Fruit Decay to Diseases: Can Induced Resistance and Priming Help?
Abstract
1. Background
2. Major Post-Harvest Threats to Agricultural Markets
3. Current Knowledge of Disease Protection
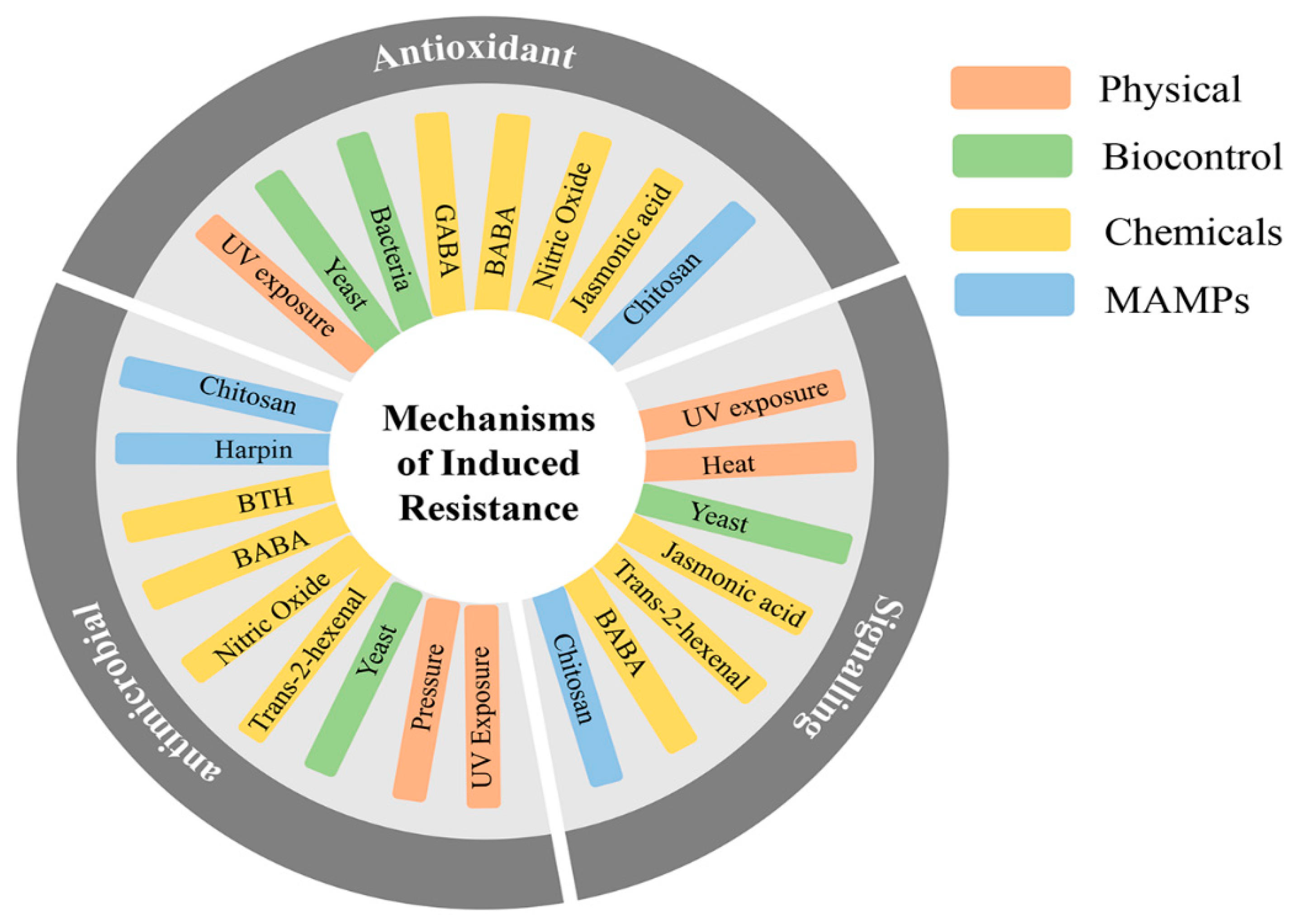
4. Induced Resistance for Post-Harvest Disease Protection
4.1. Physical Approach
4.2. Chemical Approach
4.3. Biocontrol
4.4. Microbe-Associated Molecular Patterns
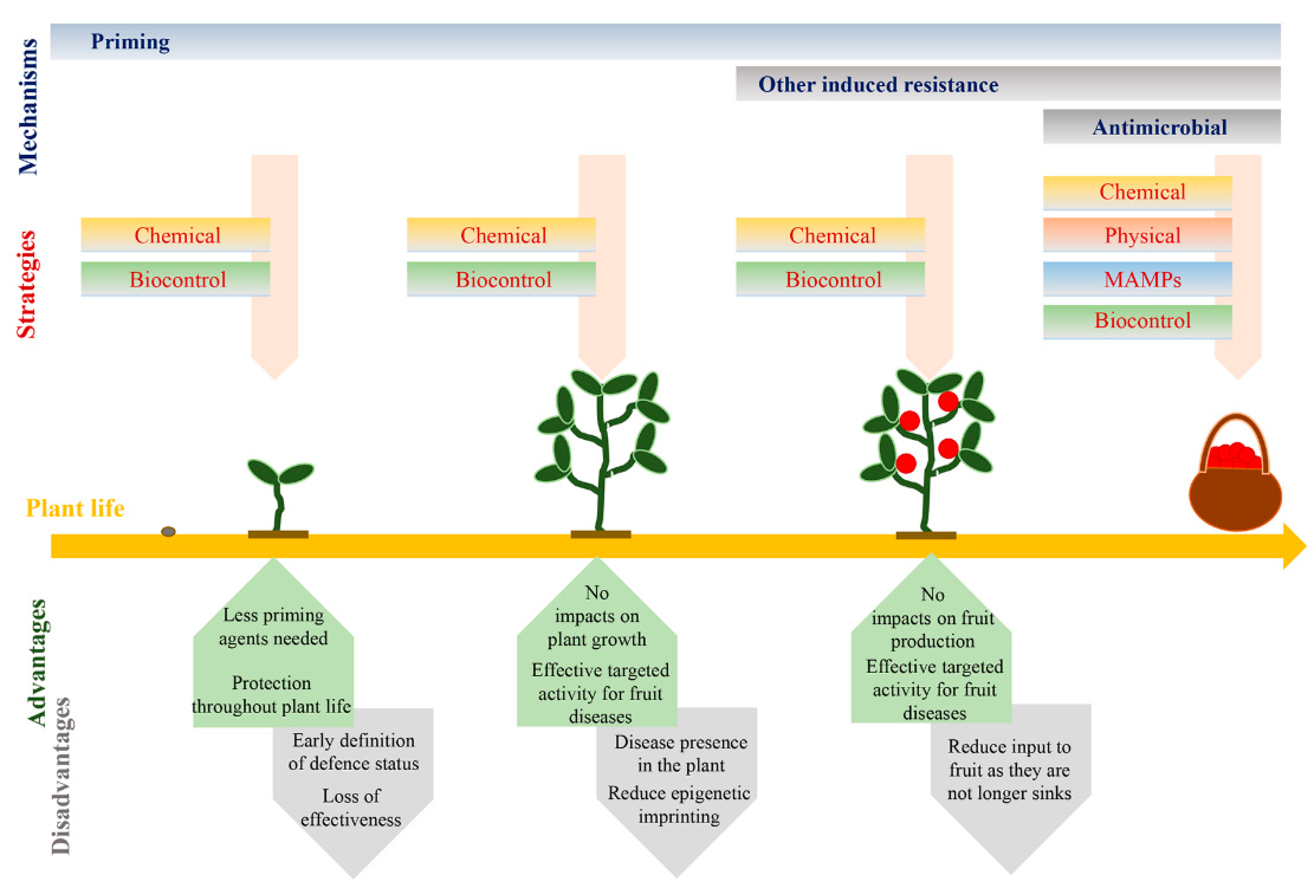
5. Impacts of Induced Resistance
6. Towards the Future: Exploiting Priming
6.1. Priming for Fruit Resistance
6.2. Key Aspects for Exploiting Priming in Fruit
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Global Food Losses and Food Waste. Extent, Causes and Prevention; Food and Agricultural Organization: Düsseldorf, Germany, 2011.
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Gullino, M.L. Postharvest Pathology; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2010; Volume 2, 211p. [Google Scholar]
- Sustainable Use of Pesticides. European Parliament and of the Council; Directive 2009/128/EC. 21 October 2009.
