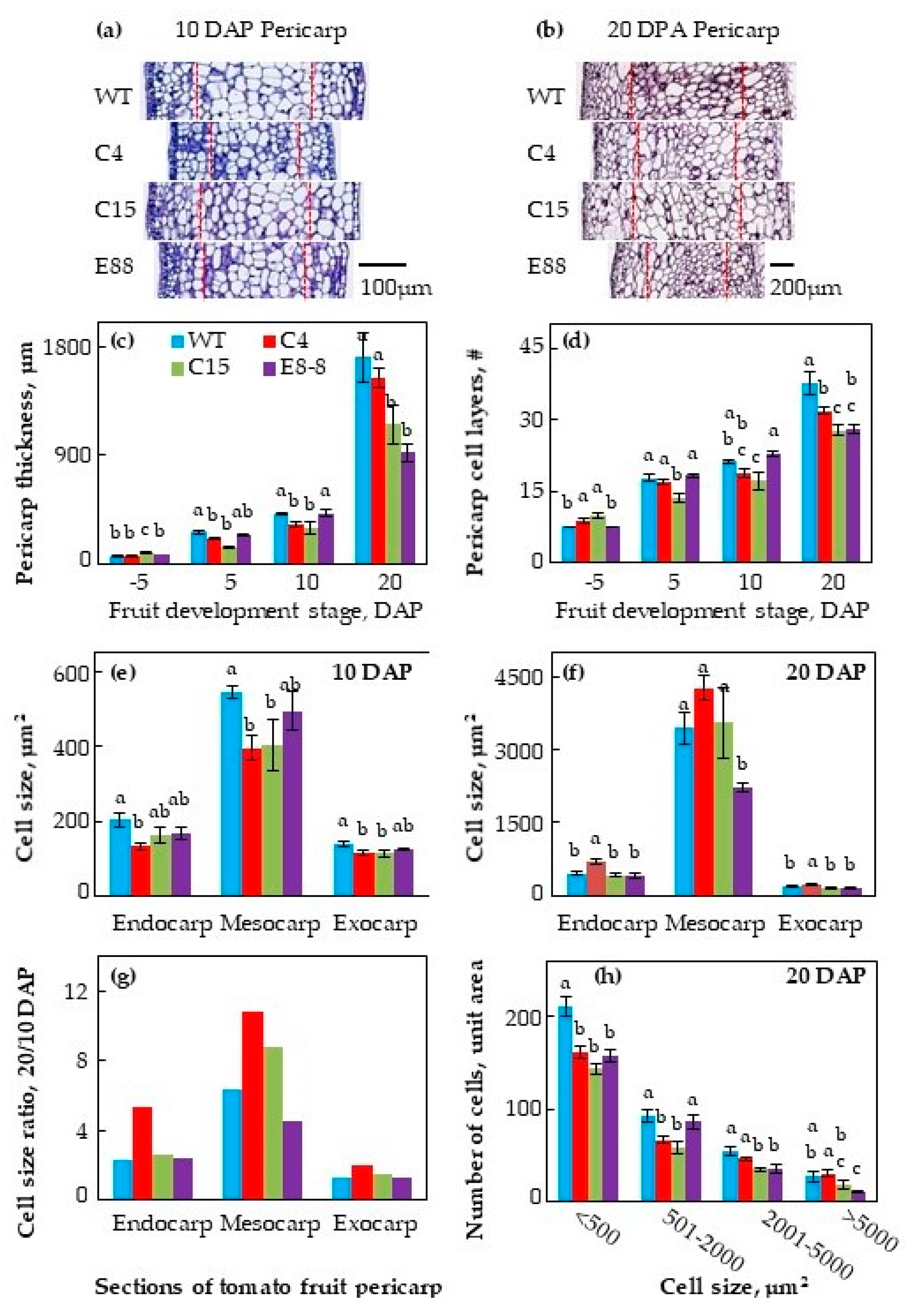
2.1. Transgenic Expression of ySpdSyn Caused Architectural Changes in Tomato Fruit
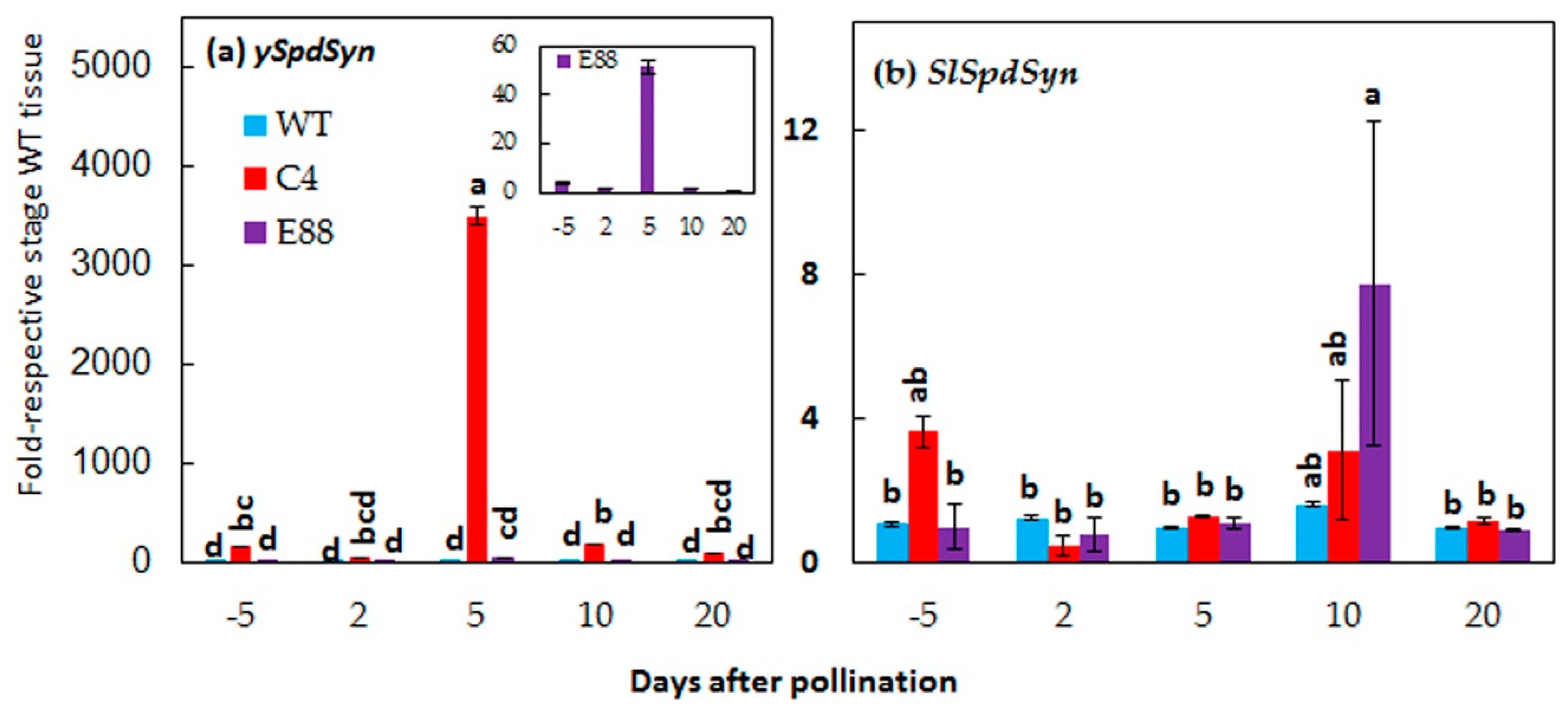
2.2. Expression of ySpdSyn Transgene and SlSpdSyn in Floral Buds and Fertilized Ovaries
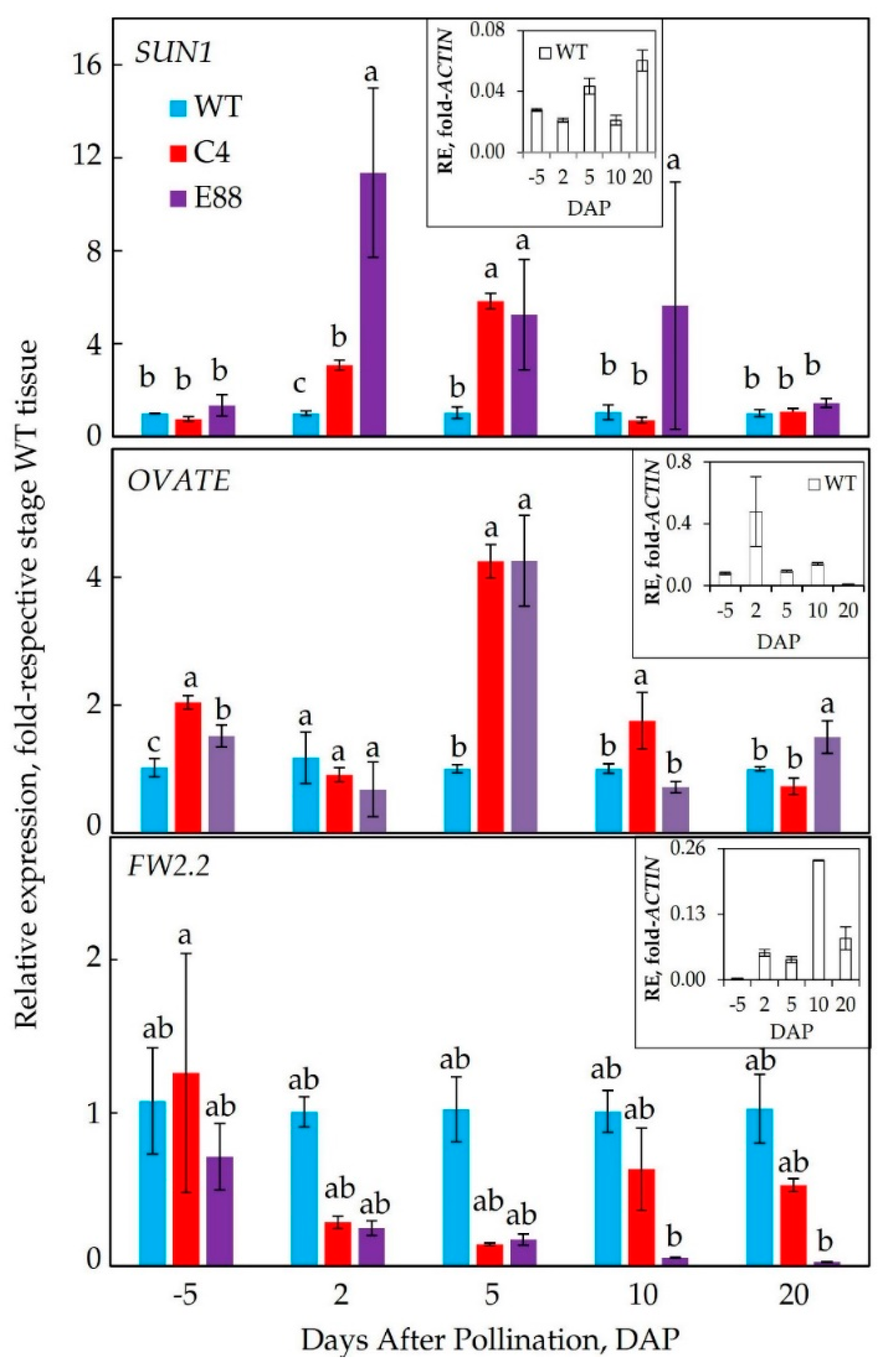
2.3. Expression Patterns of Fruit Size and Shape Genes in Transgenic Fruits
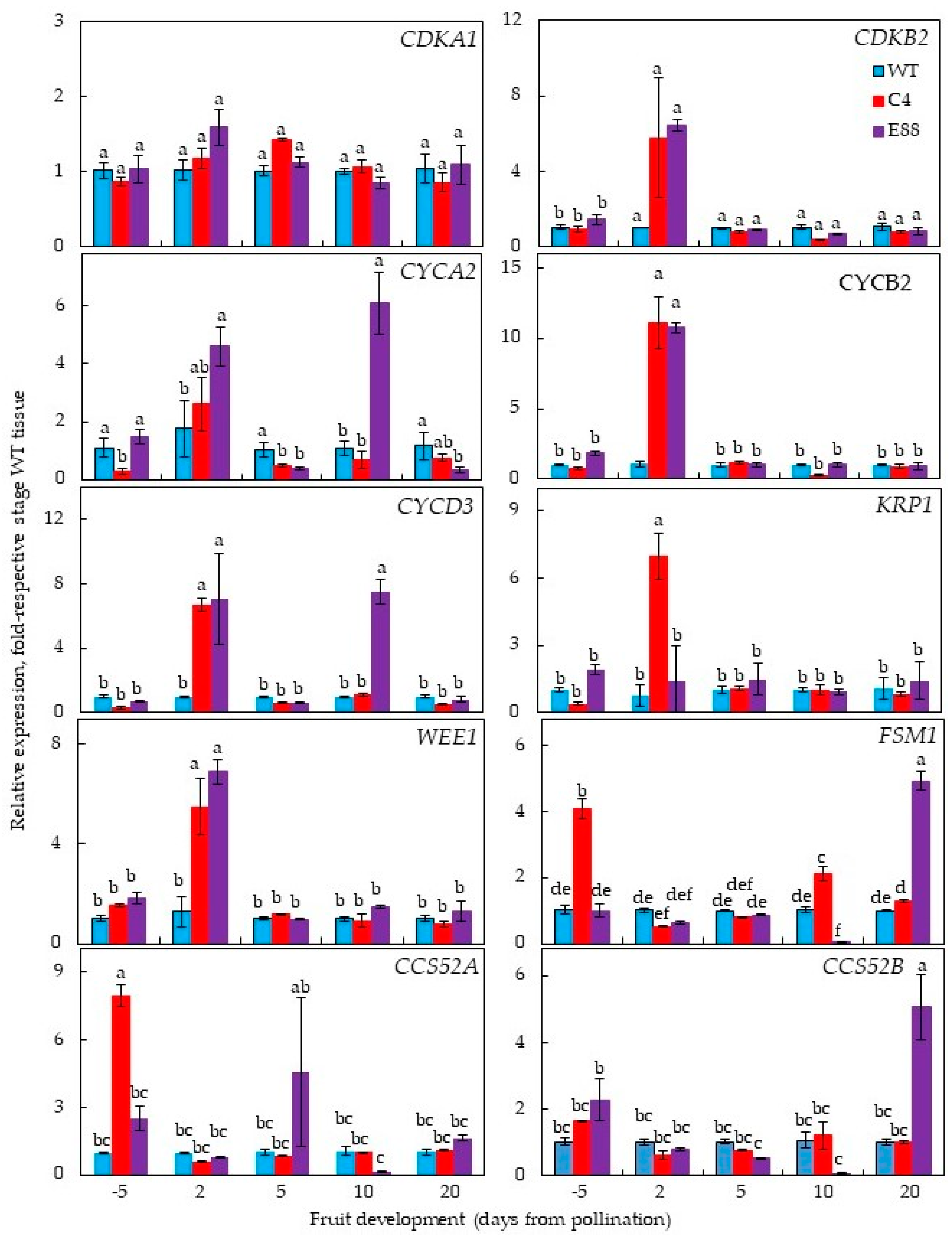
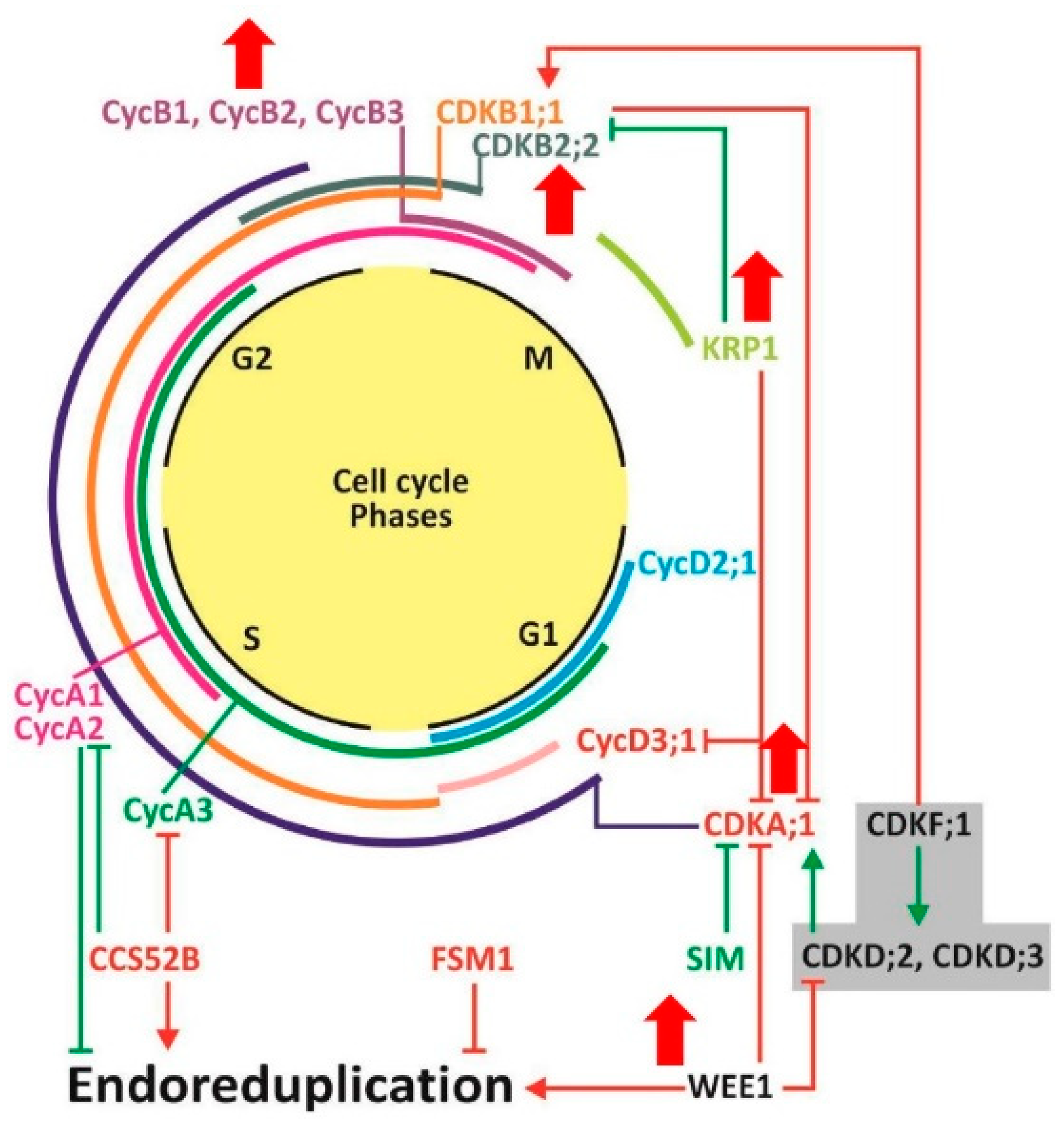
2.4. Expression Patterns of Selected Cell Division and Cell Expansion Genes in ySpdSyn Transgenic and Wild-Type Tomato
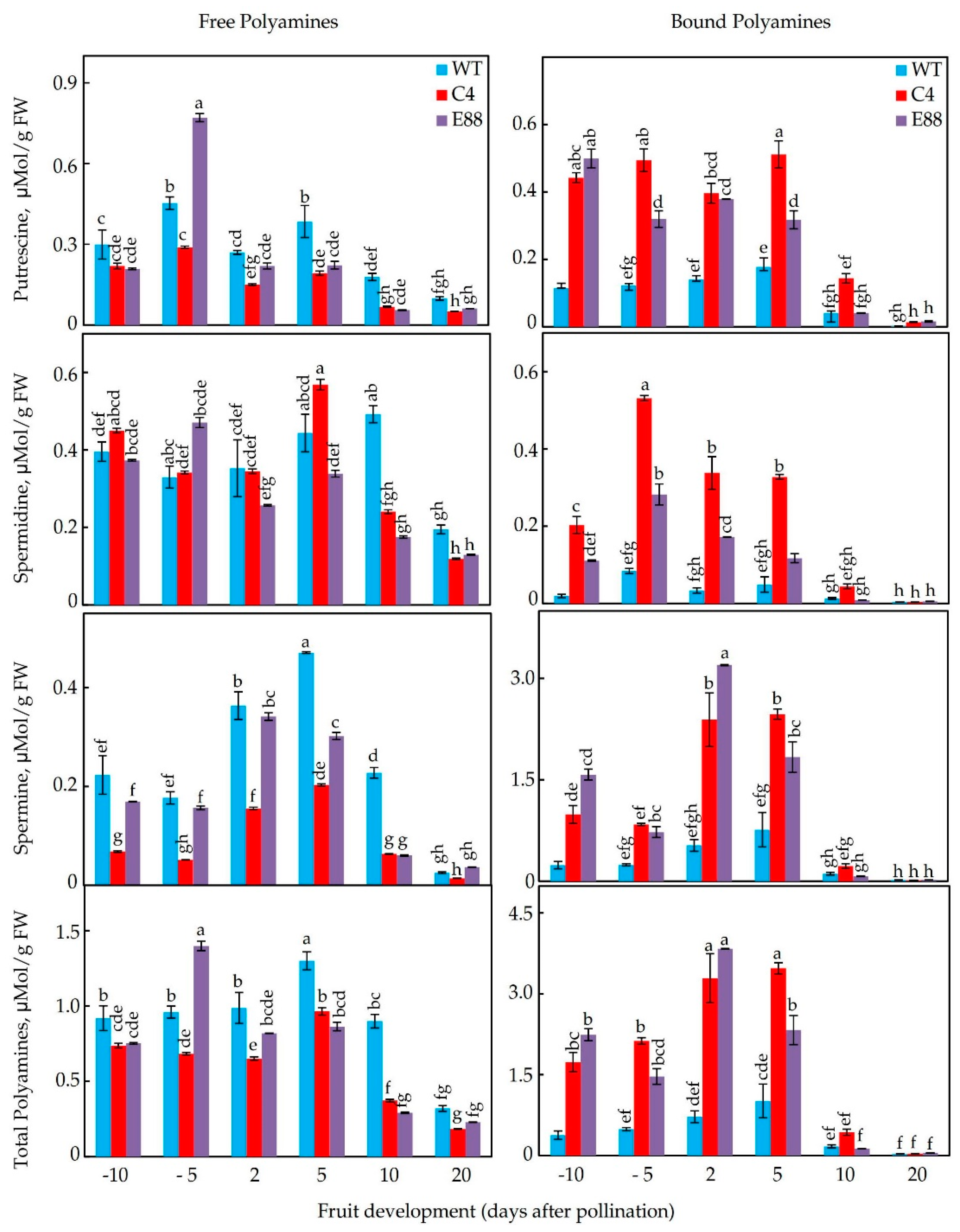
2.5. Levels of Free and Bound PAs in Floral Buds and Developing Ovaries
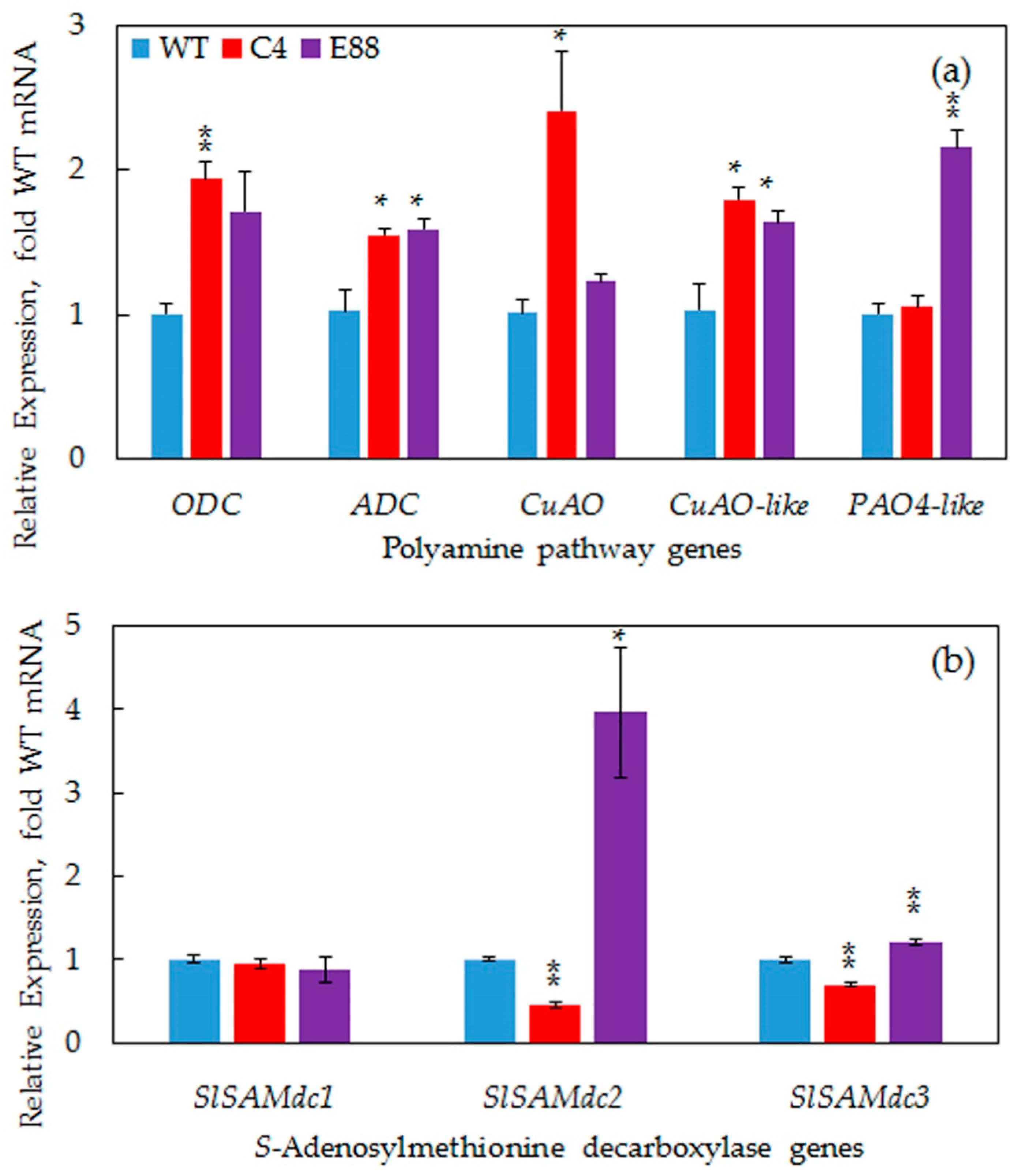
2.6. Transgene Increased Expression of PA Biosynthesis and Catabolic Pathway Genes
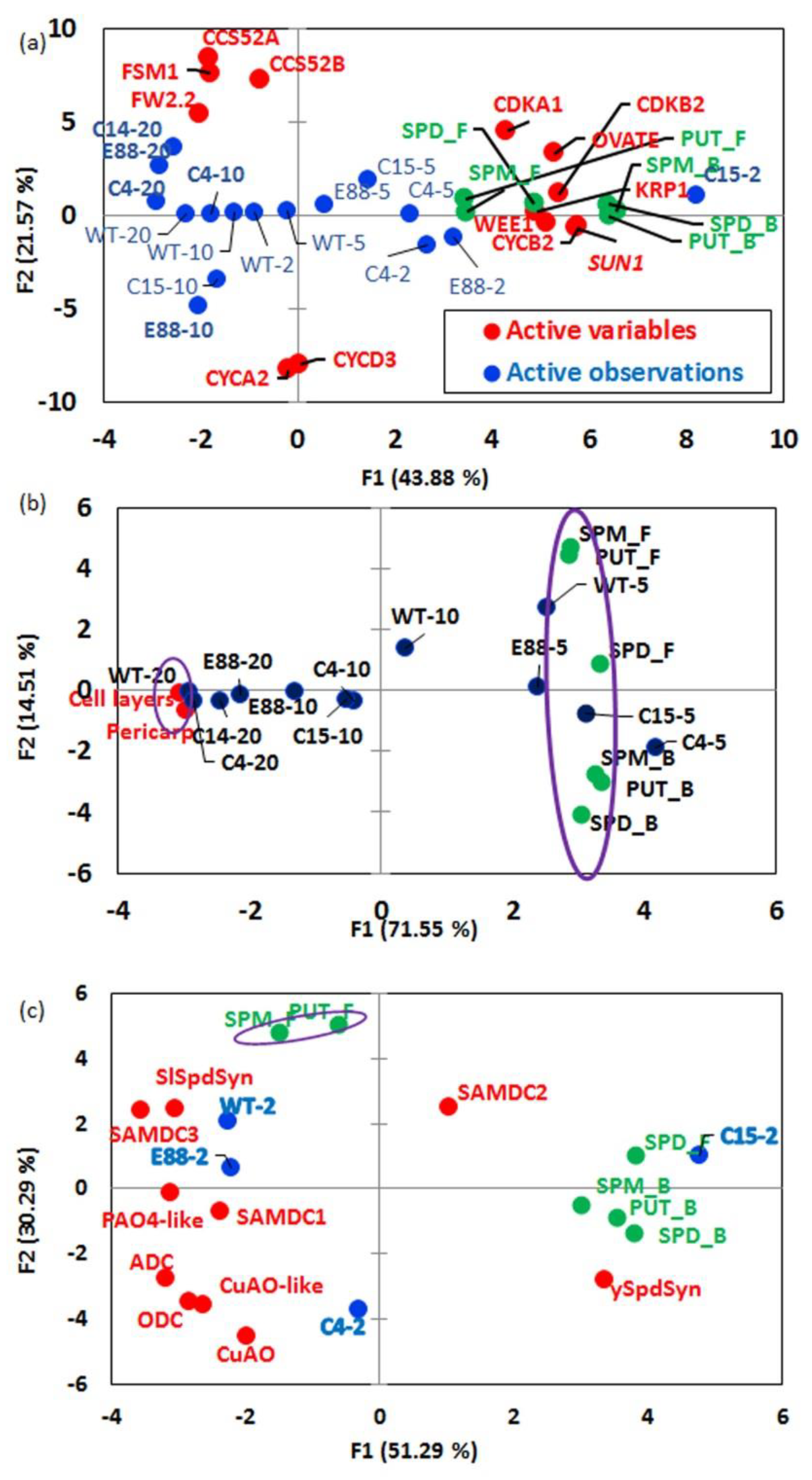
2.7. Statistical Analyses Accentuate Positive Correlations between Specific PAs levels, Gene Transcripts, and Fruit Architectural Parameters During Early Fruit Development
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Cytological Analysis
4.3. Transcript Analysis by Quantitative Real-Time PCR
4.4. Quantification of Polyamines by High Pressure Liquid Chromatography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Van Eck, J.; Cong, B.; Tanksley, S.D. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc. Natl. Acad. Sci. USA 2002, 99, 13302–13306. [Google Scholar] [CrossRef]
