Immunomodulatory and Inhibitory Effect of Immulina®, and Immunloges® in the Ig-E Mediated Activation of RBL-2H3 Cells. A New Role in Allergic Inflammatory Responses
Abstract
:1. Introduction
2. Results
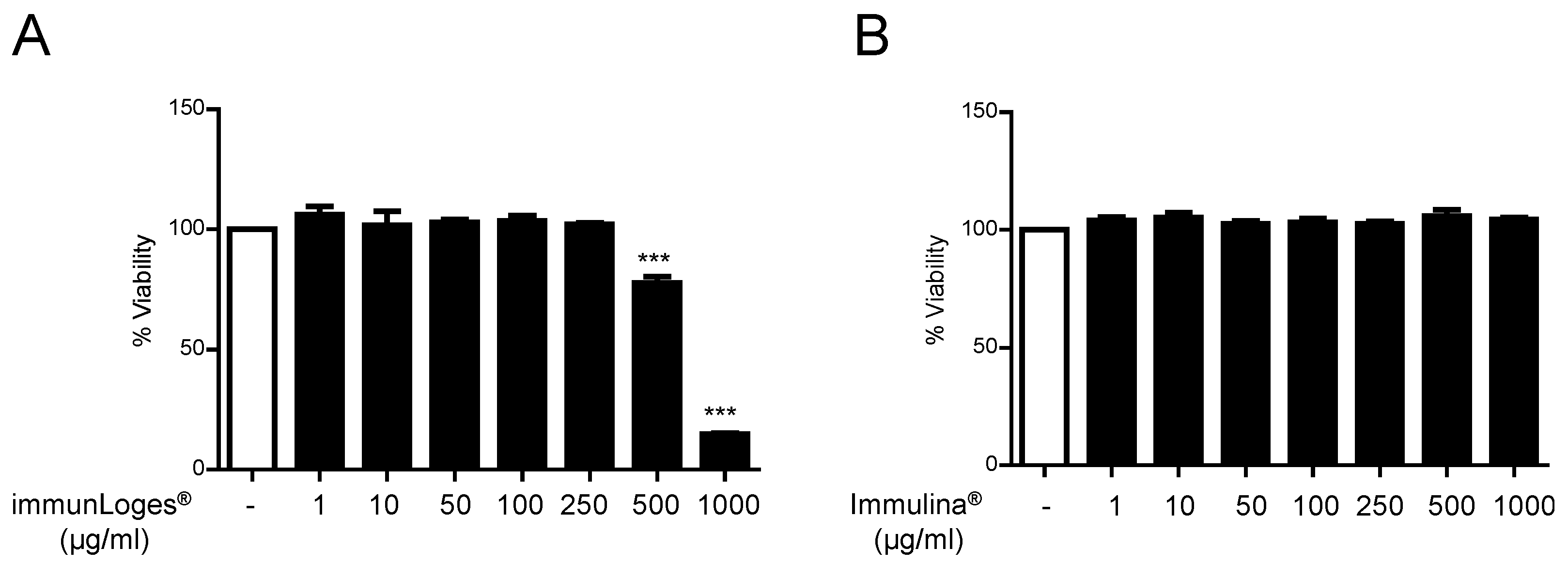
2.1. Cytotoxicity Effects of