An Efficient and Economical Protocol for Isolating, Purifying and PEG-Mediated Transient Gene Expression of Chinese Kale Hypocotyl Protoplasts
Abstract
:1. Introduction
2. Results
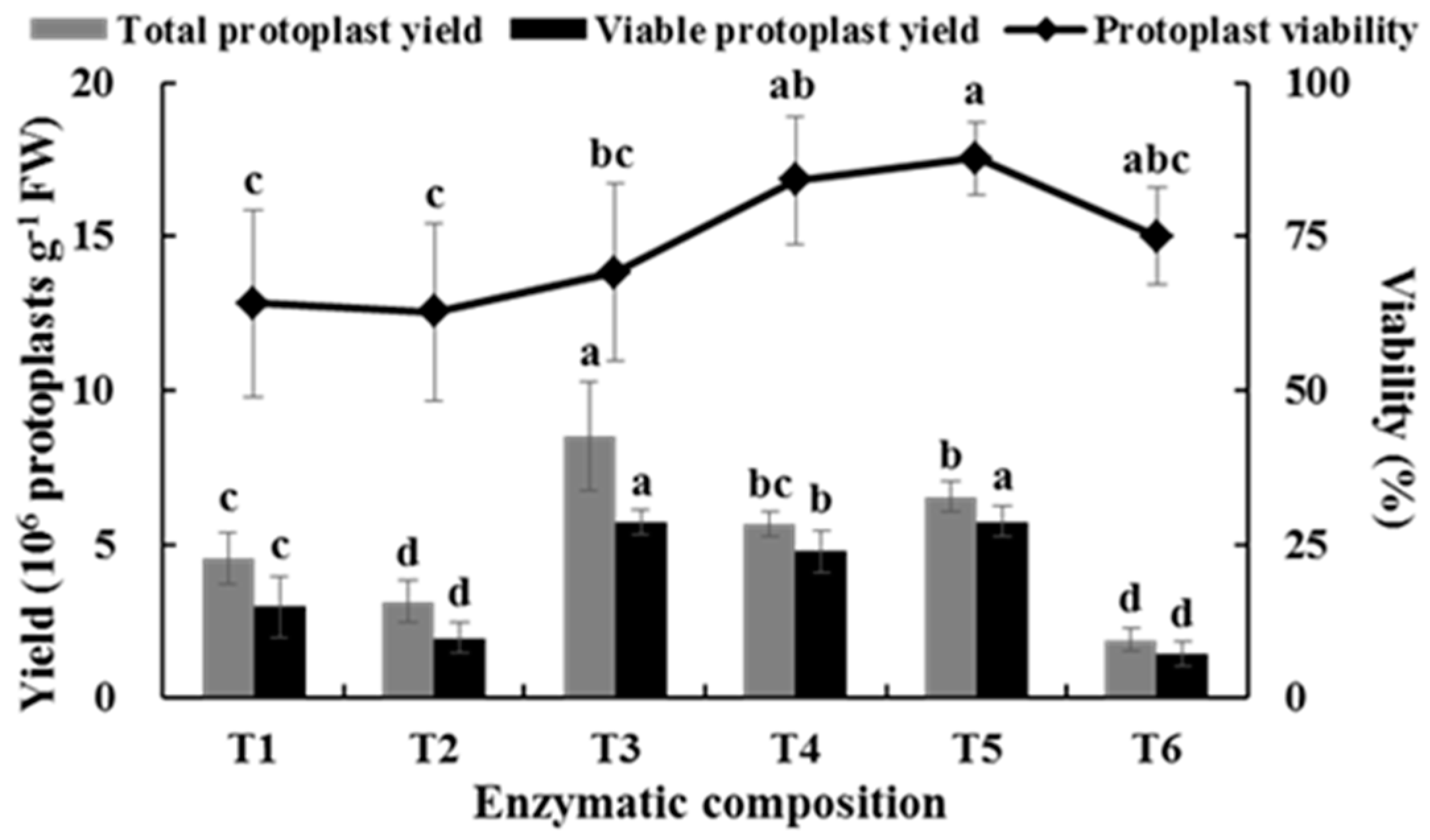
2.1. Effects of Enzyme Composition on Isolating Protoplast from Chinese Kale Hypocotyls
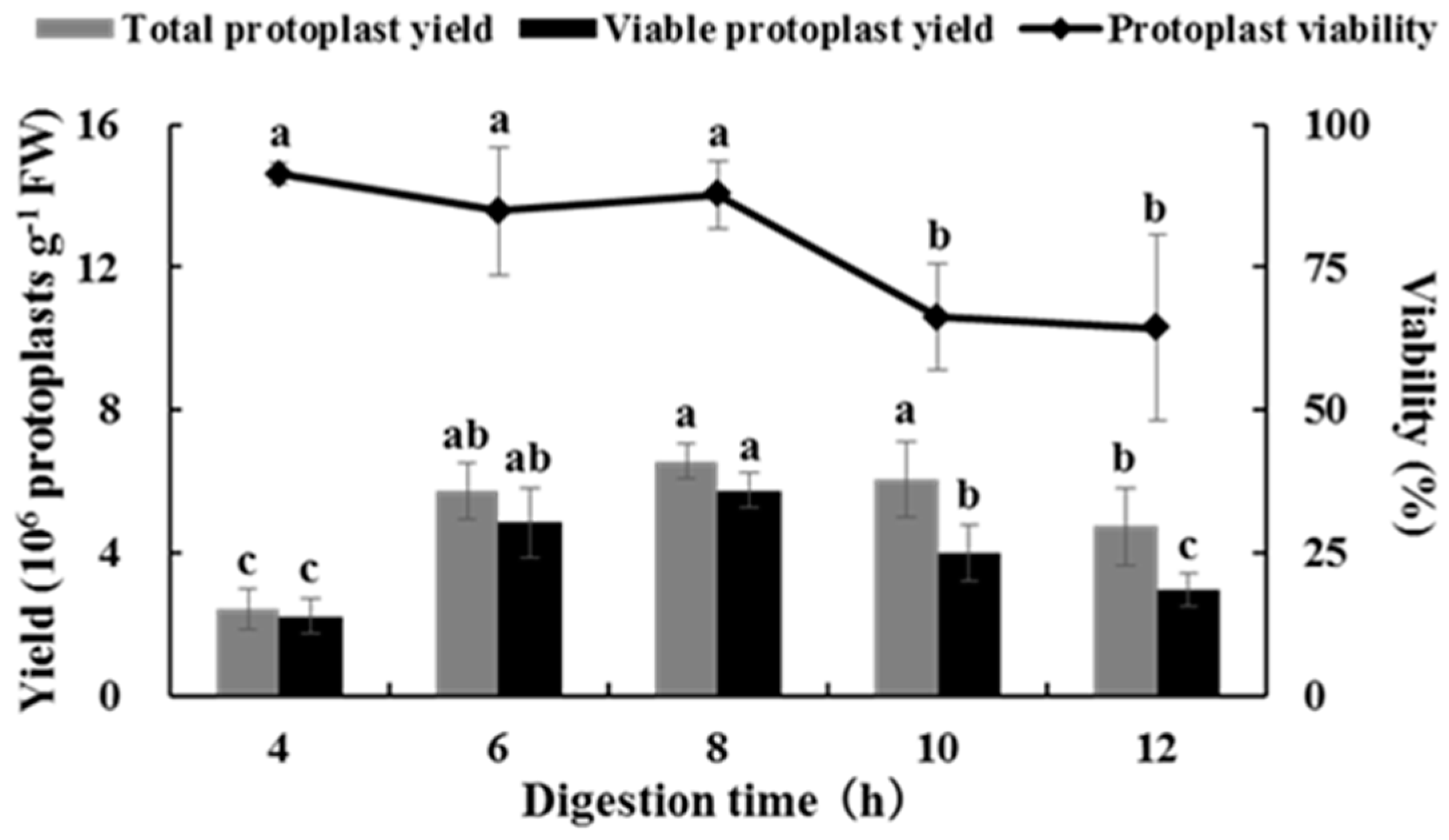
2.2. Effects of Digestion Time on Isolating Protoplasts from Chinese Kale Hypocotyls
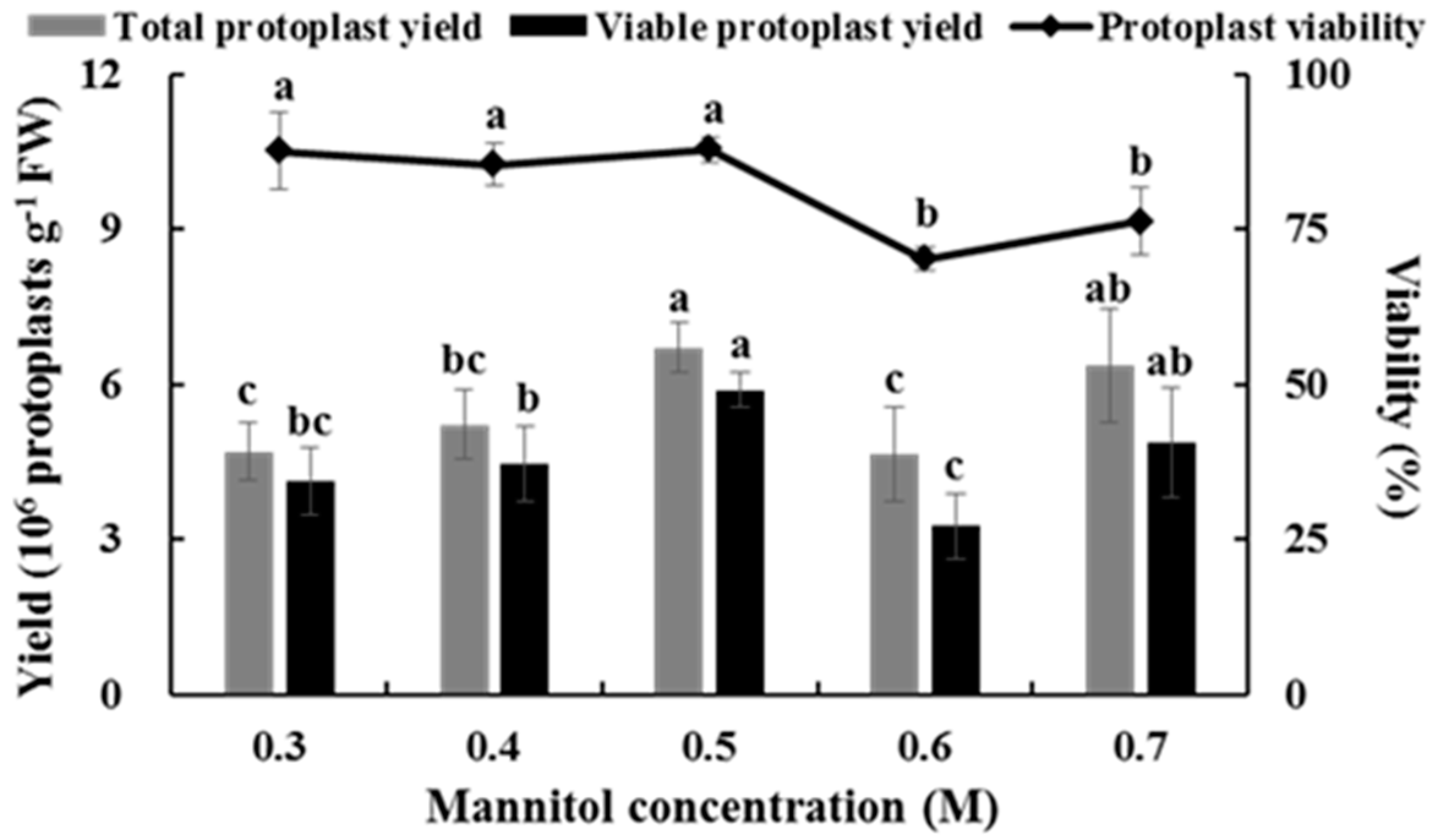
2.3. Effects of Mannitol Concentration on Isolating Protoplasts from Chinese Kale Hypocotyls
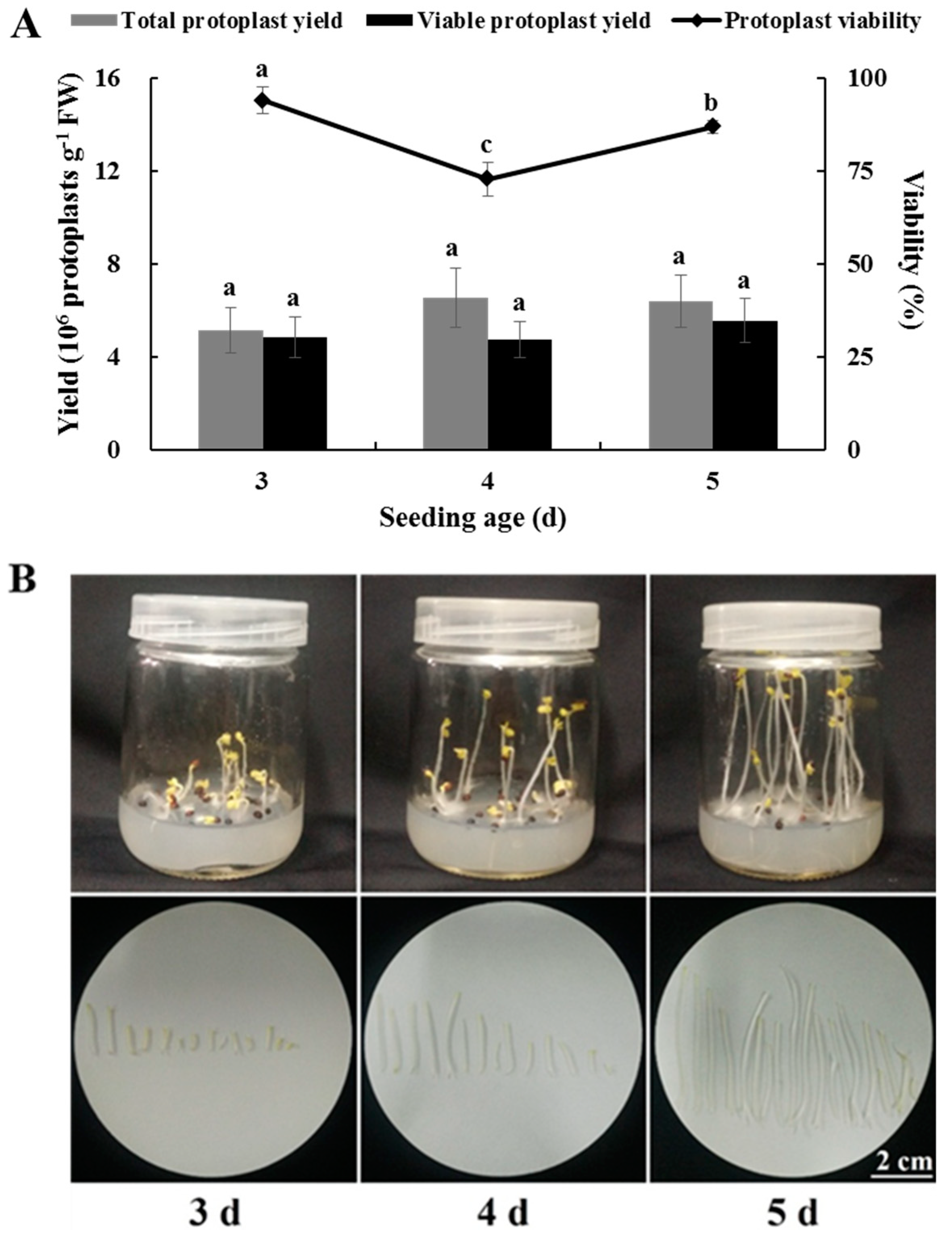
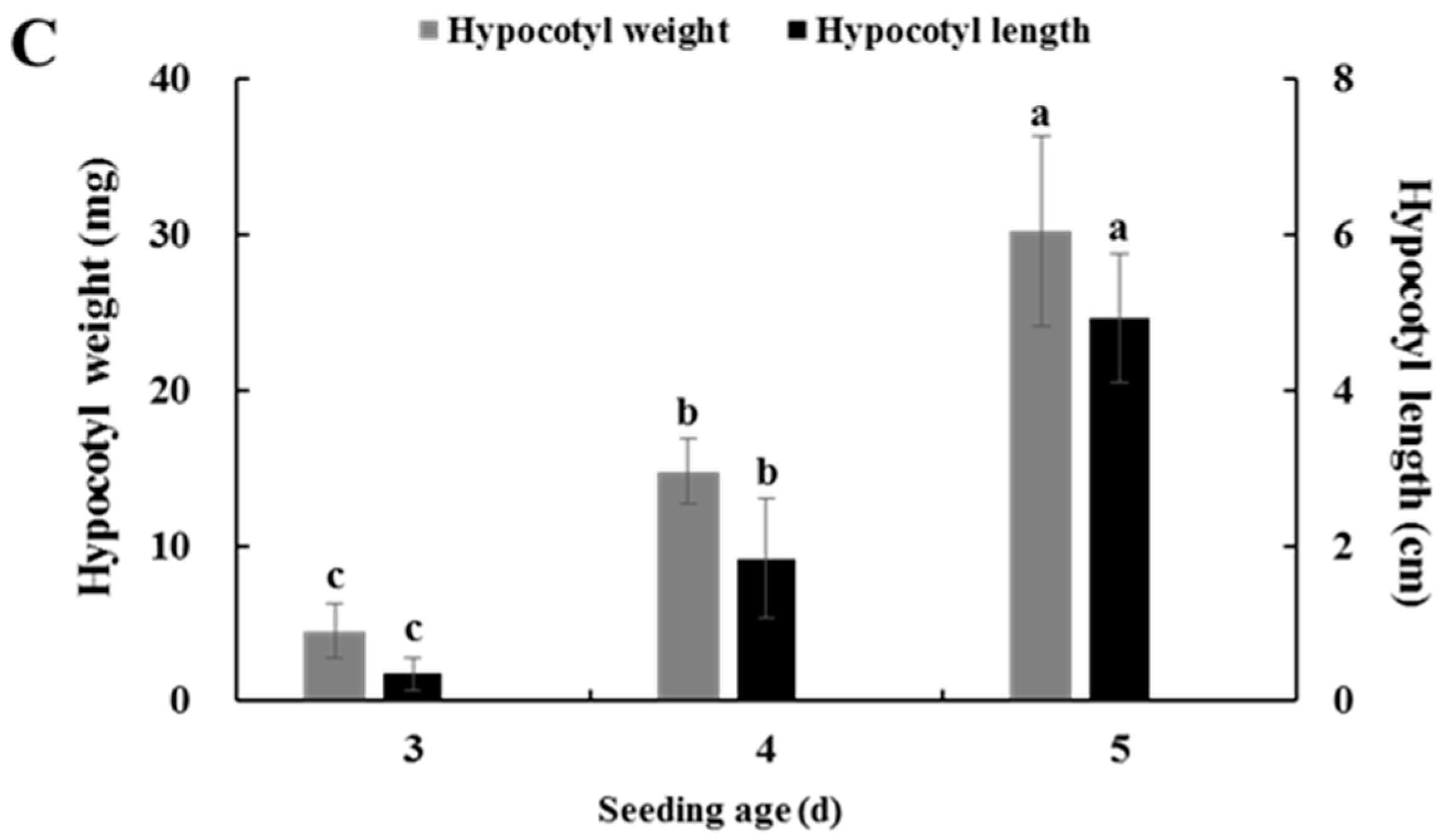
2.4. Effects of Seedling Age on Isolating Protoplasts from Chinese Kale Hypocotyls
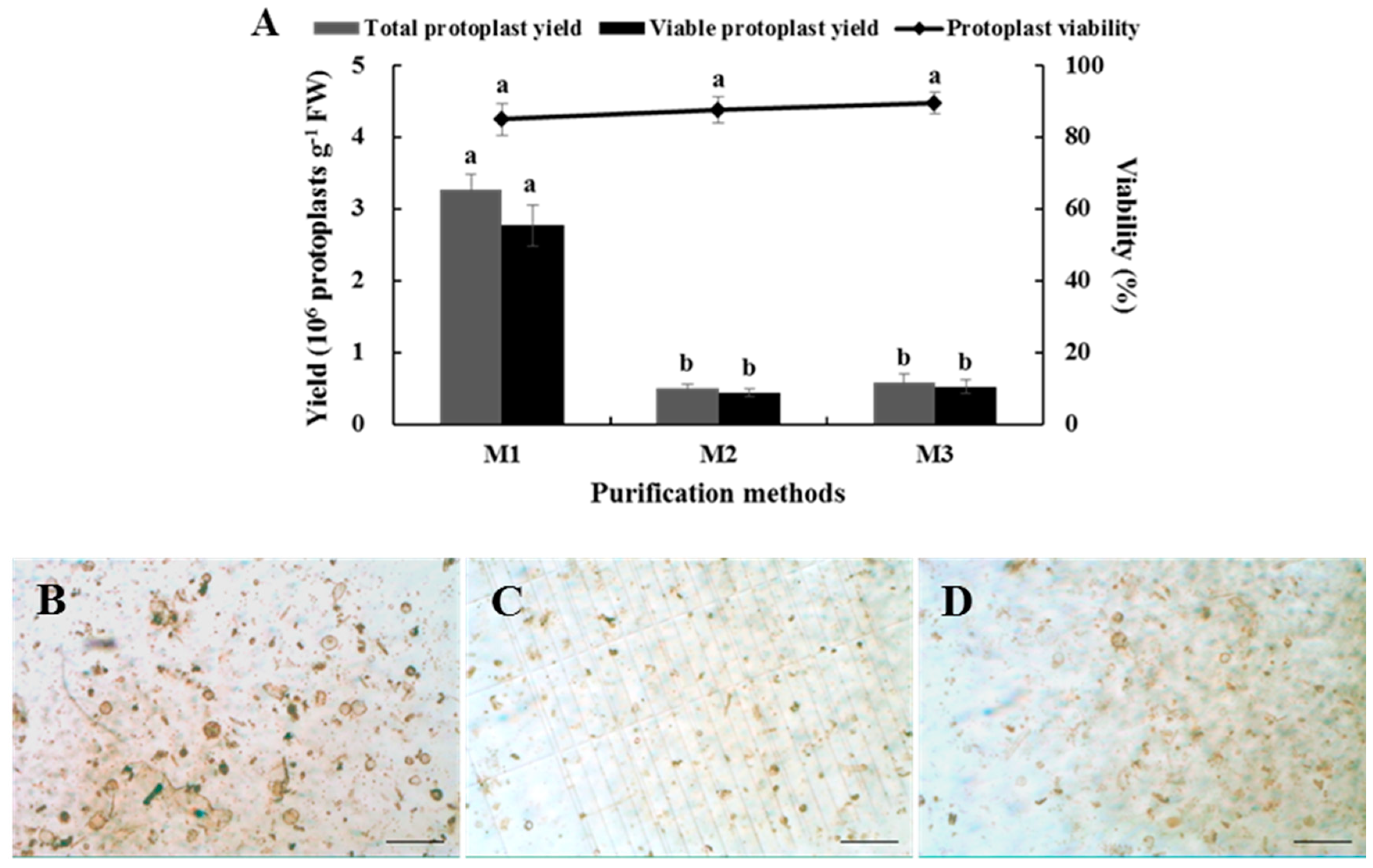
2.5. Effects of Purification Method on Isolating Protoplasts from Chinese Kale Hypocotyls
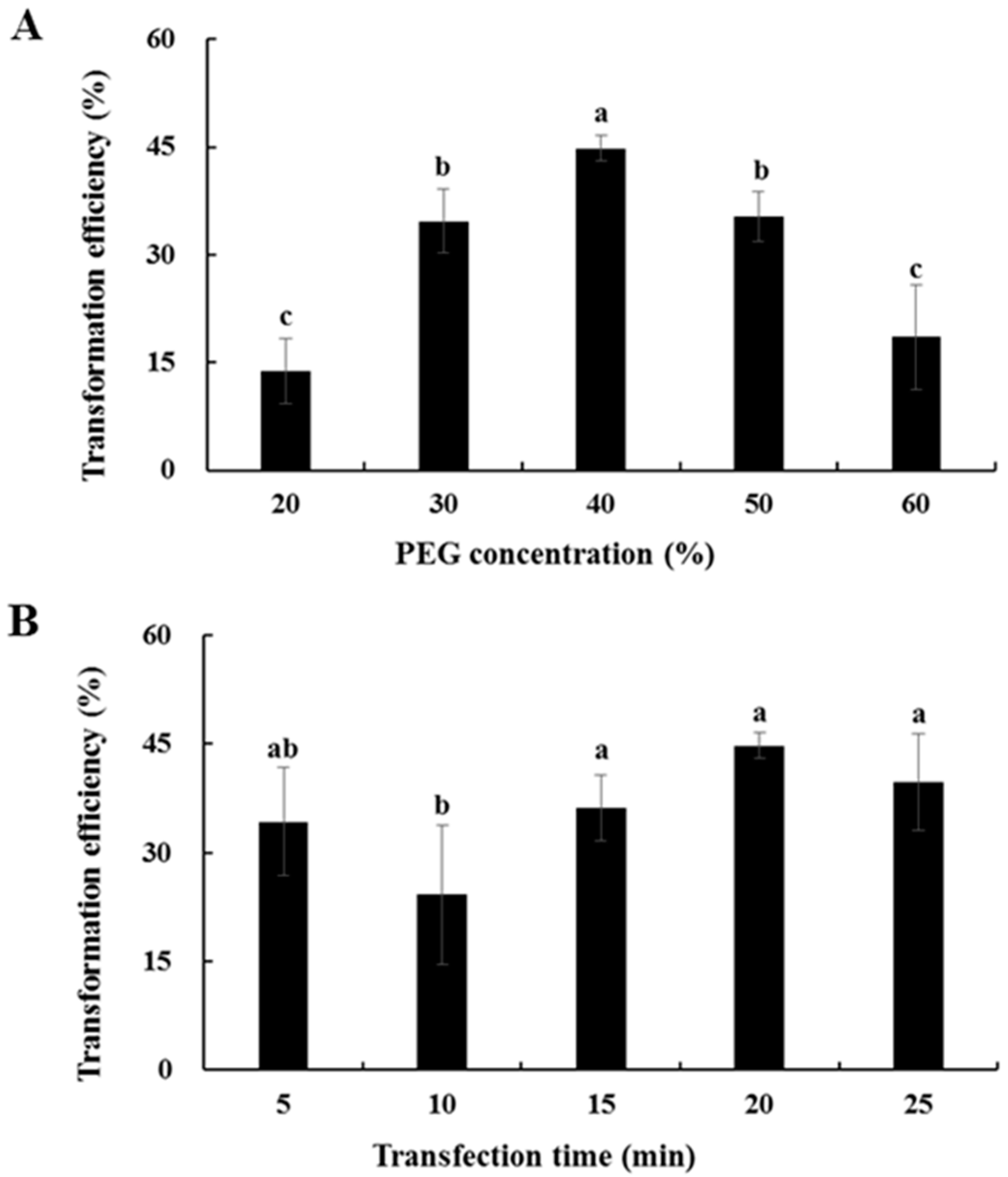
2.6. Transformation Efficiency of Chinese Kale Hypocotyl Protoplasts
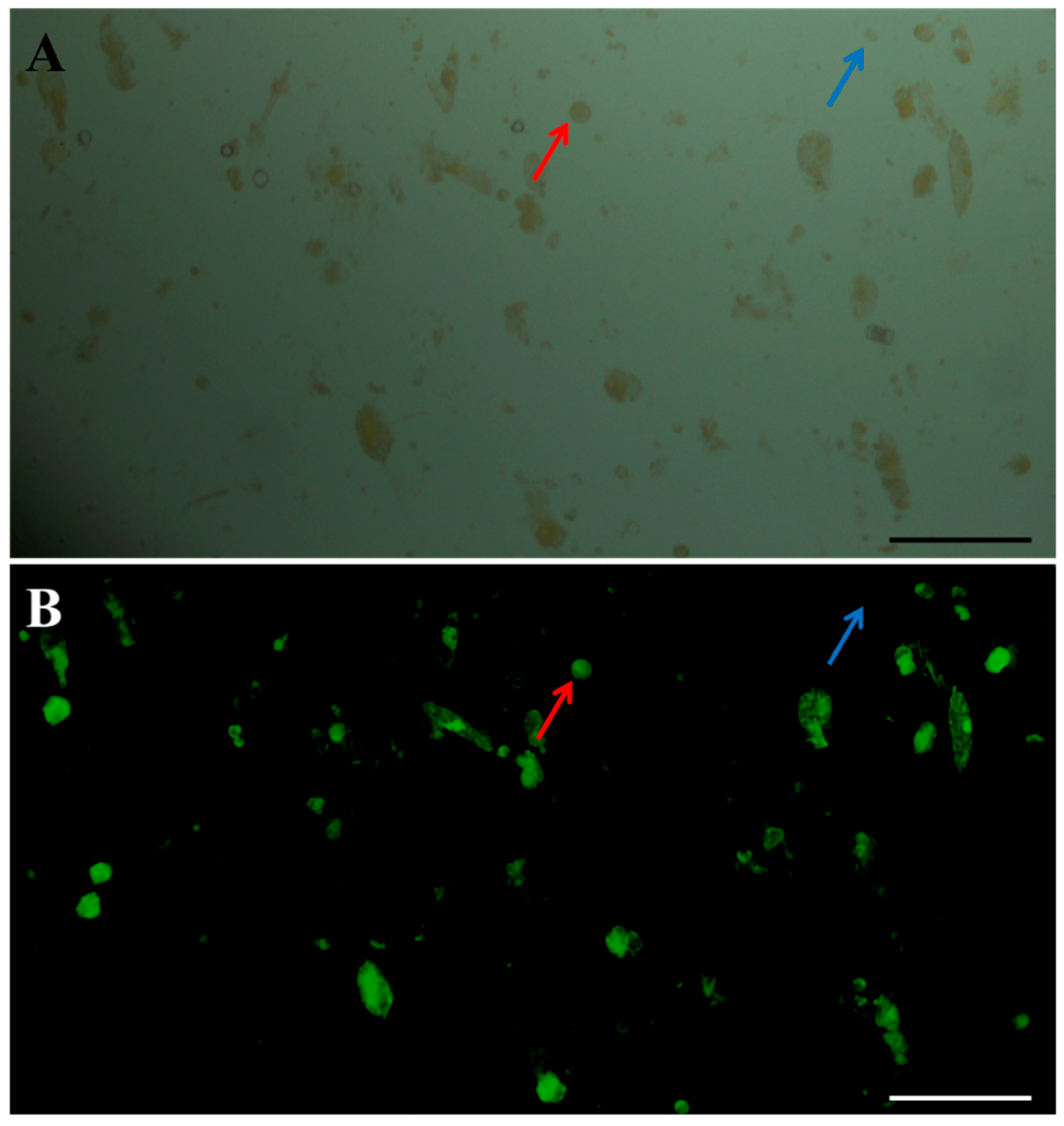
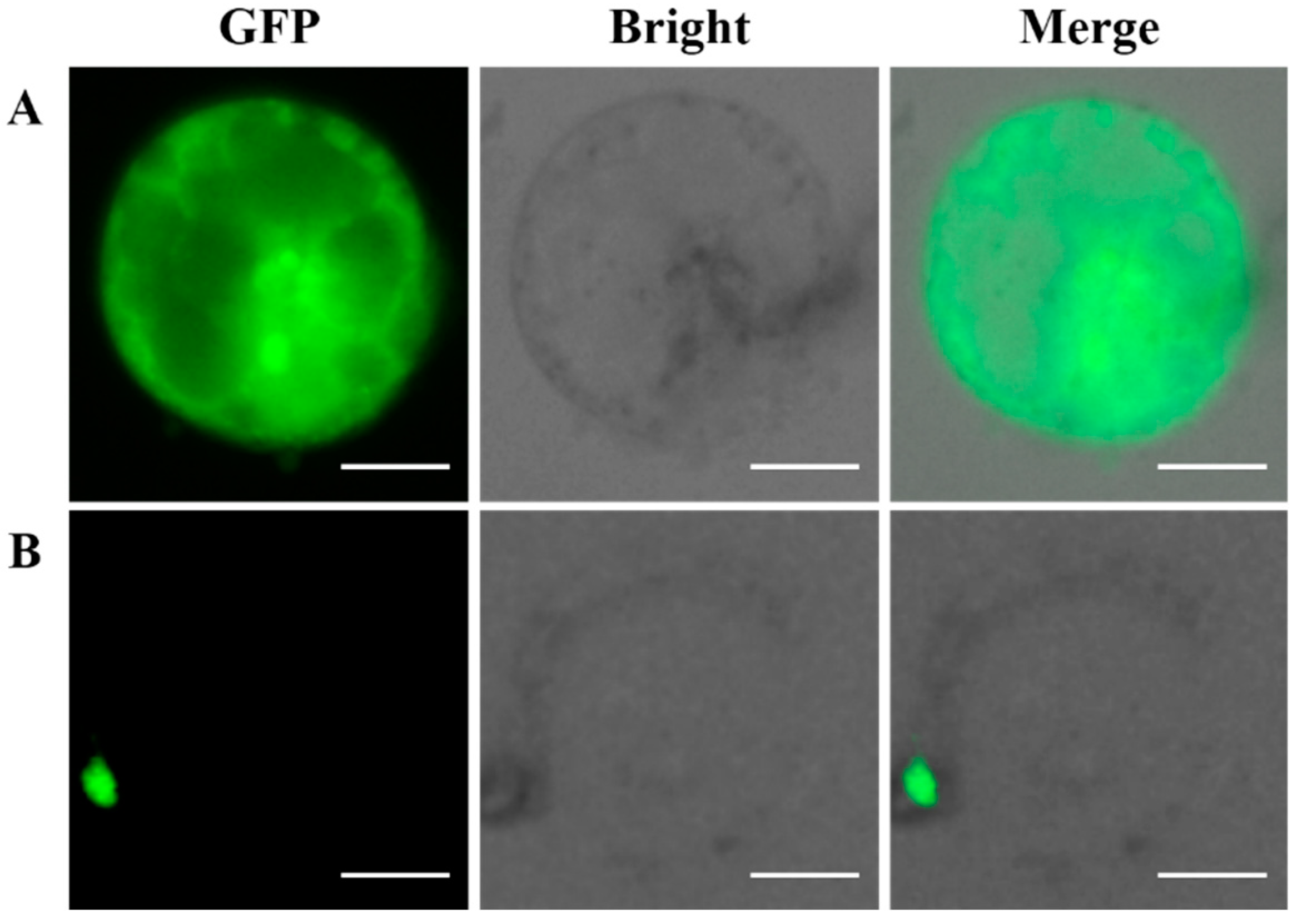
2.7. Subcellular Localization of the GFP-Fused BaMYB75 Protein in Chinese Kale Hypocotyl Protoplasts
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Protoplast Isolation and Purification
4.3. Protoplast Transformation
4.4. Subcellular Localization
