Phytochemistry of Three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling
Abstract
:1. Introduction
2. Results
2.1. EO from L. Heteromorpha Leaves
2.1.1. Chemical Analysis
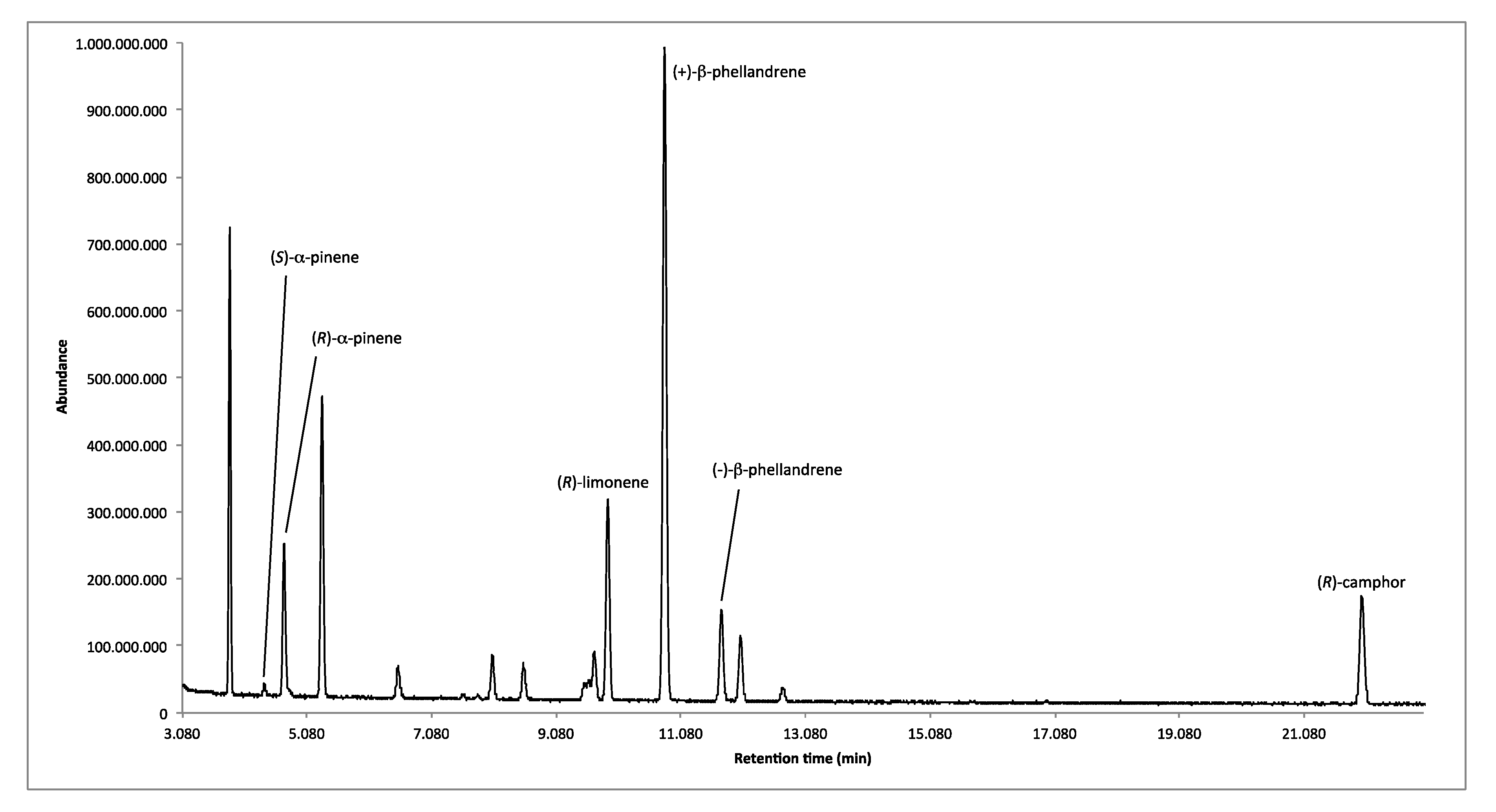
2.1.2. Enantioselective GC Analysis
2.1.3. Characterization of (−)-Ledol (1)
2.1.4. Characterization of (−)-Caryophyllene Oxide (2)
2.2. Ethyl Acetate Extract of Lepechinia Radula
2.2.1. Identification of Spathulenol (3)
2.2.2. Identification of Angustanoic Acid E (4)
2.2.3. Identification of 5-hydroxy-4′,7-dimethoxy flavone (5)
2.3. Ethyl Acetate Extract of Lepechinia paniculata
2.3.1. Identification of Ledol (3) and Guaiol (6)
2.3.2. Characterization of (−)-Carnosol (7)
3. Discussion
3.1. EO of L. Heteromorpha
3.2. Ethyl Acetate Extract of L. Radula and L. Paniculata
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. EO of L. heteromorpha
4.3.1. Distillation of the EO
4.3.2. Qualitative and Semi-quantitative Analysis
4.3.3. Enantioselective GC Analysis
4.3.4. Purification of (−)-Ledol and (−)-Caryophyllene oxide
4.4. Ethyl Acetate Extract Components of L. radula and L. paniculata Leaves
4.4.1. Obtainment of Ethyl Acetate Extracts
4.4.2. Chlorophyll Removal
4.4.3. Preparative Isolation of Secondary Metabolites from L. radula
4.4.4. Fraction Purification and Secondary Metabolites Isolation from L. paniculata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Worldatlas. 17 Most Ecologically Diverse Countries On Earth. Available online: http://www.worldatlas.com/articles/ecologically-megadiverse-countries-of-the-world.html (accessed on 6 July 2018).
