Patterning the Axes: A Lesson from the Root
Abstract
:1. Introduction
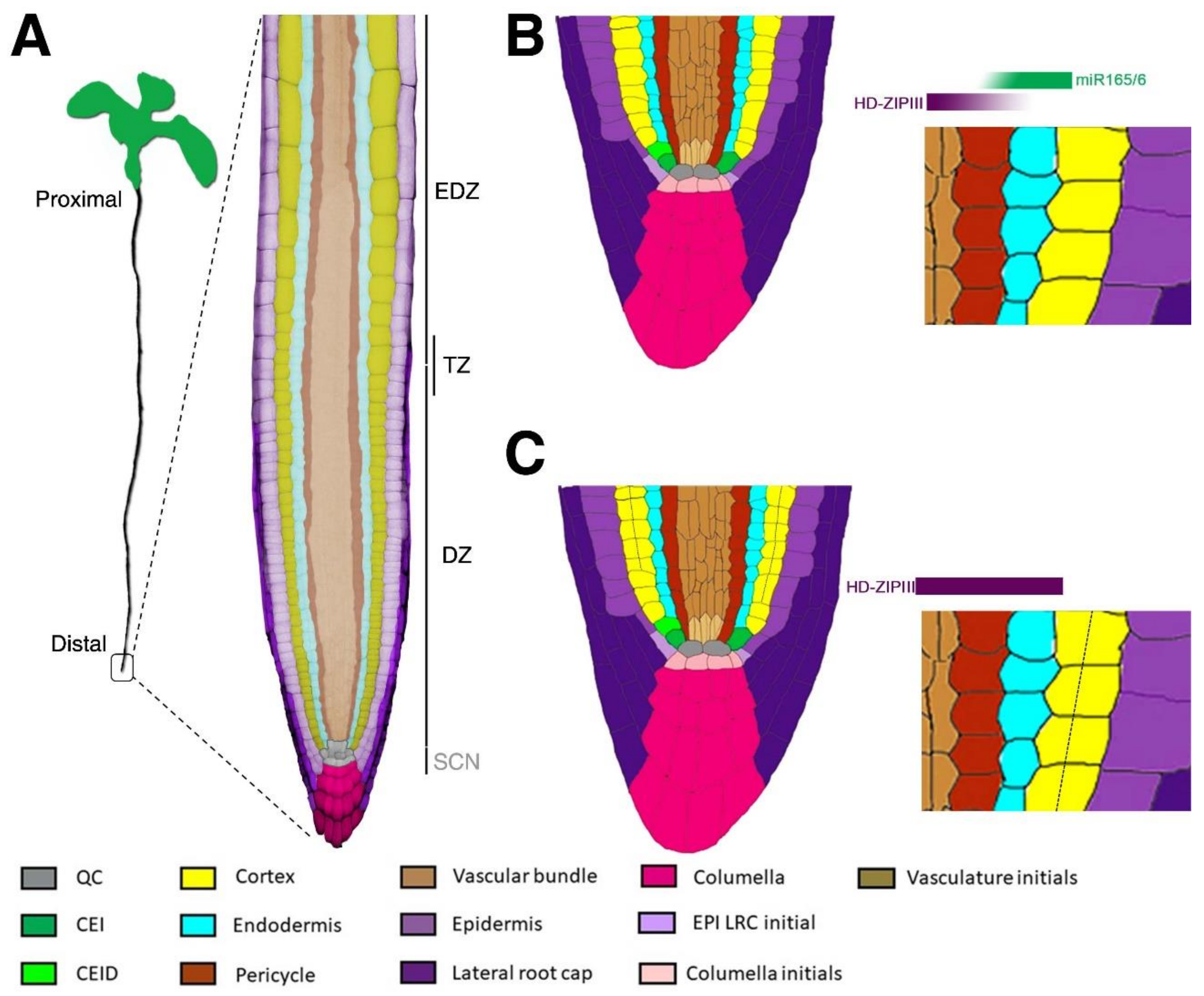
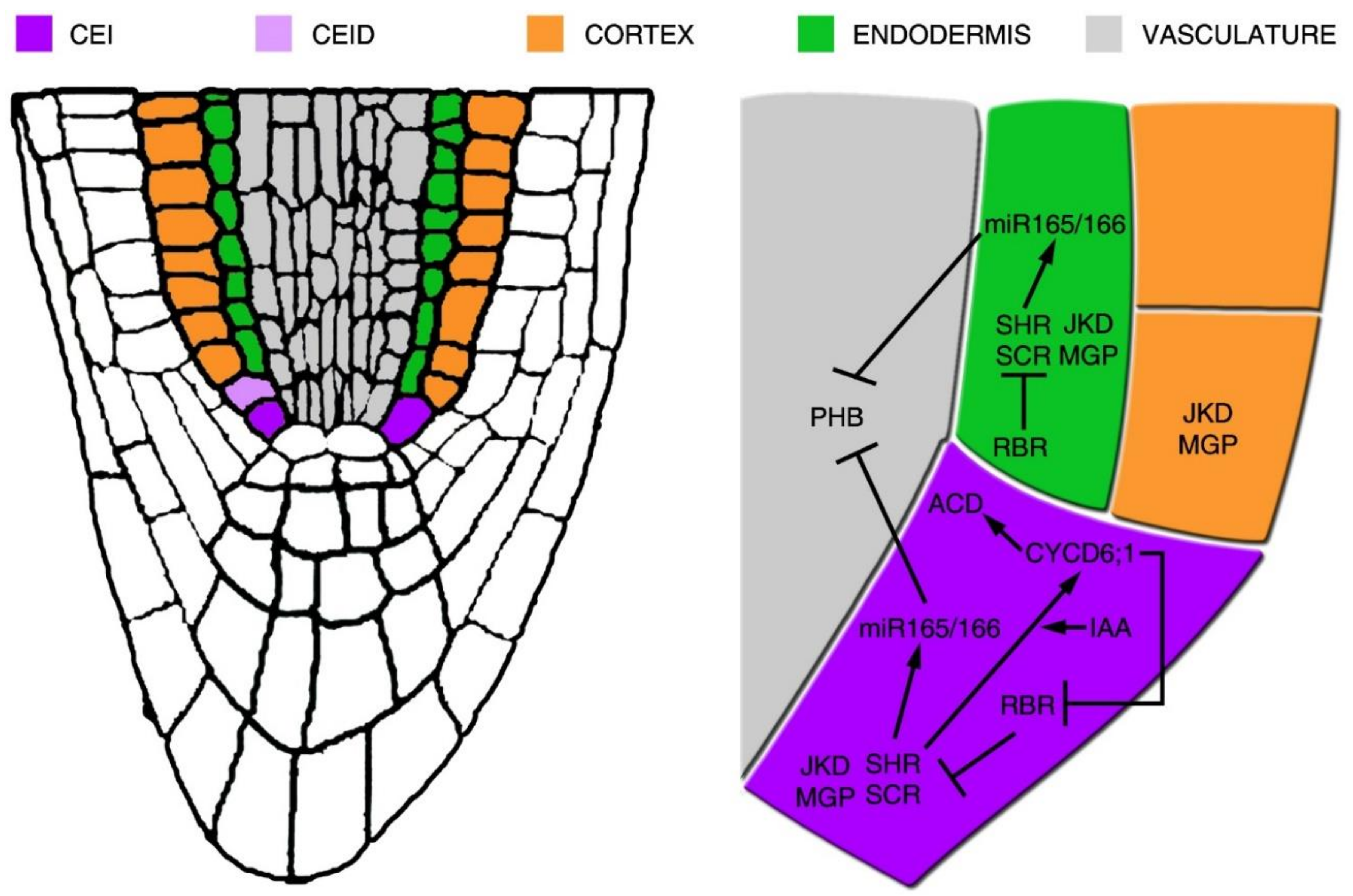
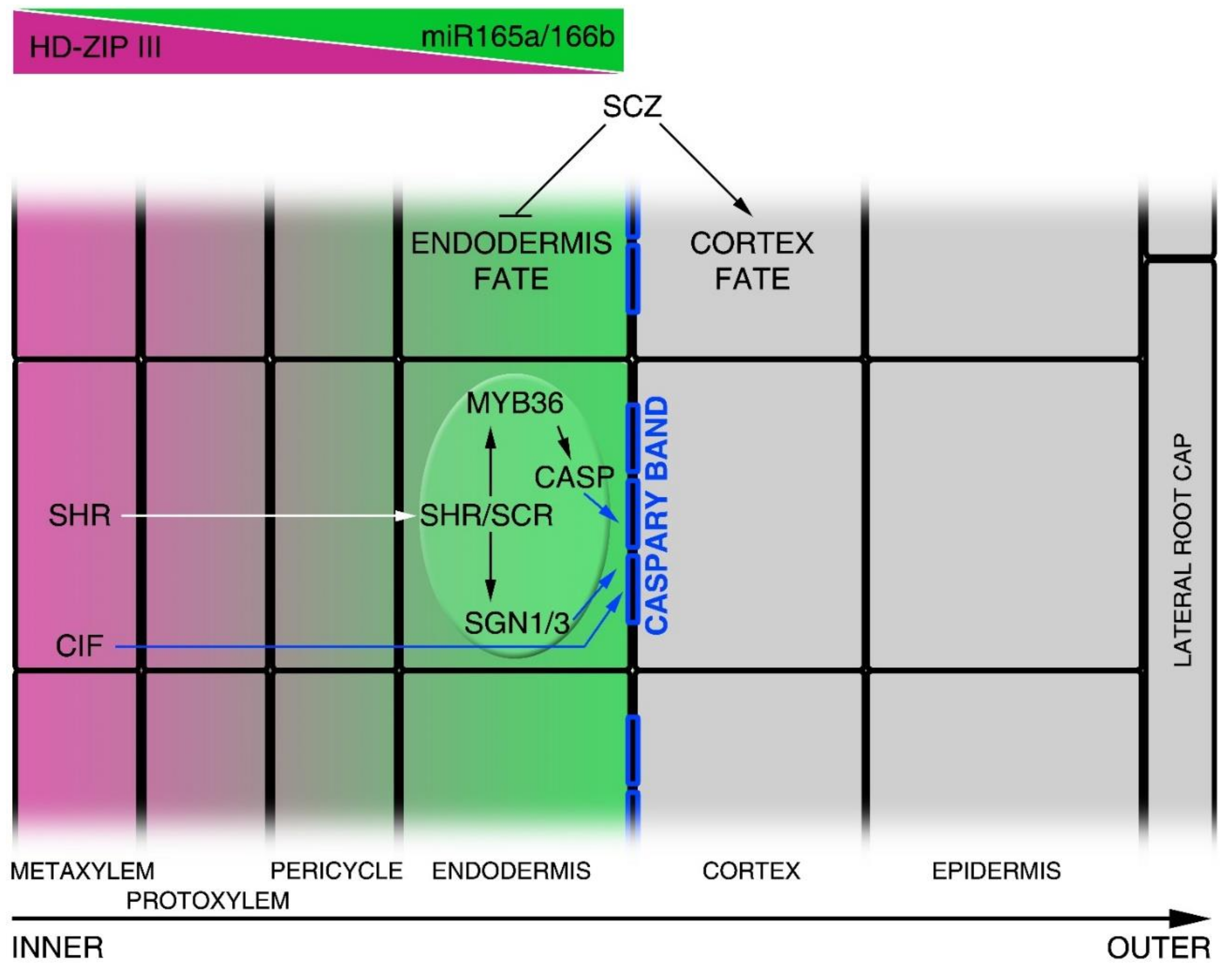
2. Root Radial Axis
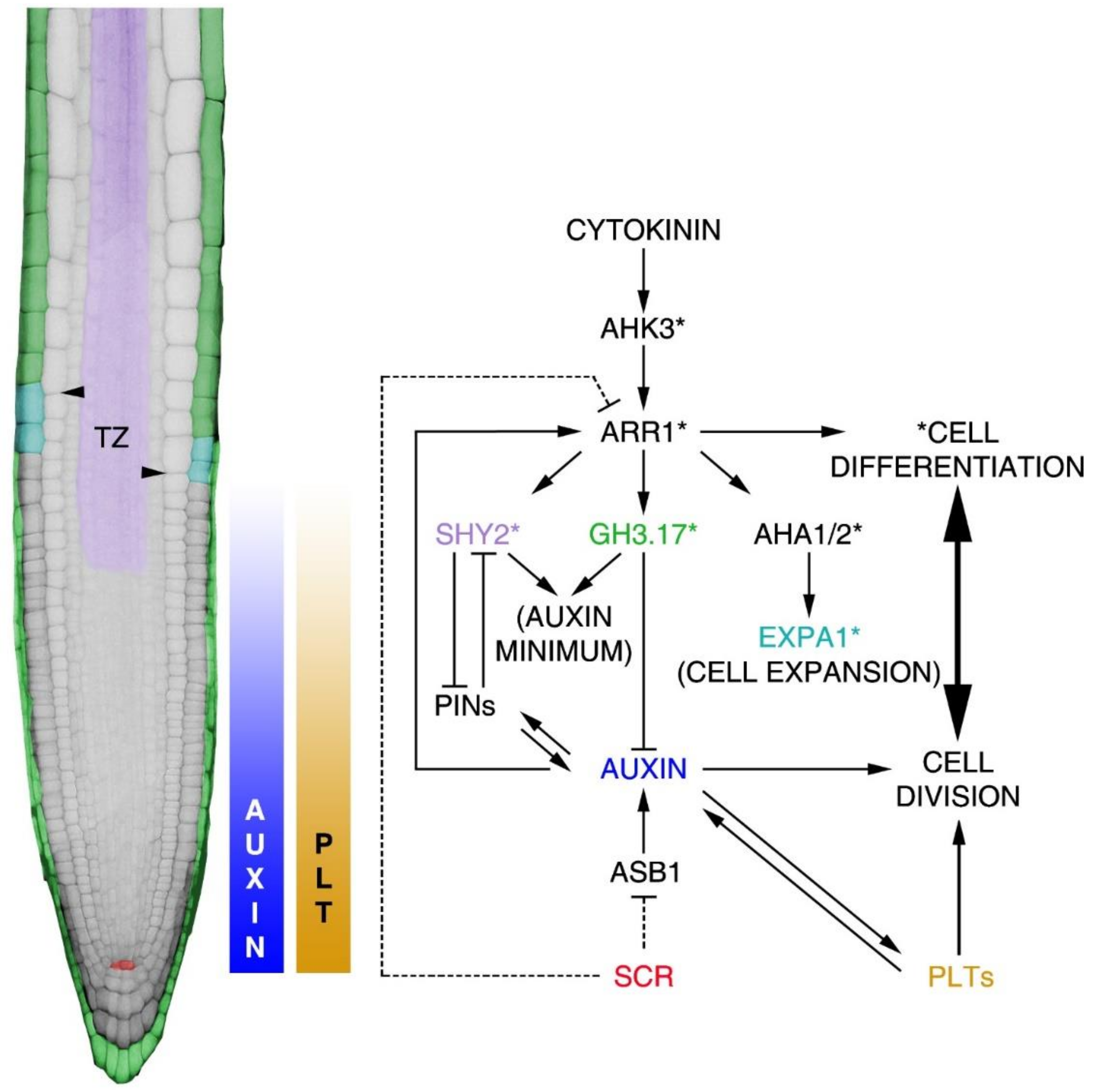
3. Root Proximodistal Axis
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabin, C.; Wolpert, L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007, 21, 1433–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuniga, A. Next generation limb development and evolution: Old questions, new perspectives. Development 2015, 142, 3810–3820. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [PubMed]
- Scheres, B.; Benfey, P.; Dolan, L. Root development. Arabidopsis Book 2002, 1, e0101. [Google Scholar] [CrossRef] [PubMed]
- Heidstra, R.; Sabatini, S. Plant and animal stem cells: Similar yet different. Nat. Rev. Mol. Cell Biol. 2014, 15, 301–312. [Google Scholar] [CrossRef]
- Ten Hove, C.A.; Lu, K.J.; Weijers, D. Building a plant: Cell fate specification in the early Arabidopsis embryo. Development 2015, 142, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. Scarecrow is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef]
- Perilli, S.; Di Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2012, 15, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, R.; Sabatini, S. Developmental analysis of Arabidopsis root meristem. Methods Mol. Biol. 2018, 1761, 33–45. [Google Scholar]
- Drapek, C.; Sparks, E.E.; Benfey, P.N. Uncovering gene regulatory networks controlling plant cell differentiation. Trends Genet. 2017, 33, 529–539. [Google Scholar] [CrossRef]
- Benfey, P.N. Defining the path from stem cells to differentiated tissue. Curr. Top. Dev. Biol. 2016, 116, 35–43. [Google Scholar] [PubMed]
- Dello Ioio, R.; Linhares, F.S.; Sabatini, S. Emerging role of cytokinin as a regulator of cellular differentiation. Curr. Opin. Plant Biol. 2008, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kumpf, R.P.; Nowack, M.K. The root cap: A short story of life and death. J. Exp. Bot. 2015, 66, 5651–5662. [Google Scholar] [CrossRef] [PubMed]