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, H.Y.; Chen, J.C.; Fang, S.C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front. Plant. Sci. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hanzawa, Y. A simple method for isolation of soybean protoplasts and application to transient gene expression analyses. JOVE J. Vis. Exp. 2018, 131, e57258. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, T.M.; Fritz, C.; Lauter, A.; Schneider, S.; Fischer, C.; Danzberger, N.; Dietrich, P.; Sauer, N.; Stadler, R. Protoplast-esculin assay as a new method to assay plant sucrose transporters: Characterization of AtSUC6 and AtSUC7 sucrose uptake activity in Arabidopsis Col-0 ecotype. Front. Plant Sci. 2018, 9, 430. [Google Scholar] [CrossRef]
- Li, J.; Liao, X.; Zhou, S.; Liu, S.; Jiang, L.; Wang, G.D. Efficient protoplast isolation and transient gene expression system for Phalaenopsis, hybrid cultivar ‘Ruili Beauty’. In Vitro Cell. Dev. Biol. Plant 2018, 54, 87–93. [Google Scholar] [CrossRef]
- Slabas, A.R.; Powell, A.J.; Lloyd, C.W. An improved procedure for the isolation and purification of protoplasts from carrot suspension culture. Planta 1980, 147, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, D.G.; Moore, A.L. Isolation and purification of functionally intact chloroplasts from leaf tissue and leaf tissue protoplasts. Methods Mol. Biol. 1993, 19, 123–131. [Google Scholar]