- Conrath, U.; Beckers, G.J.; Flors, V.; Garcia-Agustin, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Beardon, E.; Ravnskov, S.; Scholes, J.; Ton, J. Optimizing chemically induced resistance in tomato against Botrytis cinerea. Plant Dis. 2016, 100, 704–710. [Google Scholar] [CrossRef]
- Luna, E.; López, A.; Kooiman, J.; Ton, J. Role of NPR1 and KYP in long-lasting induced resistance by β-aminobutyric acid. Front. Plant Sci. 2014, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, A.; Daniel, X.; Flors, V.; Luna, E.; Hohn, B.; Mauch-Mani, B. Descendants of primed Arabidopsis plants exhibit 1 resistance to biotic stress. Plant Physiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; De Vos, M.; Casteel, C.L.; Tian, D.; Halitschke, R.; Sun, J.Y.; Agrawal, A.A.; Felton, G.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.W.; Pastor, V.; Paplauskas, S.; Pétriacq, P.; Luna, E. Long-lasting β-aminobutyric acid-induced resistance protects tomato fruit against Botrytis cinerea. Plant Pathol. 2018, 67, 30–41. [Google Scholar] [CrossRef]
- Luna, E. Using green vaccination to brighten the agronomic future. Outlooks Pest Manag. 2016, 27, 136–140. [Google Scholar] [CrossRef]
- Dean, R.; Vam Kan, J.A.L.; Pretorious, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Tannous, J.; Sionov, E.; Keller, N.; Prusky, D. Apple intrinsic factors modulating the global regulator, LaeA, the Patulin gene cluster and patulin accumulation during fruit colonization by Penicillium expansum. Front. Plant Sci. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Puel, O.; Tadrist, S.; Delaforge, M.; Oswald, I.P.; Lebrihi, A. The inability of Byssochlamys fulva to produce patulin is related to absence of 6-methylsalicylic acid synthase and isoepoxydon dehydrogenase genes. Int. J. Food Microbiol. 2007, 115, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Fierer, N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 2013, 8, e59310. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub, R.; Gartemann, K.-H. The Clavibacter michiganensis subspecies: Molecular investigation of gram-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Sen, Y.; van derWolf, J.; Visser, R.; Heusden, A. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Dis. 2015, 99, 4–13. [Google Scholar] [CrossRef]
- Basim, H.; Basim, E.; Jones, J.B.; Minsavage, G.V.; Dickstein, E.R. Bacterial Spot of Tomato and Pepper Caused by Xanthomonas axonopodis pv. vesicatoria in the Western Mediterranean Region of Turkey. Plant Dis. 2004, 88, 85. [Google Scholar] [CrossRef]
- Talibi, I.; Boubaker, H.; Boudyach, H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Köckerling, E.; Karrasch, L.; Schweitzer, A.; Razum, O.; Krause, G. Public Health Research Resulting from One of the World’s Largest Outbreaks Caused by Entero-Hemorrhagic Escherichia coli in Germany 2011: A Review. Front. Public Health 2017, 5, 332. [Google Scholar] [CrossRef] [PubMed]
- Diallo, H.; Monger, W.; Kouassi, N.; Yoro, T.D.; Jones, P. Occurrence of Papaya ringspot virus infecting papaya in Ivory Coast. Plant Viruses 2008, 2, 52–57. [Google Scholar]
- Buchholz, F.; Kostić, T.; Sessitsch, A.; Mitter, B. The potential of plant microbiota in reducing postharvest food loss. Microb. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Hamid, M.I.; Ghazanfar, M.U. Salicylic acid induced resistance in fruits to combat against postharvest pathogens: A review. Arch. Phytopathol. Plant Prot. 2015, 48, 34–42. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.-R.; Lafuente, M.T. LED Blue Light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017, 218, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A. Effectiveness of a short hyperbaric treatment to control postharvest decay of sweet cherries and table grapes. Postharvest Biol. Technol. 2008, 49, 440–442. [Google Scholar] [CrossRef]
- Pane, C.; Parisi, M.; Zaccardelli, M.; Graziani, G.; Fogliano, V. Putative role of antioxidant activity of high pigment tomato cultivars in resistance against Botrytis cinerea post-harvest infection. Acta Hortic. 2011, 914, 429–432. [Google Scholar] [CrossRef]