- Pauluzzi, G.; Divol, F.; Puig, J.; Guiderdoni, E.; Dievart, A.; Perin, C. Surfing along the root ground tissue gene network. Dev. Biol. 2012, 365, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lim, J. Control of asymmetric cell divisions during root ground tissue maturation. Mol. Cells 2016, 39, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.K.; Ten Hove, C.A.; Xiang, D.; Williams, N.; Lopez, L.G.; Yoshida, S.; Smit, M.; Datla, R.; Weijers, D. Auxin response cell-autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proc. Natl. Acad. Sci. USA 2017, 114, E2533–E2539. [Google Scholar] [CrossRef] [PubMed]
- Heidstra, R.; Welch, D.; Scheres, B. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis scarecrow action in asymmetric cell division. Genes Dev. 2004, 18, 1964–1969. [Google Scholar] [CrossRef]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.T.; Benfey, P.N. The short-root gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef]
- Nakajima, K.; Sena, G.; Nawy, T.; Benfey, P.N. Intercellular movement of the putative transcription factor shr in root patterning. Nature 2001, 413, 307–311. [Google Scholar] [CrossRef]
- Clark, N.M.; Hinde, E.; Winter, C.M.; Fisher, A.P.; Crosti, G.; Blilou, I.; Gratton, E.; Benfey, P.N.; Sozzani, R. Tracking transcription factor mobility and interaction in Arabidopsis roots with fluorescence correlation spectroscopy. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Vaten, A.; Dettmer, J.; Wu, S.; Stierhof, Y.D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R.; et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, H.F.; Heidstra, R. Transcription factor dosage: More or less sufficient for growth. Curr. Opin. Plant Biol. 2018, 45, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Di Ruocco, G.; Di Mambro, R.; Dello Ioio, R. Building the differences: A case for the ground tissue patterning in plants. Proc. Biol. Sci. 2018, 285, 20181746. [Google Scholar] [CrossRef] [PubMed]
- Sena, G.; Jung, J.W.; Benfey, P.N. A broad competence to respond to short root revealed by tissue-specific ectopic expression. Development 2004, 131, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Goedhart, J.; Schneijderberg, M.; Terpstra, I.; Shimotohno, A.; Bouchet, B.P.; Akhmanova, A.; Gadella, T.W., Jr.; Heidstra, R.; Scheres, B.; et