- Brewer, M.T.; Moyseenko, J.B.; Monforte, A.J.; van der Knaap, E. Morphological variation in tomato: A comprehensive study of quantitative trait loci controlling fruit shape and development. J. Exp. Bot. 2007, 58, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Anwar, R.; Mattoo, A.K. Biotechnology of fruit quality. In Fruit Ripening: Physiology, Signalling and Genomics; Nath, P., Bouzayen, M., Mattoo, A.K., Pech, J.C., Eds.; CAB International: Oxfordshire, UK, 2014; pp. 259–290. [Google Scholar]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Van Der Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; et al. Fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in yabby-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jiang, N.; Schaffner, E.; Stockinger, E.J.; van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008, 319, 1527–1530. [Google Scholar] [CrossRef]
- Huang, Z.J.; van der Knaap, E. Tomato fruit weight 11.3 maps close to fasciated on the bottom of chromosome 11. Theor. Appl. Genet. 2011, 123, 465–474. [Google Scholar] [CrossRef]
- Muños, S.; Ranc, N.; Botton, E.; Bérard, A.; Rolland, S.; Duffé, P.; Carretero, Y.; Le Paslier, M.-C.; Delalande, C.; Bouzayen, M.; et al. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near wuschel. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of sun, ovate, lc, and fas in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef]
- Clevenger, J. Metabolic and Genomic Analysis of Elongated Fruit Shape in Tomato (Solanum Lycopersicum). Master’s Thesis, The Ohio State University, Horticulture and Crop Science, Columbus, OH, USA, 2012. [Google Scholar]