- López-García, B.; Veyrat, A.; Pérez-Payá, E.; González-Candelas, L.; Marcos, J.F. Comparison of the activity of antifungal hexapeptides and the fungicides thiabendazole and imazalil against postharvest fungal pathogens. Int. J. Food Microbiol. 2003, 89, 163–170. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Nunes, C.; Torres, R.; Abadias, M.; Viñas, I. Preharvest strategies to control postharvest diseases in fruits. In Postharvest Pathology; Prusky, D., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 89–106. [Google Scholar] [CrossRef]
- Nunes, C.; Usall, J.; Teixidó, N.; Eribe, X.O.D.; Viñas, I. Control of post-harvest decay of apples by pre-harvest and post-harvest application of ammonium molybdate. Pest Manag. Sci. 2001, 57, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Northover, J.; Schneider, K.E. Physical modes of action of petroleum and plant oils on powdery and downy mildews of grapevines. Plant Dis. 1996, 80, 544–550. [Google Scholar] [CrossRef]
- Workneh, T.S.; Osthoff, G.; Steyn, M. Effects of preharvest treatment, disinfections, packaging and storage environment on quality of tomato. J. Food Sci. Technol. 2012, 49, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Everhart, S.; Askew, A.; Seymour, L.; Scherm, H. Spatio-temporal patterns of pre-harvest brown rot epidemics within individual peach tree canopies. Eur. J. Plant Pathol. 2013, 135, 499–508. [Google Scholar] [CrossRef]
- Ippolito, A.; Nigro, F. Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 2000, 19, 715–723. [Google Scholar] [CrossRef]
- Benbow, J.M.; Sugar, D. Fruit Surface Colonization and Biological Control of Postharvest Diseases of Pear by Preharvest Yeast Applications. Plant Dis. 1999, 83, 839–844. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Vinas, I. Efficacy of preharvest and postharvest Candida sake biocontrol treatments to prevent blue mould on apples during cold storage. Int. J. Food Microbiol. 1999, 50, 203–210. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Abdollahzadeh, G.; Damalas, C.; Rezaei, R. Farmers’ Criteria for Pesticide Selection and Use in the Pest Control Process. Agriculture 2018, 8, 24. [Google Scholar] [CrossRef]
- Chalutz, E.; Droby, S.; Wilson, C.L.; Wisniewski, M.E. UV-induced resistance to postharvest diseases of citrus fruit. J. Photochem. Photobiol. B Biol. 1992, 15, 367–371. [Google Scholar] [CrossRef]
- Rodov, V.; Ben-Yehoshua, S.; Fang, D.; D’Hallewin, G.; Castia, T. Accumulation of phytoalezxins scoparone and scopoletin in citrus fruits subjected to various postharvest treatments. Acta Hortic. 1994, 381, 517–525. [Google Scholar] [CrossRef]
- Droby, S.; Chalutz, E.; Horev, B.; Cohen, L.; Gaba, V.; Wilson, C.L.; Wisniewshi, M. Factors affecting UV-induced resistance in grapefruit against the green mould decay caused by Penicillium digitatum. Plant Pathol. 1993, 42, 418–424. [Google Scholar] [CrossRef]
- Lauxmann, M.A.; Brun, B.; Borsani, J.; Bustamante, C.A.; Budde, C.O.; Lara, M.V.; Drincovich, M.F. Transcriptomic profiling during the post-harvest of heat-treated dixiland Prunus persica fruits: Common and distinct response to heat and cold. PLoS ONE 2012, 7, e51052. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zheng, C.; Huang, Y.-P.; Wang, X.-L.; Luo, Z.-S.; Zheng, Y.-H. Hot air treatment activates defense responses and induces resistance against Botrytis cinerea in strawberry fruit. J. Integr. Agric. 2016, 15, 2658–2665. [Google Scholar] [CrossRef]
- Hashmi, M.S.; East, A.R.; Palmer, J.S.; Heyes, J.A. Strawberries inoculated after hypobaric treatment exhibit reduced fungal decay suggesting induced resistance. Acta Hortic. 2014, 1053, 163–168. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Pieterse, C.M.J. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zheng, Y.; Tang, S.; Rui, H.; Wang, C.Y. Enhancing disease resistance in peach fruit with methyl jasmonate. J. Sci. Food Agric. 2009, 89, 802–808. [Google Scholar] [CrossRef]
- Sima, P.; Fariborz, Z.-N.; Razieh, S.; Saeedeh, A.-S. Postharvest control of Rhizopus stolonifer in peach (Prunus persica L. Batsch) fruits using salicylic acid. J. Food Saf. 2012, 32, 502–507. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bi, Y.; Ge, Y.; Wang, Y.; Fan, C.; Li, D.; Deng, H. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Y.; Bi, Y.; Li, C.; Deng, H.; Hu, L.; Dong, B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014, 168, 113–119. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zeng, K. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to Colletotrichum gloeosporioides. J. Sci. Food Agric. 2016, 96, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Feng, J.; Zhang, P.; Jia, L.; Chen, K. Postharvest treatment with trans-2-hexenal induced resistance against Botrytis cinerea in tomato fruit. Australas. Plant Pathol. 2015, 44, 121–128. [Google Scholar] [CrossRef]