al. Scarecrow-like23 and scarecrow jointly specify endodermal cell fate but distinctly control short-root movement. Plant J. 2015, 84, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Smet, W.; Cruz-Ramirez, A.; Castelijns, B.; de Jonge, W.; Mahonen, A.P.; Bouchet, B.P.; Perez, G.S.; Akhmanova, A.; Scheres, B.; et al. Arabidopsis bird zinc finger proteins jointly stabilize tissue boundaries by confining the cell fate regulator short-root and contributing to fate specification. Plant Cell 2015, 27, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting shr movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef]
- Welch, D.; Hassan, H.; Blilou, I.; Immink, R.; Heidstra, R.; Scheres, B. Arabidopsis jackdaw and magpie zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting short-root action. Genes Dev. 2007, 21, 2196–2204. [Google Scholar] [CrossRef]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Postma, M.; Zhou, W.; Goedhart, J.; Sanchez-Perez, M.I.; Gadella, T.W.J.; Simon, R.; Scheres, B.; et al. In vivo fret-flim reveals cell-type-specific protein interactions in Arabidopsis roots. Nature 2017, 548, 97–102. [Google Scholar] [CrossRef]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Smet, W.; Du, Y.; Gadella, T.W.J., Jr.; Goedhart, J.; Scheres, B.; Blilou, I. Optimizing fret-flim labeling conditions to detect nuclear protein interactions at native expression levels in living Arabidopsis roots. Front. Plant Sci. 2018, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Risueno, M.A.; Sozzani, R.; Yardimci, G.G.; Petricka, J.J.; Vernoux, T.; Blilou, I.; Alonso, J.; Winter, C.M.; Ohler, U.; Scheres, B.; et al. Transcriptional control of tissue formation throughout root development. Science 2015, 350, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.A.; Busch, W.; Van Norman, J.M.; Vernoux, T.; Brady, S.M.; Dewitte, W.; Murray, J.A.; Benfey, P.N. Spatiotemporal regulation of cell-cycle genes by shortroot links patterning and growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Perilli, S.; Perez-Perez, J.M.; Di Mambro, R.; Peris, C.L.; Diaz-Trivino, S.; Del Bianco, M.; Pierdonati, E.; Moubayidin, L.; Cruz-Ramirez, A.; Costantino, P.; et al. Retinoblastoma-related protein stimulates cell differentiation in the Arabidopsis root meristem by interacting with cytokinin signaling. Plant Cell 2013, 25, 4469–4478. [Google Scholar] [CrossRef] [PubMed]