- Zhao, J.; Xu, J.; Chen, B.; Cui, W.J.; Zhou, Z.J.; Son, X.J.; Chen, Z.; Zheng, H.Y.; Lin, L.; Peng, J.J.; et al. Characterization of proteins involved in chloroplast targeting disturbed by rice stripe virus by novel protoplast-chloroplast proteomics. Int. J. Mol. Sci. 2019, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Theologis, A. Transient transformation of Arabidopsis leaf protoplasts: A versatile experimental system to study gene expression. Plant. J. 1994, 5, 421–427. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.; Zhang, Y.; Zhang, R.; et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, H.; Zhao, P.; Wu, L.F.; Zhou, J.S.; Tang, Y.P.; Luo, S.C.; Chen, G.Y. Transformation of Phomopsis asparagi with green fluorescent protein using protoplasts. Can. J. Plant Pathol. 2018, 40, 254–260. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Wang, L.; Han, L.; Zhang, X.; Feng, J. Optimization of production conditions for protoplasts and polyethylene glycol-Mediated transformation of Gaeumannomyces tritici. Molecules 2018, 23, 1253. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, F.; Xiao, N.; Jiang, M.; Yuan, Q.; Xue, S.L.; Miao, H.Y.; Chen, Q.; Li, M.Y.; Wang, X.R.; et al. An efficient mesophyll protoplast isolation, purification and PEG-mediated transient gene expression for subcellular localization in Chinese kale. Sci. Hortic. 2018, 241, 187–193. [Google Scholar] [CrossRef]
