Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance
Abstract
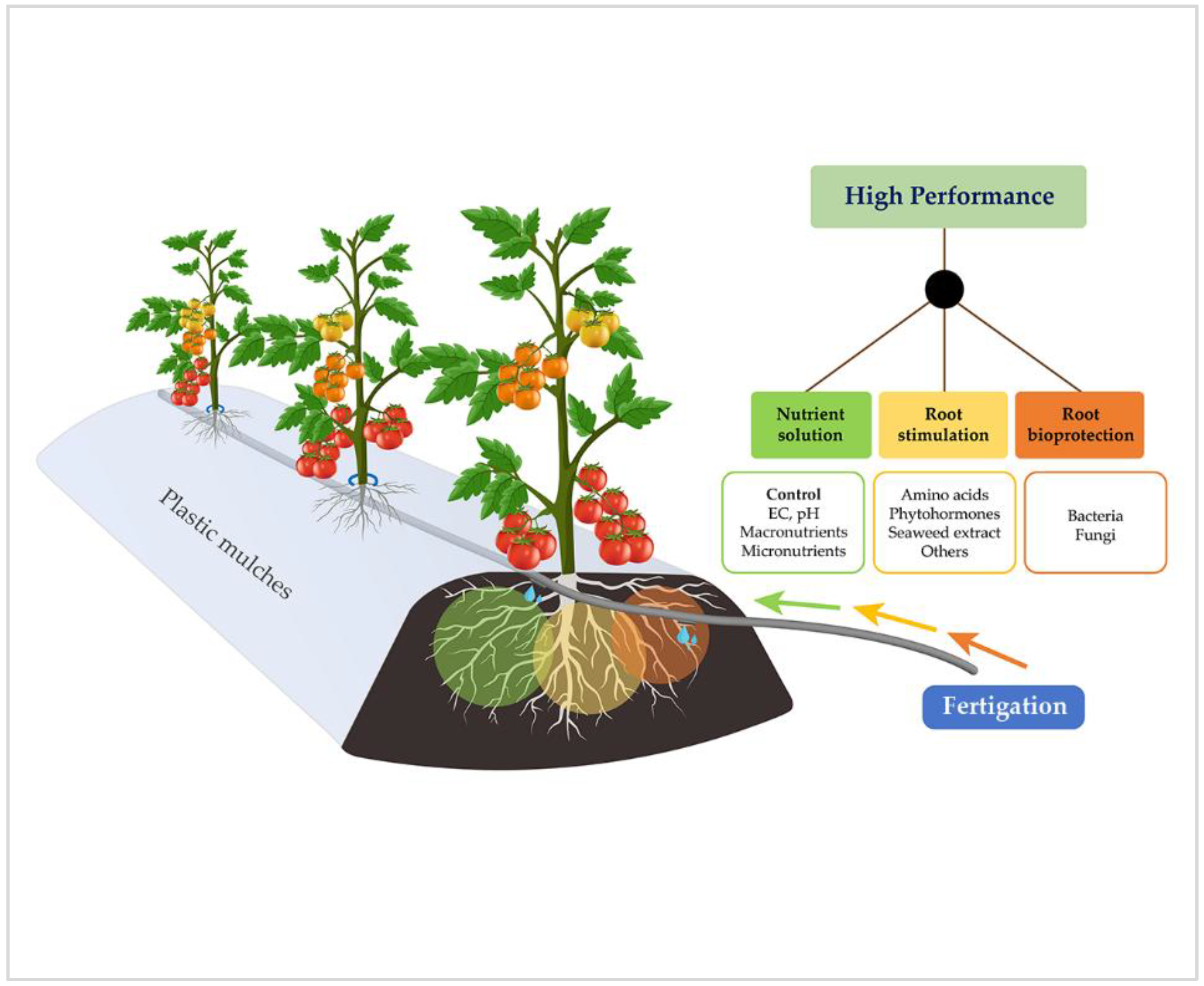
1. Introduction
2. Nutrient Solution in Fertigation
3. Biostimulants Usage in Crops
3.1. Seaweed Extract
3.2. Protein Hydrolyzates
3.3. Humic Acids
3.4. Phosphites (Phi)
3.5. Plant Hormones
4. Bioprotection in Plants
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Husson, O.; Brunet, A.; Babre, D.; Charpentier, H.; Durand, M.; Sarthou, J.P. Conservation agriculture systems alter the electrical characteristics (Eh, pH and EC) of four soil types in France. Soil Tillage Res. 2018, 176, 57–68. [Google Scholar] [CrossRef]
- Comas, L.; Becker, S.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Roots of the second green revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2010, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- De Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Rajani, M.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, E.; Calatayud, A. Optimization of nutrition in soilless systems: A review. In Advances in Botanical Research; Kader, J.C., Delseny, M., Eds.; Elsevier: New York, NY, USA, 2010; Volume 53, pp. 193–245. ISBN 0065-2296. [Google Scholar]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased Electrical Conductivity in Nutrient Solution Management Enhances Dietary and Organoleptic Qualities in Soilless Culture Tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Chrétien, S.; Gosselin, A.; Dorais, M. High electrical conductivity and radiation-based water management improve fruit quality of greenhouse tomatoes grown in rockwool. HortScience 2000, 35, 627–631. [Google Scholar]
- Mmolawa, K.; Or, D. Root zone solute dynamics under drip irrigation: A review. Plant Soil 2000, 222, 163–190. [Google Scholar] [CrossRef]
- Malhotra, S.K. Water soluble fertilizers in horticultural crops—An appraisal. Indian J. Agric. Sci. 2016, 86, 1245–1256. [Google Scholar]
- Rajput, T.; Patel, N. Water and nitrate movement in drip-irrigated onion under fertigation and irrigation treatments. Agric. Water Manag. 2006, 79, 293–311. [Google Scholar] [CrossRef]
- Suman, S.; Raina, J. Efficient use of water and nutrients through drip and mulch in apple. J. Plant Nutr. 2014, 37, 2036–2049. [Google Scholar] [CrossRef]
- Incrocci, L.; Massa, D.; Pardossi, A. New trends in the fertigation management of irrigated vegetable crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Mohammad, M.J. Squash yield, nutrient content and soil fertility parameters in response to methods of fertilizer application and rates of nitrogen fertigation. Nutr. Cycl. Agroecosys. 2004, 68, 99–108. [Google Scholar] [CrossRef]
- Silber, A.; Xu, G.; Levkovitch, I.; Soriano, S.; Bilu, A.; Wallach, R. High fertigation frequency: The effects on uptake of nutrients, water and plant growth. Plant Soil 2003, 253, 467–477. [Google Scholar] [CrossRef]
- Badr, M.; El-Yazied, A. Effect of fertigation frequency from subsurface drip irrigation on tomato yield grown on sandy soil. Aust. J. Basic Appl. Sci. 2007, 1, 279–285. [Google Scholar]
- Solaimalai, A.; Baskar, M.; Sadasakthi, A.; Subburamu, K. Fertigation in high value crops—A review. Agric. Rev. Agric. Res. Commun. Cent. INDIA 2005, 26, 1. [Google Scholar]