- Wildwater, M.; Campilho, A.; Perez-Perez, J.M.; Heidstra, R.; Blilou, I.; Korthout, H.; Chatterjee, J.; Mariconti, L.; Gruissem, W.; Scheres, B. The retinoblastoma-related gene regulates stem cell maintenance in Arabidopsis roots. Cell 2005, 123, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramirez, A.; Diaz-Trivino, S.; Blilou, I.; Grieneisen, V.A.; Sozzani, R.; Zamioudis, C.; Miskolczi, P.; Nieuwland, J.; Benjamins, R.; Dhonukshe, P.; et al. A bistable circuit involving scarecrow-retinoblastoma integrates cues to inform asymmetric stem cell division. Cell 2012, 150, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, W.; Chen, Q.; Fang, M.; Zheng, S.; Scheres, B.; Li, C. Mediator subunit med31 is required for radial patterning of Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2018, 115, E5624–E5633. [Google Scholar] [CrossRef]
- Wu, S.; Lee, C.M.; Hayashi, T.; Price, S.; Divol, F.; Henry, S.; Pauluzzi, G.; Perin, C.; Gallagher, K.L. A plausible mechanism, based upon short-root movement, for regulating the number of cortex cell layers in roots. Proc. Natl. Acad. Sci. USA 2014, 111, 16184–16189. [Google Scholar] [CrossRef]
- Koizumi, K.; Hayashi, T.; Wu, S.; Gallagher, K.L. The short-root protein acts as a mobile, dose-dependent signal in patterning the ground tissue. Proc. Natl. Acad. Sci. USA 2012, 109, 13010–13015. [Google Scholar] [CrossRef]
- Mylona, P.; Linstead, P.; Martienssen, R.; Dolan, L. Schizoriza controls an asymmetric cell division and restricts epidermal identity in the Arabidopsis root. Development 2002, 129, 4327–4334. [Google Scholar]
- Ten Hove, C.A.; Willemsen, V.; de Vries, W.J.; van Dijken, A.; Scheres, B.; Heidstra, R. Schizoriza encodes a nuclear factor regulating asymmetry of stem cell divisions in the Arabidopsis root. Curr. Biol. 2010, 20, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Pernas, M.; Ryan, E.; Dolan, L. Schizoriza controls tissue system complexity in plants. Curr. Biol. 2010, 20, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vaten, A.; Thitamadee, S.; et