- Chakrabarti, M.; Zhang, N.; Sauvage, C.; Muños, S.; Blanca, J.; Cañizares, J.; Diez, M.J.; Schneider, R.; Mazourek, M.; McClead, J.; et al. A cytochrome p450 regulates a domestication trait in cultivated tomato. Proc. Natl. Acad. Sci. USA 2013, 110, 17125–17130. [Google Scholar] [CrossRef] [PubMed]
- Alpert, K.B.; Grandillo, S.; Tanksley, S.D. Fw 2.2: A major qtl controlling fruit weight is common to both red- and green-fruited tomato species. TAG Theor. Appl. Genet. 1995, 91, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, T.C.; Tanksley, S.D. Comparative sequencing in the genus lycopersicon: Implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 2002, 162, 365–379. [Google Scholar] [PubMed]
- van der Knaap, E.; Tanksley, S.D. Identification and characterization of a novel locus controlling early fruit development in tomato. Theor. Appl. Genet. 2001, 103, 353–358. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, H.; Cabrera, A.; Meulia, T.; van der Knaap, E. Sun regulates vegetative and reproductive organ shape by changing cell division patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar] [CrossRef]
- Wang, Y.; Clevenger, J.P.; Illa-Berenguer, E.; Meulia, T.; van der Knaap, E.; Sun, L. A comparison of sun, ovate, fs8.1 and auxin application on tomato fruit shape and gene expression. Plant Cell Physiol. 2019, 60, 1067–1081. [Google Scholar] [CrossRef]
- Ku, H.M.; Doganlar, S.; Chen, K.Y.; Tanksley, S.D. The genetic basis of pear-shaped tomato fruit. TAG Theor. Appl. Genet. 1999, 99, 844–850. [Google Scholar] [CrossRef]
- Tsaballa, A.; Pasentsis, K.; Darzentas, N.; Tsaftaris, A. Multiple evidence for the role of an ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 2011, 11, 46. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Alcazar, R. Potential Applications of Polyamines in Agriculture and Plant Biotechnology. Methods Mol. Biol. 2018, 1694, 489–508. [Google Scholar]