- Yamada, Y.; Yang, Z.Q.; Tang, D.T. Plant regeneration from protoplast-derived callus of rice (Oryza sativa, L.). Plant. Cell Rep. 1986, 5, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.K.; Hammatt, N.; Power, J.B.; Davey, M.R. A reproducible procedure for plant regeneration from seedling hypocotyl protoplasts of Vigna sublobata, L. Plant Cell Rep. 1994, 14, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, P.; Fry, S.C.; Spyropoulos, C.G. Protoplast isolation and culture from carob (Ceratonia siliqua) hypocotyls: Ability of regenerated protoplasts to produce mannose-containing polysaccharides. Physiol. Plant. 2010, 130, 11–22. [Google Scholar] [CrossRef]
- Grzebelus, E.; Szklarczyk, M.; Baranski, R. An improved protocol for plant regeneration from leaf-and hypocotyl-derived protoplasts of carrot. Plant Cell Tissue Organ Cult. 2012, 109, 101–109. [Google Scholar] [CrossRef]
- Jin, H.; Martin, C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol. Biol. 1999, 41, 577–585. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Y.; Wu, Z.; Lu, W.J.; Han, J.L.; Wu, P.Z.; Chen, Y.P.; Li, M.R.; Jiang, H.W.; Wu, G.J. Genome-wide analysis of the MYB gene family in physic nut (Jatropha curcas L.). Gene 2015, 1, 63–71. [Google Scholar] [CrossRef]