Immunloges® and Immulina® in Raw264.7 Cells
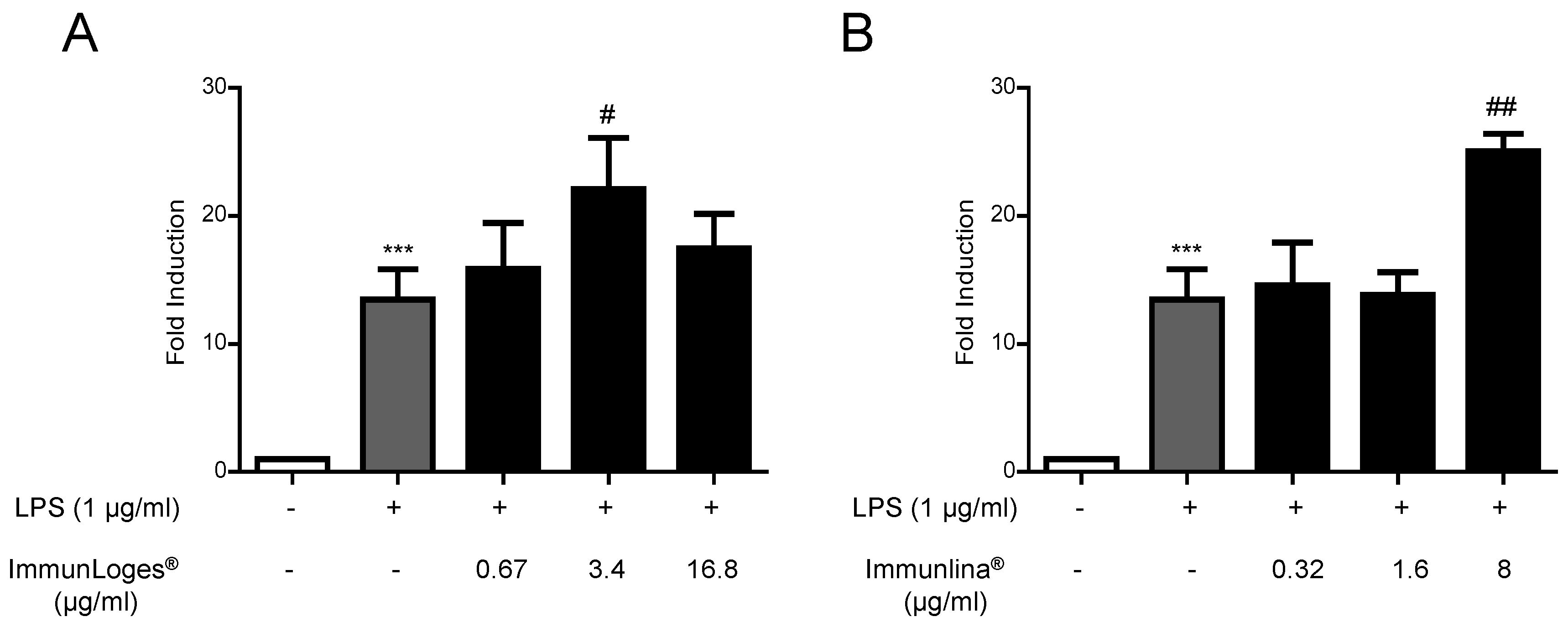
2.2. NF-ĸB Activation in Raw264.7 Cells
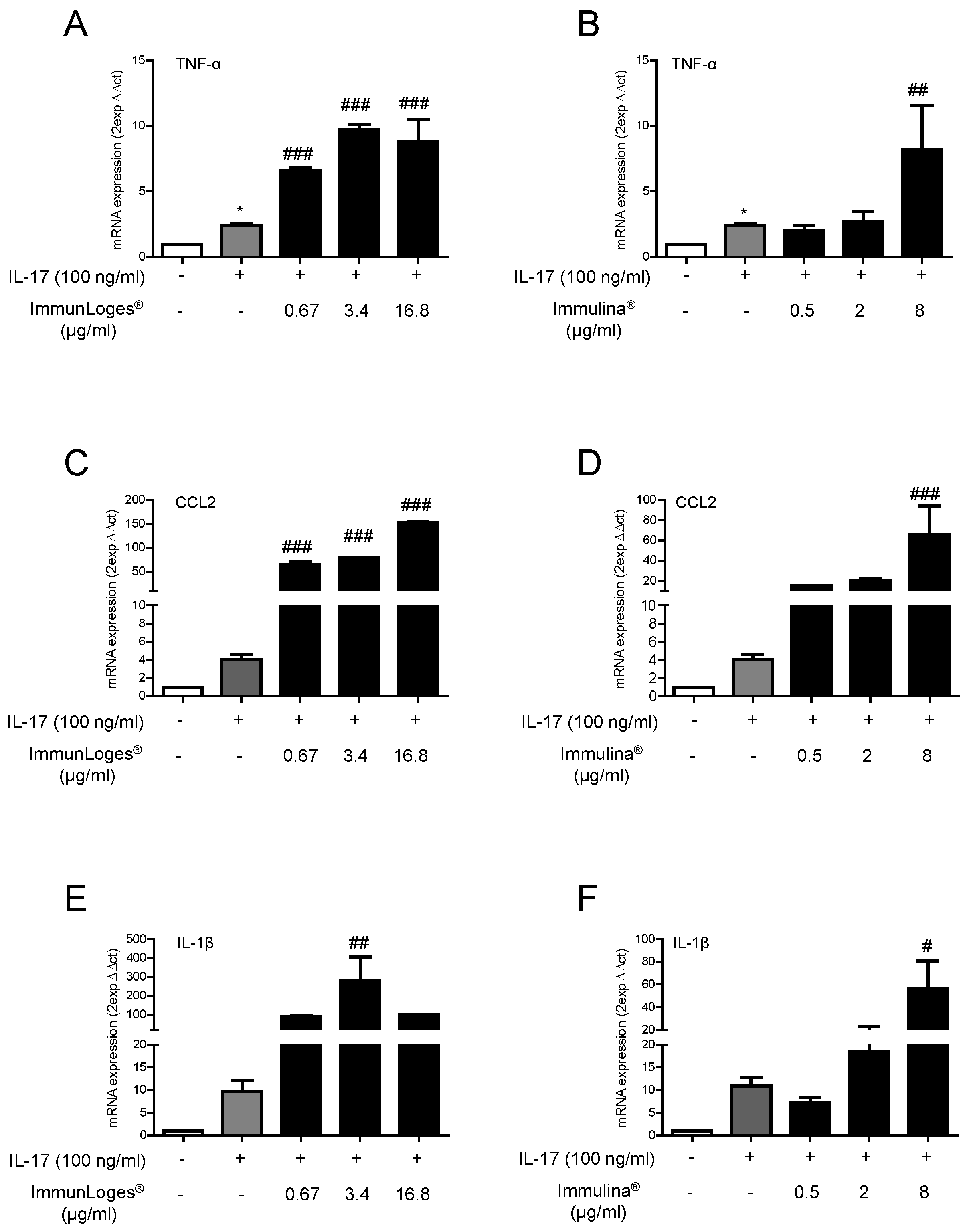