- Pegg, A.E. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J. Biol. Chem. 2018, 293, 18681–18692. [Google Scholar] [CrossRef]
- Miyamoto, M.; Shimao, S.; Tong, W.; Motose, H.; Takahashi, T. Effect of Thermospermine on the Growth and Expression of Polyamine-Related Genes in Rice Seedlings. Plants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Nambeesan, S.; Mattoo, A.K. Nexus between spermidine and floral organ identity and fruit/seed set in tomato. Front. Plant Sci. 2019, 10, 1033. [Google Scholar]
- Nambeesan, S.; Datsenka, T.; Ferruzzi, M.G.; Malladi, A.; Mattoo, A.K.; Handa, A.K. Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J. 2010, 63, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Sobieszczuk-Nowicka, E. Polyamine as signaling molecules and leaf senescence. In Senescence Signalling and Control in Plants; Sarwat, M., Tuteja, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–138. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef]
- Mehta, R.A.; Cassol, T.; Li, N.; Ali, N.; Handa, A.K.; Mattoo, A.K. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 2002, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Cho, H.; Park, K.; Kim, Y.-B. Changes in cellular polyamine contents and activities of their biosynthetic enzymes at each phase of the cell cycle in by-2 cells. J. Plant Biol 2006, 49, 153–159. [Google Scholar] [CrossRef]