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [PubMed]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric natural products: Occurrence and biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron Asymmetr. 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Liberto, E.; Cagliero, C.; Sgorbini, B.; Bicchi, C.; Sciarrone, D.; d’Acampora-Zellner, B.; Mondello, L.; Rubiolo, P. Enantiomer identification in the flavour and fragrance fields by “interactive” combination of linear retention indices from enantioselective gas chromatography and mass spectrometry. J. Chromatogr. A. 2008, 1195, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Gilardoni, G.; Ramón, E.; Tosi, S.; Picco, A.; Bicchi, C.; Vidari, G. Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae. Pharmaceuticals 2018, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical Composition, Enantiomeric Analysis, AEDA Sensorial Evaluation and Antifungal Activity of the Essential Oil from the Ecuadorian Plant Lepechinia mutica Benth (Lamiaceae). Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Caprai, E.; Bastida, J.; Viladomat, F.; Jáuregui, O.; Codina, C. Investigation of Lepechinia graveolens for its antioxidant activity and phenolic composition. J. Ethnopharmacol. 2004, 94, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.G.; Muñoz, J.L.; Martínez, A.; García, A.M.; Martínez, G.; Peñalosa, I. In vitro anti-Vibrio cholerae activity of essential oil from Lepechinia caulescens. Fitoterapia 2005, 76, 104–107. [Google Scholar]
- Martins, F.O.; Esteves, P.F.; Mendes, G.S.; Barbi, N.S.; Menezes, F.S.; Romanos, M.T. Verbascoside isolated from Lepechinia speciosa has inhibitory activity against HSV-1 and HSV-2 in vitro. Nat. Prod. Commun. 2009, 4, 1693–1696. [Google Scholar] [PubMed]
- Cicció, J.F.; Soto, V.H.; Poveda, L.J. Essential oil of Lepechinia schiedeana (Lamiaceae) from Costa Rica. Rev. Biol. Trop. 1999, 47, 373–375. [Google Scholar] [PubMed]
- Jonathan, L.T.; Che, C.T.; Pezzuto, J.M.; Fong, H.H.S.; Farnsworth, N.R. 7-O-Methylhorminone and Other Cytotoxic Diterpene Quinones From Lepechinla Bullata. J. Nat. Prod. 1989, 52, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17602035?tab=acceptednames (accessed on 6 July 2018).
- Jørgesen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; p. 521. [Google Scholar]
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17602047?tab=acceptednames (accessed on 6 July 2018).
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17602047?tab=synonyms (accessed on 6 July 2018).
- Jørgesen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; p. 522. [Google Scholar]
- Morocho, V.; Toro, M.; Cartuche, L.; Guaya, D.; Valarezo, E.; Malagon, O.; Ramirez, J. Chemical Composition and Antimicrobial Activity of Essential Oil of Lepechinia radula Benth Epling. Rec. Nat. Prod. 2017, 11, 57–62. [Google Scholar]
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17607770?tab=synonyms (accessed on 6 July 2018).
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17607770?tab=acceptednames (accessed on 6 July 2018).
- Valarezo, E.; Castillo, A.; Guaya, D.; Morocho, V.; Malagón, O. Chemical composition of essential oils of two species of Lamiaceae family: Scutellaria volubilis and Lepechinia paniculata from Loja, Ecuador. J. Essent. Oil Res. 2012, 24, 31–37. [Google Scholar] [CrossRef]
- de la Torre, L.; Navarrete, H.; Muriel, M.P.; Macía, M.J.; Balslev, H. (Eds.) Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Denmark, 2008; ISBN 978-9978-77-135-8. [Google Scholar]
- Rios, M.; Koziol, M.J.; Pedersen, H.B.; Granda, G. (Eds.) Useful Plants of Ecuador: Applications, Challenges, and Perspectives, 1st ed.; Herbario QCA, Pontificia Universidad Católica del Ecuador, and Herbario AAU, Universidad de Aarhus: Quito, Ecuador, 2007; ISBN 978-9978-22-684-1. [Google Scholar]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C1139306&Mask=2000#Gas-Chrom (accessed on 9 July 2018).