- Yao, L.P.; Liao, X.; Gan, Z.Z.; Peng, X.; Wang, P.; Li, S.J.; Li, T.H. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Sci. Hortic. 2016, 209, 14–21. [Google Scholar] [CrossRef]
- Zhao, F.L.; Li, Y.J.; Hu, Y.; Gao, Y.R.; Zang, X.W.; Ding, Q.; Wang, Y.J.; Wen, Y.Q. A highly efficient grapevine mesophyll protoplast system for transient gene expression and the study of disease resistance proteins. Plant Cell Tissue Organ Cult. 2016, 125, 43–57. [Google Scholar] [CrossRef]
- Cheng, X.H.; Wang, P.F.; Peng, Y.F.; Chen, M.Q.; Tian, H.Y. Analysis of chemical components in tobacco stem and correlation with crotonaldehyde. Chin. Tob. Sci. 2018, 39, 85–90. [Google Scholar]
- Zhao, Y.Y.; Chen, S.S.; Fang, K.F.; Xing, Y.; Cao, Q.Q. Isolation and purification of protoplasts from chestnut hypocotyl. J. Fruit Sci. 2013, 30, 994–997. [Google Scholar]
- Huang, H.Y.; Wang, Z.Y.; Cheng, J.T.; Zhao, W.C.; Li, X.; Wang, H.Y.; Zhang, Z.X.; Sui, X.L. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. 2013, 150, 206–212. [Google Scholar] [CrossRef]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis, Sandwich-a simpler Arabidopsis, protoplast isolation method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Millam, S.; Burns, A.T.H.; Hocking, T.J. A comparative assessment of purification techniques for mesophyll protoplasts of Brassica napus L. Plant Cell Tissue Organ Cult. 1991, 24, 43–47. [Google Scholar] [CrossRef]