- Verkest, A.; Weinl, C.; Inzé, D.; De Veylder, L.; Schnittger, A. Switching the cell cycle. Kip-related proteins in plant cell cycle control. Plant Physiol. 2005, 139, 1099–1106. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R. Polyamines promote root elongation and growth by increasing root cell division in regenerated virginia pine (pinus virginiana mill.) plantlets. Plant Cell Rep. 2005, 24, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Polyamine interactions with plant hormones: Crosstalk at several levels. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Kusano, T., Suzuki, H., Eds.; Springer: London, UK, 2015; pp. 267–302. [Google Scholar]
- Czerednik, A.; Busscher, M.; Angenent, G.C.; de Maagd, R.A. The cell size distribution of tomato fruit can be changed by overexpression of cdka1. Plant Biotechnol. J. 2015, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Barg, R.; Sobolev, I.; Eilon, T.; Gur, A.; Chmelnitsky, I.; Shabtai, S.; Grotewold, E.; Salts, Y. The tomato early fruit specific gene lefsm1 defines a novel class of plant-specific sant/myb domain proteins. Planta 2005, 221, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Balbi, V.; Lomax, T.L. Regulation of early tomato fruit development by the diageotropica gene. Plant Physiol. 2003, 131, 186–197. [Google Scholar] [CrossRef]
- Azzi, L.; Deluche, C.; Gévaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit growth-related genes in tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef]