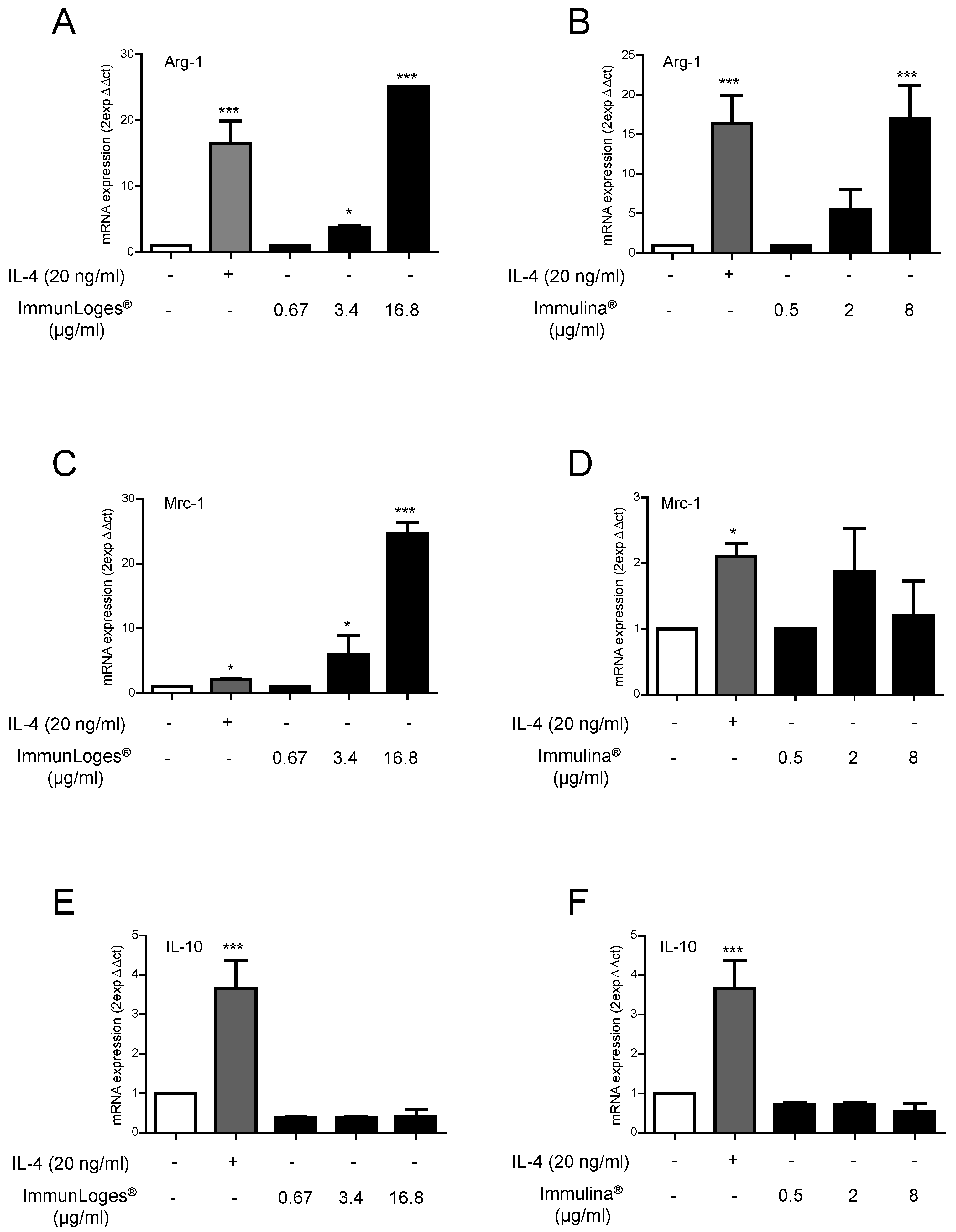
2.3. Effect of Immunloges® and Immulina® on M1 and M2 Polarization