al. Cell signalling by microrna165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Muraro, D.; Mellor, N.; Pound, M.P.; Help, H.; Lucas, M.; Chopard, J.; Byrne, H.M.; Godin, C.; Hodgman, T.C.; King, J.R.; et al. Integration of hormonal signaling networks and mobile micrornas is required for vascular patterning in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2014, 111, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benkova, E.; Mahonen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Miyashima, S.; Koi, S.; Hashimoto, T.; Nakajima, K. Non-cell-autonomous microrna165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 2011, 138, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Di Ruocco, G.; Bertolotti, G.; Pacifici, E.; Polverari, L.; Tsiantis, M.; Sabatini, S.; Costantino, P.; Dello Ioio, R. Differential spatial distribution of mir165/6 determines variability in plant root anatomy. Development 2018, 145. [Google Scholar] [CrossRef]
- Miyashima, S.; Hashimoto, T.; Nakajima, K. Argonaute1 acts in Arabidopsis root radial pattern formation independently of the shr/scr pathway. Plant Cell Physiol. 2009, 50, 626–634. [Google Scholar] [CrossRef]
- Muller, C.J.; Valdes, A.E.; Wang, G.; Ramachandran, P.; Beste, L.; Uddenberg, D.; Carlsbecker, A. Phabulosa mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol. 2016, 170, 956–970. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Gu, X.; Xu, C.; Qi, S.; Wang, H.; Zhong, F.; Baskin, T.I.; Rahman, A.; Wu, S. Construction of a functional casparian strip in non-endodermal lineages is orchestrated by two parallel signaling systems in Arabidopsis thaliana. Curr. Biol. 2018, 28, 2777.e2.–2786.e2. [Google Scholar] [CrossRef]
- Drapek, C.; Sparks, E.E.; Marhavy, P.; Taylor, I.; Andersen, T.G.; Hennacy, J.H.; Geldner, N.; Benfey, P.N. Minimum requirements for changing and maintaining endodermis cell identity in the Arabidopsis root. Nat. Plants 2018, 4, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Borghi, M.; Wang, P.; Danku, J.M.; Kalmbach, L.; Hosmani, P.S.; Naseer, S.; Fujiwara, T.; Geldner, N.; Salt, D.E. The myb36 transcription factor orchestrates casparian strip formation. Proc. Natl. Acad. Sci. USA 2015, 112, 10533–10538. [Google Scholar] [CrossRef] [PubMed]