- Wang, S.; Chang, Y.; Guo, J.; Zeng, Q.; Ellis, B.E.; Chen, J.-G. Arabidopsis ovate family proteins, a novel transcriptional repressor family, control multiple aspects of plant growth and development. PLoS ONE 2011, 6, e23896. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, J.; Zhang, J.; Miao, H.; Jia, C.; Wang, Z.; Xu, B.; Jin, Z. Mumads1 and maofp1 regulate fruit quality in a tomato ovate mutant. Plant Biotechnol. J. 2018, 16, 989–1001. [Google Scholar] [CrossRef]
- Fellenberg, C.; Handrick, V.; Ziegler, J.; Vogt, T. Polyamine homeostasis in wild type and phenolamide deficient arabidopsis thaliana stamens. Front. Plant Sci. 2012, 3, 180. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Plant polyamine catabolism: The state of the art. Plant Signal. Behav. 2008, 3, 1061–1066. [Google Scholar] [CrossRef]
- Takahashi, T.; Tong, W. Regulation and diversity of polyamine biosynthesis in plants. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Kusano, T., Suzuki, H., Eds.; Springer: Japan, Tokyo, 2015; pp. 27–44. [Google Scholar]
- Tobias, K.E.; Kahana, C. Exposure to ornithine results in excessive accumulation of putrescine and apoptotic cell-death in ornithine decarboxylase overproducing mouse myeloma cells. Cell Growth Differ. 1995, 6, 1279–1285. [Google Scholar] [PubMed]
- Handa, A.K.; Mattoo, A.K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Sandusky-Beltran, L.A.; Kovalenko, A.; Ma, C.; Calahatian, J.I.T.; Placides, D.S.; Watler, M.D.; Hunt, J.B.; Darling, A.L.; Baker, J.D.; Blair, L.J.; et al. Spermidine/spermine-n1-acetyltransferase ablation impacts tauopathy-induced polyamine stress response. Alzheimer’s Res. Ther. 2019, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Spermidine/spermine n1-acetyltransferase--the turning point