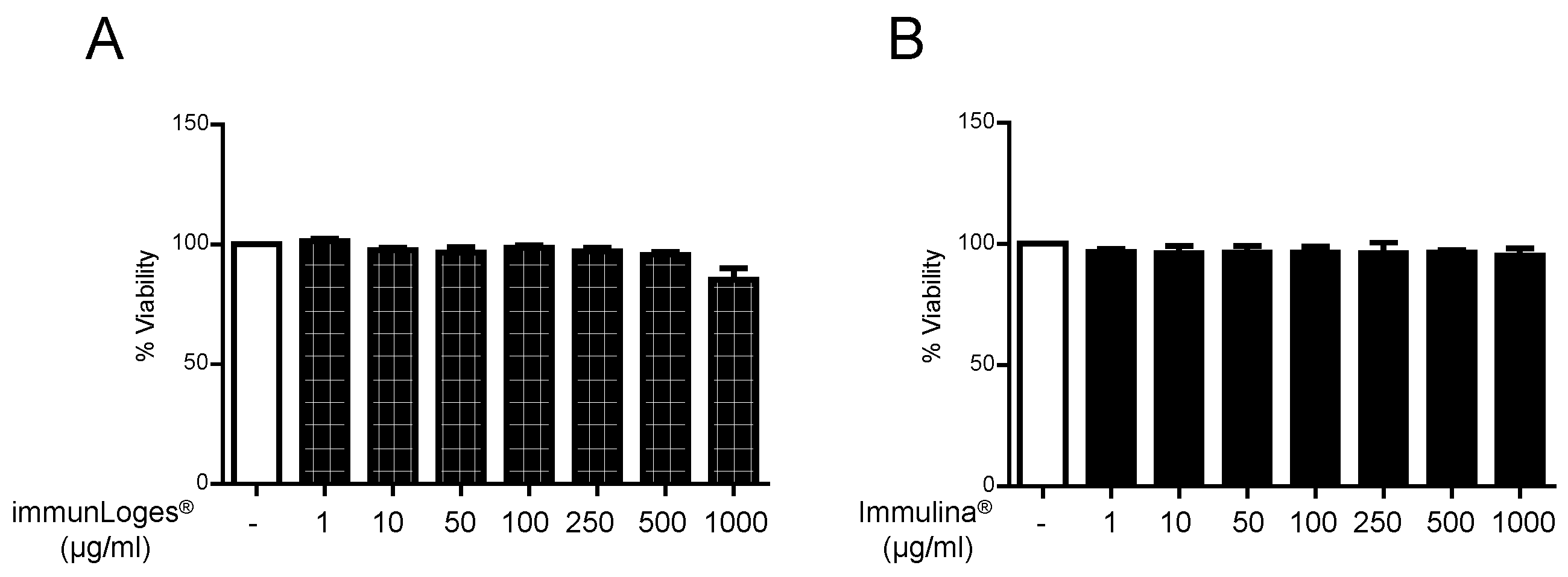
2.4. Cytotoxicity Effects of Immunloges® and Immulina® in RBL-2H3 Cells
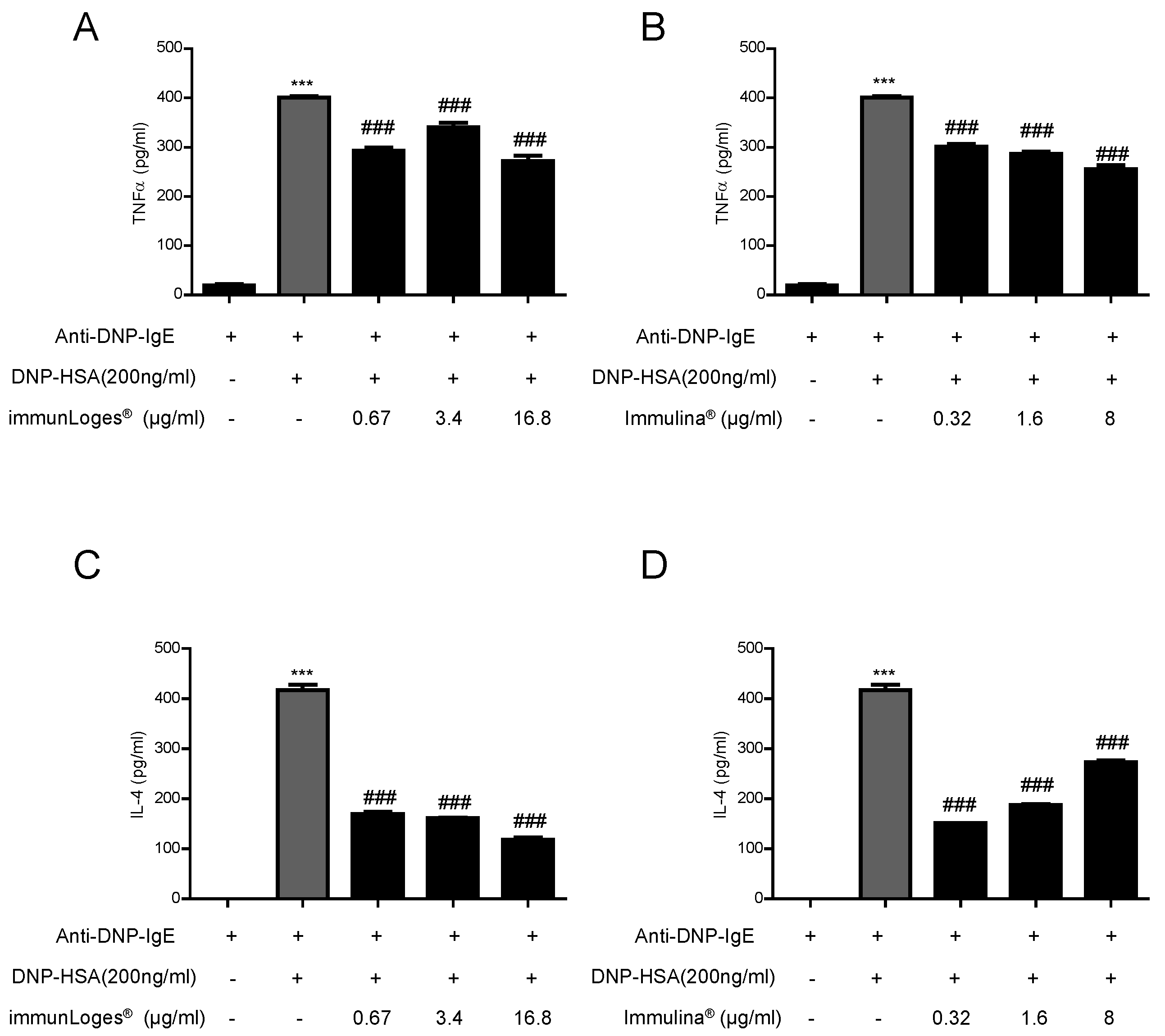