- Cao, S.G.; Sim, K.Y.; Goh, S.H. (−)-Ledol from Calophyllum teysmanii: Structure and stereochemistry. Nat. Prod. Lett. 2000, 14, 447–452. [Google Scholar] [CrossRef]
- Costa, E.V.; De Assis Marques, F.; Pinheiro, M.L.B.; Braga, R.M.; Delarmelina, C.; Duarte, M.C.T.; Ruiz, A.L.; De Carvalho, J.E.; Maia, B. Chemical constituents isolated from the bark of Guatteria blepharophylla (Annonaceae) and their antiproliferative and antimicrobial activities. J. Braz. Chem. Soc. 2011, 22, 1111–1117. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Ragasa, C.Y.; Ganzon, J.; Hofilena, J.; Tamboong, B.; Rideout, J.A. A New Furanoid Diterpene from Caesaipinia puicherrima. Chem. Pharm. Bull. 2003, 51, 1208–1210. [Google Scholar] [CrossRef]
- Sy, L.-K.; Geoffrey, D.B. Abietane Diterpenes from Illicium angustisepalum. J. Nat. Prod. 1998, 3864, 907–912. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef]
- Momozane, T.; Kawamura, T.; Itoh, Y.; Sanosaka, M.; Sasaki, T; Kanzaki, R.; Ose, N.; Funaki, S.; Shintani, Y.; Minami, M.; et al. Carnosol suppresses IL-6 production in mouse lungs injured by ischemia-reperfusion operation and in RAW264.7 macrophages treated with lipopolysaccharide. Biochem. Cell Biol. 2011, 9, 1–24. [Google Scholar]
- Vlavcheski, F.; Baron, D.; Vlachogiannis, I.A.; Macpherson, R.E.K.; Tsiani, E. Carnosol increases skeletal muscle cell glucose uptake via ampk-dependent glut4 glucose transporter translocation. Int. J. Mol. Sci. 2018, 19, 3–6. [Google Scholar] [CrossRef]
- Tong, L.; Wu, S. The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation. Sci. Rep. 2018, 8, 3574. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Natali, L.; Iofrida, C.; Flamini, G.; Braca, A.; Trincavelli, M.L.; Martini, C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Tang, F.; Zhao, Y.; Feng, D.; Li, Y.; Hu, Y.; Wang, C.; Zhou, J.; Tian, X.; et al. Carnosol-mediated Sirtuin 1 activation inhibits Enhancer of Zeste Homolog 2 to attenuate liver fibrosis. Pharmacol. Res. 2018, 128, 327–337. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Suárez, A.I.; Bec, N.; Armijos, C.; Gilardoni, G.; Larroque, C.; Vidari, G. Carnosol from Lepechinia mutica (Benth.) Epling and Tiliroside from Vallea stipularis L.f.: Two promising inhibitors of BuChE. Rev. Bras. Farmacogn. 2018, in press. [Google Scholar]
- Toyota, M.; Koyama, H.; Mizutani, M.; Asakawa, Y. (−)-ent-spathulenol isolated from liverworts is an artefact. Phytochemistry 1996, 41, 1347–1350. [Google Scholar] [CrossRef]
- Esteves, P.F.; Kuster, R.M.; Barbi, N.D.S.; Menezes, F.D.S. Chemical composition and cytotoxic activity of Lepechinia speciosa (St. Hill) Epling. Lat. Am. J. Pharmacy 2010, 29, 38–44. [Google Scholar]
- Delgado, G.; Hernández, J.; Chávez, M.I.; Álvarez, L.; Gonzaga, V.; Martínez, E. Di- and triterpenpoid acids from Lepechinia caulescens. Phytochemistry 1994, 37, 1119–1121. [Google Scholar] [CrossRef]
- Delgado, G.; Sánchez, E.; Hernández, J.; Chávez, M.I.; Álvarez, L.; Martínez, E. Abietanoid acid from Lepechinia caulescens. Phytochemistry 1992, 31, 3159–3161. [Google Scholar] [CrossRef]
- Bruno, M.; Savona, G.; Piozzi, F.; De la Torre, M.; Rodríguez, B.; Marlier, M. Abietane diterpenoids from Lepechinia meyeni and Lepechinia hastata. Phytochemistry 1991, 30, 2339–2343. [Google Scholar] [CrossRef]
- Areche, C.; Schmeda-Hirschmann, G.; Theoduloz, C.; Rodríguez, J.A. Gastroprotective effect and cytotoxicity of abietane diterpenes from the Chilean Lamiaceae Sphacele chamaedryoides (Balbis) Briq. J. Pharmacy Pharmacol. 2009, 61, 1689–1697. [Google Scholar] [CrossRef]