in polyamine metabolism. FASEB J. 1993, 7, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk-Nowicka, E.; Paluch-Lubawa, E.; Mattoo, A.K.; Arasimowicz-Jelonek, M.; Gregersen, P.L.; Pacak, A. Polyamines—A new metabolic switch: Crosstalk with networks involving senescence, crop improvement, and mammalian cancer therapy. Front. Plant Sci. 2019, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Pooja; Goyal, V.; Devi, S.; Munjal, R. Role of polyamines in protecting plants from oxidative stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 143–157. [Google Scholar]
- Gonzalo, M.J.; Brewer, M.T.; Anderson, C.; Sullivan, D.; Gray, S.; van der Knaap, E. Tomato fruit shape analysis using morphometric and morphology attributes implemented in tomato analyzer software program. J. Am. Soc. Hortic. Sci. 2009, 134, 77–87. [Google Scholar] [CrossRef]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology; Battelle Press: Columbus, OH, USA, 1987; Volume II, p. 481. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Torrigiani, P.; Bressanin, D.; Beatriz Ruiz, K.; Tadiello, A.; Trainotti, L.; Bonghi, C.; Ziosi, V.; Costa, G. Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signaling. Physiol. Plant 2012, 146, 86–98. [Google Scholar] [CrossRef]
- Xiao, L.; Cui, Y.-H.; Rao, J.N.; Zou, T.; Liu, L.; Smith, A.; Turner, D.J.; Gorospe, M.; Wang, J.-Y. Regulation of cyclin-dependent kinase 4 translation through cug-binding protein 1 and microrna-222 by polyamines. Mol. Biol. Cell 2011, 22, 3055–3069. [Google Scholar] [CrossRef]
- Gutierrez, C. The Arabidopsis cell division cycle. Arab. Book 2009, 7, e0120. [Google Scholar] [CrossRef] [PubMed]