- Hatfield, J.L.; Sauer, T.J.; Prueger, J.H. Managing soils to achieve greater water use efficiency. Agron. J. 2001, 93, 271–280. [Google Scholar] [CrossRef]
- Kleinert, A.; Benedito, V.; Morcillo, R.; Dames, J.; Cornejo-Rivas, P.; Zuniga-Feest, A.; Delgado, M.; Muñoz, G. Morphological and Symbiotic Root Modifications for Mineral Acquisition from Nutrient-Poor Soils. In Root Biology; Giri, B., Prasad, R., Varma, A., Eds.; Springer: Berlin, Germany, 2018; pp. 85–142. [Google Scholar]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; George, T.S.; Dupuy, L.X.; Karley, A.J.; Valentine, T.A.; Wiesel, L.; Wishart, J. Root traits for infertile soils. Front. Plant Sci. 2013, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Jungk, A.O. Dynamics of nutrient movement at the soil-root interface. In Plant Roots; CRC Press: Florida, FL, USA, 2002; pp. 919–1016. [Google Scholar]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Cadahia, C. Fertirrigación: Cultivos hortícolas, frutales y ornamentales; Mundi-prensa Libros: Madrid, Spain, 2005; ISBN 84-8476-247-5. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Hamza, B.; Suggars, A. Biostimulants: Myths and realities. TurfGrass Trends 2001, 8, 6–10. [Google Scholar]
- Stirk, W.; Arthur, G.; Lourens, A.; Novak, O.; Strnad, M.; Van Staden, J. Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J. Appl. Phycol. 2004, 16, 31. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, Ł.; Chojnacka, K. Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem. 2015, 13, 1183–1195. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts—The prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.; Mandolino, G. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Piero, A.R.L.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2018, 1–6. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.; Anand, K.V.; Kubavat, D.; Kumar, R.; Vaghela, P.; Ghosh, A. Crop stage selection is vital to elicit optimal response of maize to seaweed bio-stimulant application. J. Appl. Phycol. 2017, 29, 2135–2144. [Google Scholar] [CrossRef]
- Trivedi, K.; Anand, K.V.; Kubavat, D.; Patidar, R.; Ghosh, A. Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J. Appl. Phycol. 2018, 30, 2001–2015. [Google Scholar] [CrossRef]
- Rathore, S.; Chaudhary, D.; Boricha, G.; Ghosh, A.; Bhatt, B.; Zodape, S.; Patolia, J. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Critchley, A.T. A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J. Appl. Phycol. 2018, 1–15. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum nodosum spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant. Sci. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; De Lucia, B. Effects of an Animal-Derived Biostimulant on the Growth and Physiological Parameters of Potted Snapdragon (Antirrhinum majus L.). Front. Plant Sci. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Horii, A.; McCue, P.; Shetty, K. Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Bioresour. Technol. 2007, 98, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy, P. The principles of humic substances: An introduction to the first principle. Spec. Publ. R. Soc. Chem. 2001, 273, 19–30. [Google Scholar]