- Eggers, M.D.; Sinnwell, V.; Stahl-Biskup, E. (−)-Spirolepechinene, a spirosesquiterpene from Lepechinia bullata (Lamiaceae). Phytochemistry 1999, 51, 987–990. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Dec. Kratz, P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- D’Acampora-Zellner, B.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review. Flavour Fragr. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Bicchi, C.; Liberto, E.; Matteodo, M.; Sgorbini, B.; Mondello, L.; d’Acampora-Zellner, B.; Costa, R.; Rubiolo, P. Quantitative analysis of essential oils: A complex task. Flavour Fragr. J. 2008, 23, 382–391. [Google Scholar] [CrossRef]
- De Saint Laumer, J.I.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
| Reference LRIs | Calculated LRIs | Compounds | % 1 | σ |
|---|---|---|---|---|
| 932 | 931 | α-Pinene | 1.2 | 0.71 |
| 946 | 946 | Camphene | 2.4 | 0.45 |
| 969 | 976 | β-Pinene | 0.4 | 0.18 |
| 988 | 988 | Myrcene | 0.4 | 0.22 |
| 1003 | 1003 | Mentha-1(7),8-diene | 0.4 | 0.23 |
| 1007 | 1005 | iso-Sylvestrene | 0.2 | 0.13 |
| 1020 | 1015 | p-Cymene | 0.5 | 0.26 |
| 1022 | 1017 | o-Cymene | 1.0 | 0.01 |
| 1024 | 1029 | Limonene | 1.3 | 0.41 |
| 1025 | 1029 | β-Phellandrene | 4.6 | 1.02 |
| 1141 | 1145 | Camphor | 1.7 | 0.46 |
| 1400 | 1404 | Sibirene | 3.2 | 0.32 |
| 1417 | 1411 | (E)-β-Caryophyllene | 7.1 | 0.77 |
| 1439 | 1439 | Aromadendrene | 1.0 | 0.18 |
| 1449 | 1445 | Spirolepechinene | 7.1 | 0.93 |
| 1458 | 1451 | allo-Aromadendrene | 6.1 | 1.00 |
| 1452 | 1453 | α-Humulene | 1.2 | 0.19 |
| 1496 | 1484 | Valencene | 1.6 | 0.23 |
| - | 1487 | Undetermined (MW 204) | 3.7 | 1.84 |
| 1492 | 1489 | cis-β-Guaiene | 0.3 | 0.16 |
| 1496 | 1497 | Viridiflorene | 27.3 | 1.80 |
| 1505 | 1503 | (E,E)-α-Farnesene | 1.4 | 0.41 |
| - | 1509 | Undetermined (MW 204) | 0.2 | 0.12 |
| 1511 | 1516 | δ-Amorphene | 1.0 | 0.98 |
| 1559 | 1549 | Germacrene B | 1.6 | 0.83 |
| 1592 2 | 1593 | Caryophyllene oxide | 1.0 | 0.59 |
| 1602 | 1601 | (−)-Ledol 3 | 21.2 | 4.32 |
| Monoterpene hydrocarbons | 12.4 | |||
| Oxygenated monoterpenes | 1.7 | |||
| Sesquiterpene hydrocarbons | 62.8 | |||
| Oxygenated sesquiterpenes | 22.2 | |||
| Others | - | |||
| Total identified | 99.1 | |||
| LRIs | Enantiomers | Enantiomeric Distribution (%) | ee (%) |
|---|---|---|---|
| 917 | (S)-α-pinene | 8.7 | 82.6 |
| 926 | (R)-α-pinene | 91.3 | |
| 1054 | (R)-limonene | 100.0 | 100.0 |
| 1072 | (+)-β-phellandrene | 88.3 | 76.6 |
| 1091 | (−)-β-phellandrene | 11.7 | |
| 1262 | (R)-camphor | 100.0 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilardoni, G.; Ramírez, J.; Montalván, M.; Quinche, W.; León, J.; Benítez, L.; Morocho, V.; Cumbicus, N.; Bicchi, C. Phytochemistry of Three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling. Plants 2019, 8, 1. https://doi.org/10.3390/plants8010001
Gilardoni G, Ramírez J, Montalván M, Quinche W, León J, Benítez L, Morocho V, Cumbicus N, Bicchi C. Phytochemistry of Three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling. Plants. 2019; 8(1):1. https://doi.org/10.3390/plants8010001
Chicago/Turabian StyleGilardoni, Gianluca, Jorge Ramírez, Mayra Montalván, Willan Quinche, Jackeline León, Lita Benítez, Vladimir Morocho, Nixon Cumbicus, and Carlo Bicchi. 2019. "Phytochemistry of Three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling" Plants 8, no. 1: 1. https://doi.org/10.3390/plants8010001