- Zhang, C.; Zong, H.; Zhuge, B.; Lu, X.Y.; Fang, H.Y.; Zhu, J.L.; Zhu, J. Protoplast preparation and polyethylene glycol (PEG)-mediated transformation of Candida glycerinogenes. Biotechnol. Bioprocess Eng. 2016, 21, 95–102. [Google Scholar] [CrossRef]
- Choury, Z.; Meschini, R.; Dell’Orso, A.; Fardusi, M.J.; Mugnozza, G.S.; Kuzminsky, E. Optimized conditions for the isolation of mesophyll protoplasts along the growing season from Arbutus unedo and their use in single cell gel electrophoresis. Plant Cell Tissue Organ Cult. 2017, 132, 535–543. [Google Scholar] [CrossRef]
- Hammatt, N.; Davey, M.R. Isolation and culture of soybean hypocotyl protoplasts. In Vitro Cell. Dev. Biol. 1988, 24, 601–604. [Google Scholar] [CrossRef]
- Oi, T.; Enomoto, S.; Nakao, T.; Arai, S.; Yamane, K.; Taniguchi, M. Three-dimensional intracellular structure of a whole rice mesophyll cell observed with FIB-SEM. Ann. Bot. 2017, 120, 21. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, H.W.; Zhou, R.Y.; Tian, Z.H. Isolation and culture of hypocotyls protoplast of Kenaf. Hubei Agric. Sci. 2006, 45, 149–152. [Google Scholar]