- Motta, F.L.; Santana, M. Production of humic acids from oil palm empty fruit bunch by submerged fermentation with Trichoderma viride: Cellulosic substrates and nitrogen sources. Biotechnol. Prog. 2013, 29, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Sławiñska, D.; Polewski, K.; Rolewski, P.; Sławiñski, J. Synthesis and properties of model humic substances derived from gallic acid. Int. Agrophysics 2007, 21, 199–208. [Google Scholar]
- Kiprop, A.; Pourtier, E.; Kimutai, S.; Kirui, S. Synthesis of Humic and Fulvic Acids and their Characterization using Optical Spectroscopy (ATR-FTIR and UV-Visible). Int. J. Appl. 2013, 3, 28–35. [Google Scholar]
- Klučáková, M.; Pekař, M. Solubility and dissociation of lignitic humic acids in water suspension. Colloids Surf. Physicochem. Eng. Asp. 2005, 252, 157–163. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: California, CA, USA, 1994; ISBN 0-471-59474-1. [Google Scholar]
- García, A.C.; De Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef] [PubMed]
- Martins Gomes, E.T.; Berbara, R.L.L.; Pereira, M.G.; Urquiaga, S.S.; Tavares, O.C.H.; Assunção, S.A.; Zonta, E.; do Amaral Sobrinho, N.M.B.; García, A.C. Effects of farmed managements in sandy soils on composition and stabilization of soil humic substances. Land Degrad. Dev. 2018, 29, 68–79. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.; Ali, S. Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, A.; Kafi, M.; Babalar, M.; Xia, Y.P.; Luo, A.; Etemadi, N. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Waqas, M.; Ahmad, B.; Arif, M.; Munsif, F.; Khan, A.L.; Amin, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Evaluation of humic acid application methods for yield and yield components of mungbean. Am. J. Plant Sci. 2014, 5, 2269. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Francioso, O. Soil–root cross-talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Bahtiyar, M.; Kucukoduk, M. The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol. Environ. Saf. 2018, 155, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant. Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A novel P-fertilizer for weed management and pathogen control. Plant Biotechnol. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Liljeroth, E.; Lankinen, Å.; Wiik, L.; Burra, D.D.; Alexandersson, E.; Andreasson, E. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Prot. 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Mofidnakhaei, M.; Abdossi, V.; Dehestani, A.; Pirdashti, H.; Babaeizad, V. Potassium phosphite affects growth, antioxidant enzymes activity and alleviates disease damage in cucumber plants inoculated with Pythium ultimum. Arch. Phytopathol. Plant Prot. 2016, 49, 207–221. [Google Scholar] [CrossRef]
- Dalio, R.J.; Fleischmann, F.; Humez, M.; Osswald, W. Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 2014, 9, e87860. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, B.; Lin, M.; Chen, G.; Ding, X.; Weng, Q.; Chen, Q. Phosphite-induced reactive oxygen species production and ethylene and ABA biosynthesis, mediate the control of Phytophthora capsici in pepper (Capsicum annuum). Funct. Plant Biol. 2016, 43, 563–574. [Google Scholar] [CrossRef]