- Roppolo, D.; De Rybel, B.; Denervaud Tendon, V.; Pfister, A.; Alassimone, J.; Vermeer, J.E.; Yamazaki, M.; Stierhof, Y.D.; Beeckman, T.; Geldner, N. A novel protein family mediates casparian strip formation in the endodermis. Nature 2011, 473, 380–383. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef]
- Leyser, O. Auxin distribution and plant pattern formation: How many angels can dance on the point of pin? Cell 2005, 121, 819–822. [Google Scholar] [CrossRef]
- Yoshida, S.; Saiga, S.; Weijers, D. Auxin regulation of embryonic root formation. Plant Cell Physiol. 2013, 54, 325–332. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The pin auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Wisniewska, J.; Xu, J.; Seifertova, D.; Brewer, P.B.; Ruzicka, K.; Blilou, I.; Rouquie, D.; Benkova, E.; Scheres, B.; Friml, J. Polar pin localization directs auxin flow in plants. Science 2006, 312, 883. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jurgens, G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Weijers, D.; Schlereth, A.; Ehrismann, J.S.; Schwank, G.; Kientz, M.; Jurgens, G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell 2006, 10, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T.; Mayer, U.; Jurgens, G. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 1999, 126, 1387–1395. [Google Scholar] [PubMed]
- Hamann, T.; Benkova, E.; Baurle, I.; Kientz, M.; Jurgens, G. The Arabidopsis bodenlos gene encodes an auxin response protein inhibiting monopteros-mediated embryo patterning. Genes Dev. 2002, 16, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The plethora genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. Plethora proteins as dose-dependent master regulators of Arabidopsis root development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Nawy, T.; Bayer, M.; Mravec, J.; Friml, J.; Birnbaum, K.D.; Lukowitz, W. The gata factor hanaba taranu is required to position the proembryo boundary in the early Arabidopsis embryo. Dev. Cell 2010, 19, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Grigg, S.P.; Galinha, C.; Kornet, N.; Canales, C.; Scheres, B.; Tsiantis, M. Repression of apical homeobox genes is required for embryonic root development in Arabidopsis. Curr. Biol. 2009, 19, 1485–1490. [Google Scholar] [CrossRef]