- Ma, Z.Q. Study on hypocotyl protoplast isolation of Matthiola incana. Yunnan Agric. Univ. 2005, 20, 155–158. [Google Scholar]
- Li, G.; Li, B.Y.; Wang, W.H.; Yue, Z.C.; Zhong, X.M.; Hou, X.L. Plant regeneration from hypocotyl protoplast culture of Cabbage (Brassica oleracea L. var. capitata). Acta Bot. Boreal. Occident. Sin. 2012, 32, 2438–2443. [Google Scholar]
- Yuan, B.; Pan, X.J. Protoplast isolation and purification of Vitis quinquangularis Rehd. J. Southwest Univ. 2010, 32, 97–101. [Google Scholar]
- Zhang, Z.R.; Chen, P. Isolation, purification and transient expression of mesophyll protoplast in Tartary Buckwheat. Acta Bot. Boreal. Occident. Sin. 2016, 36, 183–189. [Google Scholar]
- Nanjareddy, K.; Arthikala, M.K.; Blanco, L.; Arellano, E.S.; Lara, M. Protoplast isolation, transient transformation of leaf mesophyll protoplasts and improved Agrobacterium-mediated leaf disc infiltration of Phaseolus vulgaris: Tools for rapid gene expression analysis. BMC Biotechnol. 2016, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Wetzstein, H.Y. Protoplast isolation and callus production from leaves of tissue-cultured Vitis spp. Plant Cell Rep. 1988, 7, 531. [Google Scholar] [CrossRef]