- Machinandiarena, M.F.; Lobato, M.C.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B. Potassium phosphite primes defense responses in potato against Phytophthora infestans. J. Plant Physiol. 2012, 169, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium phosphite increases tolerance to UV-B in potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Rahmani, F.; Dehestani, A. Study of physio-biochemical responses elicited by potassium phosphite in downy mildew-infected cucumber plants. Arch. Phytopathol. Plant Prot. 2017, 50, 540–554. [Google Scholar] [CrossRef]
- Constán-Aguilar, C.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Camacho, M.; Romero, L.; Ruiz, J.; Blasco, B. Physiological and nutritional evaluation of the application of phosphite as a phosphorus source in cucumber plants. Commun. Soil Sci. Plant Anal. 2014, 45, 204–222. [Google Scholar] [CrossRef]
- Pandeya, D.; López-Arredondo, D.L.; Janga, M.R.; Campbell, L.M.; Estrella-Hernández, P.; Bagavathiannan, M.V.; Herrera-Estrella, L.; Rathore, K.S. Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proc. Natl. Acad. Sci. USA. 2018, 115, E6946–E6955. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Achary, V.M.M.; Islam, T.; Agrawal, P.K.; Reddy, M.K. The development of a phosphite-mediated fertilization and weed control system for rice. Sci. Rep. 2016, 6, 24941. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Elias, J.; Salas, M.; Urrestarazu, M. Application of indole-3-butyric acid by fertigation on pepper plants in soilless culture grown in a greenhouse. Acta Hortic. 2004, 697, 475–479. [Google Scholar] [CrossRef]
- Salas, M.; Fernandez, M.; Urrestaraz, M. Sweet pepper yield and fruit quality affected by different auxin application methods. Acta Hortic. 2008, 807, 401–406. [Google Scholar] [CrossRef]
- Salas, M.; Urrestarazu, M.; Lopez, J. Effect of IBA application by fertigation on melon in soilless culture. Acta Hortic. 2003, 609, 225–228. [Google Scholar] [CrossRef]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. CMLS 2006, 63, 2738–2754. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Benková, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jürgens, G. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 108, 661–673. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramirez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant. Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, C.-A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Liberman, L.M. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant. Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic press: Massachusetts, MA, USA, 2010; ISBN 0-08-055934-4. [Google Scholar]
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Charyulu, P.; Rao, V.R. Influence of various soil factors on nitrogen fixation by Azospirillum spp. Soil Biol. Biochem. 1980, 12, 343–346. [Google Scholar] [CrossRef]
- Payne, P.; Asher, M.; Kershaw, C. The incidence of Pythium spp. and Aphanomyces cochlioides associated with the sugar-beet growing soils of Britain. Plant Pathol. 1994, 43, 300–308. [Google Scholar] [CrossRef]