- John, P.C.L.; Qi, R. Cell division and endoreduplication: Doubtful engines of vegetative growth. Trends Plant Sci. 2008, 13, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Gévaudant, F.; Sicard, A.; Salar, S.; Do, P.T.; Mouras, A.; Fernie, A.R.; Gibon, Y.; Rothan, C.; Chevalier, C.; et al. Functional analysis of the anaphase promoting complex activator ccs52a highlights the crucial role of endo-reduplication for fruit growth in tomato. Plant J. 2010, 62, 727–741. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, R.; Fatima, S.; Mattoo, A.K.; Handa, A.K. Fruit Architecture in Polyamine-Rich Tomato Germplasm Is Determined via a Medley of Cell Cycle, Cell Expansion, and Fruit Shape Genes. Plants 2019, 8, 387. https://doi.org/10.3390/plants8100387
Anwar R, Fatima S, Mattoo AK, Handa AK. Fruit Architecture in Polyamine-Rich Tomato Germplasm Is Determined via a Medley of Cell Cycle, Cell Expansion, and Fruit Shape Genes. Plants. 2019; 8(10):387. https://doi.org/10.3390/plants8100387
Chicago/Turabian StyleAnwar, Raheel, Shazia Fatima, Autar K. Mattoo, and Avtar K. Handa. 2019. "Fruit Architecture in Polyamine-Rich Tomato Germplasm Is Determined via a Medley of Cell Cycle, Cell Expansion, and Fruit Shape Genes" Plants 8, no. 10: 387. https://doi.org/10.3390/plants8100387
APA StyleAnwar, R., Fatima, S., Mattoo, A. K., & Handa, A. K. (2019). Fruit Architecture in Polyamine-Rich Tomato Germplasm Is Determined via a Medley of Cell Cycle, Cell Expansion, and Fruit Shape Genes. Plants, 8(10), 387. https://doi.org/10.3390/plants8100387