- Scholz, S.S.; Malabarba, J.; Reichelt, M.; Heyer, M.; Ludewig, F.; Mithöfer, A. Evidence for GABA-Induced Systemic GABA Accumulation in Arabidopsis upon Wounding. Front. Plant Sci. 2017, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2017, 213, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.R. β-Aminobutyric acid-induced resistance against plant pathogens. Plant Dis. 2002, 86, 448–457. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.; Zhou, T.; Zheng, X.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Lacombe, B.T.; Fromm, H. GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol. 2003, 13, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Wisniewski, M. Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu. Rev. Phytopathol. 2003, 27, 425–441. [Google Scholar] [CrossRef]
- Chan, Z.; Qin, G.; Xu, X.; Li, B.; Tian, S. Proteome Approach To Characterize Proteins Induced by Antagonist Yeast and Salicylic Acid in Peach Fruit. J. Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, A.; El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 2000, 19, 265–272. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, J.; Jin, P.; Liu, H.; Zheng, Y. Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 2014, 9, e112494. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, Y.; Wang, Y.; Deng, J.; Zhang, H.; Zhang, Z. Multiple preharvest treatments with harpin reduce postharvest disease and maintain quality in muskmelon fruit (cv. Huanghemi). Phytoparasitica 2014, 42, 155–163. [Google Scholar] [CrossRef]
- Lucon, C.M.M.; Guzzo, S.D.; de Jesus, C.O.; Pascholati, S.F.; de Goes, A. Postharvest harpin or Bacillus thuringiensis treatments suppress citrus black spot in ‘Valencia’ oranges. Crop Prot. 2010, 29, 766–772. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; van Hulten, M.; Zhang, Y.; Berkowitz, O.; López, A.; Pétriacq, P.; Sellwood, M.A.; Chen, B.; Burrell, M.; van de Meene, A.; et al. Plant perception of β-aminobutyric acid is mediated by an aspartyl-tRNA synthetase. Nat. Chem. Biol. 2014, 10, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Mohammadi, S.; Aminifard, M.H. Effect of postharvest salicylic acid treatment on fungal decay and some postharvest quality factors of kiwi fruit. Arch. Phytopathol. Plant Prot. 2013, 46, 1338–1345. [Google Scholar] [CrossRef]
- Tzortakis, N.G.; Economakis, C.D. Maintaining postharvest quality of the tomato fruit by employing methyl jasmonate and ethanol vapor treatment. J. Food Qual. 2007, 30, 567–580. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, L.; Jiang, S.; Dong, Y.; Zheng, X. Biocontrol of gray mold decay in peach fruit by integration of antagonistic yeast with salicylic acid and their effects on postharvest quality parameters. Biol. Control 2008, 47, 60–65. [Google Scholar] [CrossRef]
- Wanigasekara, U.; Adikaram, N.; Abayasekara, C. Pre-harvest chemical elicitor treatment enhances induced resistance in harvested banana fruit cv. ‘Embul’ and reduces anthracnose caused by Colletotrichum musae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 101–110. [Google Scholar] [CrossRef]
- Neri, F.; Mari, M.; Brigati, S.; Bertolini, P. Fungicidal Activity of Plant Volatile Compounds for Controlling Monilinia laxa in Stone Fruit. Plant Dis. 2007, 91, 30–35. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology 2003, 93, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Buswell, W.; Schwarzenbacher, R.E.; Luna, E.; Sellwood, M.; Chen, B.; Flors, V.; Pétriacq, P.; Ton, J. Chemical priming of immunity without costs to plant growth. New Phytol. 2018, 218, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Adikaram, N.K.B.; Joyce, D.C.; Terryc, L.A. Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas. Plant Pathol. 2002, 31, 223–229. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; González, M.B.; Garat, M.F.; Wisniewski, M. Aureobasidium pullulans as a biocontrol agent of postharvest pathogens of apples in Uruguay. Biocontrol Sci. Technol. 2009, 19, 1033–1049. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Tanou, G.; Minas, I.S.; Scossa, F.; Belghazi, M.; Xanthopoulou, A.; Ganopoulos, I.; Madesis, P.; Fernie, A.; Molassiotis, A. Exploring priming responses involved in peach fruit acclimation to cold stress. Sci. Rep. 2017, 7, 11358. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, J.; Nie, Y.; Fan, B.; Wu, S.; Zhang, Y.; Sheng, J.; Shen, L.; Zhao, R.; Tang, X. Salicylic-Acid-Induced Chilling- and Oxidative-Stress Tolerance in Relation to Gibberellin Homeostasis, C-Repeat/Dehydration-Responsive Element Binding Factor Pathway, and Antioxidant Enzyme Systems in Cold-Stored Tomato Fruit. J. Agric. Food Chem. 2016, 64, 8200–8206. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Singh, Z.; Khangura, R.; Ahmad, S. Management of citrus blue and green moulds through application of organic elicitors. Australas. Plant Pathol. 2012, 41, 69–77. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef] [PubMed]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M.; Abramowski, D. BABA-primed defense responses to Phytophthora infestans in the next vegetative progeny of potato. Front. Plant Sci. 2015, 6, 844. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Carrasco, G.; Martínez-Aguilar, K.; Alvarez-Venegas, R. Transgenerational defense priming for crop protection against plant pathogens: A hypothesis. Front. Plant Sci. 2017, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Gallusci, P.; Hodgman, C.; Teyssier, E.; Seymour, G.B. DNA methylation and chromatin regulation during fleshy fruit development and ripening. Front. Plant Sci. 2016, 7, 807. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; Rasori, A.; Varotto, S.; Bonghi, C. Rosaceae fruit development, ripening and post-harvest: An epigenetic perspective. Front. Plant Sci. 2017, 8, 1247. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Samiotaki, M.; Karamanoli, K.; Avramidou, E.; Ganopoulos, I.; Madesis, P.; Molassiotis, A. Ethylene-dependent and -independent superficial scald resistance mechanisms in ‘Granny Smith’ apple fruit. Sci. Rep. 2018, 8, 11436. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Liu, Y.; Wang, X.; Xu, Q.; Deng, X. Genome-wide identification of sweet orange (Citrus sinensis) histone modification gene families and their expression analysis during the fruit development and fruit-blue mold infection process. Front. Plant Sci. 2015, 6, 607. [Google Scholar] [CrossRef] [PubMed]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef] [PubMed]
| Pathogenic Microbes | Threats | Crops |
|---|---|---|
| Fungi | Botrytis cinerea | Tomatoes, citrus fruit, grapes, strawberries |
| Penicillium expansum | Apples, citrus fruit | |
| Penicillium digitatum | Apples, citrus fruit | |
| Penicillium italicum | Citrus fruit | |
| Plasmopara viticola | Grapes | |
| Rhizopus stolonifera | Strawberries | |
| Alternaria alternata | Tomatoes, grapes | |
| Fusarium spp. | Melons | |
| Trichothecium roseum | Cucurbits (e.g., melon) | |
| Colletotrichum gloeosporioides | Citrus fruit, bananas, mangoes, papayas | |
| Colletotrichum acutatum | Loquats | |
| Guignardia citricarpa | Citrus fruit | |
| Bacteria | Clavibacter michiganensis | Tomatoes |
| Xanthomonas axonopodis | Tomatoes, peppers | |
| Salmonella enterica | Tomatoes, melons | |
| Escherichi coli | Tomatoes, strawberries | |
| Viruses | Ringspot virus | Papayas |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pétriacq, P.; López, A.; Luna, E. Fruit Decay to Diseases: Can Induced Resistance and Priming Help? Plants 2018, 7, 77. https://doi.org/10.3390/plants7040077
Pétriacq P, López A, Luna E. Fruit Decay to Diseases: Can Induced Resistance and Priming Help? Plants. 2018; 7(4):77. https://doi.org/10.3390/plants7040077
Chicago/Turabian StylePétriacq, Pierre, Ana López, and Estrella Luna. 2018. "Fruit Decay to Diseases: Can Induced Resistance and Priming Help?" Plants 7, no. 4: 77. https://doi.org/10.3390/plants7040077
APA StylePétriacq, P., López, A., & Luna, E. (2018). Fruit Decay to Diseases: Can Induced Resistance and Priming Help? Plants, 7(4), 77. https://doi.org/10.3390/plants7040077