- Larkin, P.J. Purification and viability determinations of plant protoplasts. Planta 1976, 128, 213–216. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Yuan, Q.; Zheng, H.; Liang, S.; Jiang, M.; Wang, M.-M.; Chen, Q.; Li, M.-Y.; Zhang, Y.; Luo, Y.; et al. An Efficient and Economical Protocol for Isolating, Purifying and PEG-Mediated Transient Gene Expression of Chinese Kale Hypocotyl Protoplasts. Plants 2019, 8, 385. https://doi.org/10.3390/plants8100385
Sun B, Yuan Q, Zheng H, Liang S, Jiang M, Wang M-M, Chen Q, Li M-Y, Zhang Y, Luo Y, et al. An Efficient and Economical Protocol for Isolating, Purifying and PEG-Mediated Transient Gene Expression of Chinese Kale Hypocotyl Protoplasts. Plants. 2019; 8(10):385. https://doi.org/10.3390/plants8100385
Chicago/Turabian StyleSun, Bo, Qiao Yuan, Hao Zheng, Sha Liang, Min Jiang, Mei-Mei Wang, Qing Chen, Meng-Yao Li, Yong Zhang, Ya Luo, and et al. 2019. "An Efficient and Economical Protocol for Isolating, Purifying and PEG-Mediated Transient Gene Expression of Chinese Kale Hypocotyl Protoplasts" Plants 8, no. 10: 385. https://doi.org/10.3390/plants8100385
APA StyleSun, B., Yuan, Q., Zheng, H., Liang, S., Jiang, M., Wang, M.-M., Chen, Q., Li, M.-Y., Zhang, Y., Luo, Y., Gong, R.-G., Zhang, F., & Tang, H.-R. (2019). An Efficient and Economical Protocol for Isolating, Purifying and PEG-Mediated Transient Gene Expression of Chinese Kale Hypocotyl Protoplasts. Plants, 8(10), 385. https://doi.org/10.3390/plants8100385