- Höper, H.; Steinberg, C.; Alabouvette, C. Involvement of clay type and pH in the mechanisms of soil suppressiveness to fusarium wilt of flax. Soil Biol. Biochem. 1995, 27, 955–967. [Google Scholar] [CrossRef]
- Lee, K.J.; Kamala-Kannan, S.; Sub, H.S.; Seong, C.K.; Lee, G.W. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillussubtilis. World J. Microbiol. Biotechnol. 2008, 24, 1139–1145. [Google Scholar] [CrossRef]
- Idris, H.A.; Labuschagne, N.; Korsten, L. Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol. Control 2007, 40, 97–106. [Google Scholar] [CrossRef]
- Karthiba, L.; Saveetha, K.; Suresh, S.; Raguchander, T.; Saravanakumar, D.; Samiyappan, R. PGPR and entomopathogenic fungus bioformulation for the synchronous management of leaffolder pest and sheath blight disease of rice. Pest Manag. Sci. 2010, 66, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment–Plants–Molecules; Springer: Berlin, Germany, 2002; pp. 3–20. [Google Scholar]
- Flowers, T. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Moyer, M. How much is left? Sci. Am. 2010, 303, 74–81. [Google Scholar] [CrossRef] [PubMed]
- García-Gaytán, V.; Gómez-Merino, F.C.; Trejo-Téllez, L.I.; Baca-Castillo, G.A.; García-Morales, S. The chilhuacle chili (Capsicum annuum L.) in Mexico: Description of the variety, its cultivation, and uses. Int. J. Agron. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Yang, J.; Zang, J. Crop management techniques to enhance harvest index in rice. J. Exp. Bot. 2010, 61, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Dickin, S.K.; Schuster-Wallace, C.J.; Qadir, M.; Pizzacalla, K. A review of health risks and pathways for exposure to wastewater use in agriculture. Environ. Health Perspect. 2016, 124, 900. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Mare, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Elsevier: New York, NY, USA, 2015; Volume 130, pp. 141–174. ISBN 0065-2113. [Google Scholar]
- Olivares, F.L.; Busato, J.G.; Paula, A.M.; Lima, L.S.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gaytán, V.; Hernández-Mendoza, F.; Coria-Téllez, A.V.; García-Morales, S.; Sánchez-Rodríguez, E.; Rojas-Abarca, L.; Daneshvar, H. Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance. Plants 2018, 7, 88. https://doi.org/10.3390/plants7040088
García-Gaytán V, Hernández-Mendoza F, Coria-Téllez AV, García-Morales S, Sánchez-Rodríguez E, Rojas-Abarca L, Daneshvar H. Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance. Plants. 2018; 7(4):88. https://doi.org/10.3390/plants7040088
Chicago/Turabian StyleGarcía-Gaytán, Víctor, Fanny Hernández-Mendoza, Ana Velia Coria-Téllez, Soledad García-Morales, Esteban Sánchez-Rodríguez, Luis Rojas-Abarca, and Hadiseh Daneshvar. 2018. "Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance" Plants 7, no. 4: 88. https://doi.org/10.3390/plants7040088
APA StyleGarcía-Gaytán, V., Hernández-Mendoza, F., Coria-Téllez, A. V., García-Morales, S., Sánchez-Rodríguez, E., Rojas-Abarca, L., & Daneshvar, H. (2018). Fertigation: Nutrition, Stimulation and Bioprotection of the Root in High Performance. Plants, 7(4), 88. https://doi.org/10.3390/plants7040088