- Smith, Z.R.; Long, J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 2010, 464, 423–426. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L.; et al. The plethora gene regulatory network guides growth and cell differentiation in Arabidopsis roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Mahonen, A.P.; Ten Tusscher, K.; Siligato, R.; Smetana, O.; Diaz-Trivino, S.; Salojarvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. Plethora gradient formation mechanism separates auxin responses. Nature 2014, 515, 125–129. [Google Scholar] [CrossRef]
- Di Mambro, R.; De Ruvo, M.; Pacifici, E.; Salvi, E.; Sozzani, R.; Benfey, P.N.; Busch, W.; Novak, O.; Ljung, K.; Di Paola, L.; et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 2017, 114, E7641–E7649. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Wang, X.J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Di Mambro, R.; Sozzani, R.; Pacifici, E.; Salvi, E.; Terpstra, I.; Bao, D.; van Dijken, A.; Dello Ioio, R.; Perilli, S.; et al. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev. Cell 2013, 26, 405–415. [Google Scholar] [CrossRef]
- Salvi, E.; Di Mambro, R.; Pacifici, E.; Dello Ioio, R.; Costantino, P.; Moubayidin, L.; Sabatini, S. Scarecrow and shortroot control the auxin/cytokinin balance necessary for embryonic stem cell niche specification. Plant Signal Behav. 2018, 13, e1507402. [Google Scholar]
- Dello Ioio, R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A phabulosa/cytokinin feedback loop controls root growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef]
- Pacifici, E.; Di Mambro, R.; Dello Ioio, R.; Costantino, P.; Sabatini, S. Acidic cell elongation drives cell differentiation in the Arabidopsis root. EMBO J. 2018, 37, e99134. [Google Scholar] [CrossRef]
- Vuolo, F.; Kierzkowski, D.; Runions, A.; Hajheidari, M.; Mentink, R.A.; Gupta, M.D.; Zhang, Z.; Vlad, D.; Wang, Y.; Pecinka, A.; et al. Lmi1 homeodomain protein regulates organ proportions by spatial modulation of endoreduplication. Genes Dev. 2018, 32, 1361–1366. [Google Scholar] [CrossRef]
- Hayashi, K.; Hasegawa, J.; Matsunaga, S. The boundary of the meristematic and elongation zones in roots: Endoreduplication precedes rapid cell expansion. Sci. Rep. 2013, 3, 2723. [Google Scholar] [CrossRef]
- Takahashi, N.; Kajihara, T.; Okamura, C.; Kim, Y.; Katagiri, Y.; Okushima, Y.; Matsunaga, S.; Hwang, I.; Umeda, M. Cytokinins control endocycle onset by promoting the expression of an apc/c activator in Arabidopsis roots. Curr. Biol. 2013, 23, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; Wiley Interscience, John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 601. ISBN 978-0-471-73843-5.
- Ron, M.; Dorrity, M.W.; de Lucas, M.; Toal, T.; Hernandez, R.I.; Little, S.A.; Maloof, J.N.; Kliebenstein, D.J.; Brady, S.M. Identification of novel loci regulating interspecific variation in root morphology and cellular development in tomato. Plant Physiol. 2013, 162, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Dievart, A.; Divol, F.; Pauluzzi, G.; Meynard, D.; Swarup, R.; Wu, S.; Gallagher, K.L.; Perin, C. Shr overexpression induces the formation of supernumerary cell layers with cortex cell identity in rice. Dev. Biol. 2017, 425, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Fischer, A.M.; Roettger, M.; Rommel, S.; Schluepmann, H.; Brautigam, A.; Carlsbecker, A.; Gould, S.B. Cytokinin-induced promotion of root meristem size in the fern azolla supports a shoot-like origin of euphyllophyte roots. New Phytol. 2016, 209, 705–720. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mambro, R.; Sabatini, S.; Dello Ioio, R. Patterning the Axes: A Lesson from the Root. Plants 2019, 8, 8. https://doi.org/10.3390/plants8010008
Di Mambro R, Sabatini S, Dello Ioio R. Patterning the Axes: A Lesson from the Root. Plants. 2019; 8(1):8. https://doi.org/10.3390/plants8010008
Chicago/Turabian StyleDi Mambro, Riccardo, Sabrina Sabatini, and Raffaele Dello Ioio. 2019. "Patterning the Axes: A Lesson from the Root" Plants 8, no. 1: 8. https://doi.org/10.3390/plants8010008
APA StyleDi Mambro, R., Sabatini, S., & Dello Ioio, R. (2019). Patterning the Axes: A Lesson from the Root. Plants, 8(1), 8. https://doi.org/10.3390/plants8010008