2.5. Effect on Cytokines Release in IgE-Antigen Complex Stimulated RBL-2H3 Cells
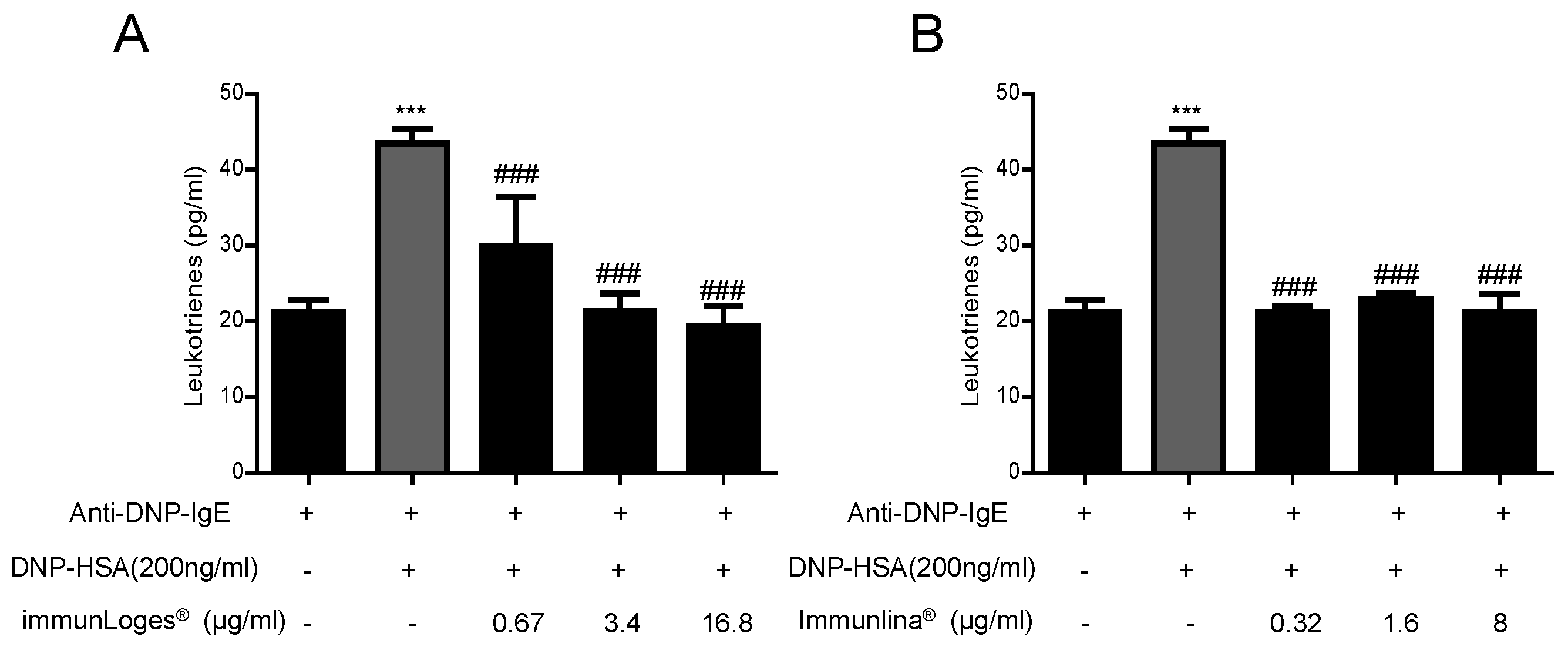
2.6. Effect on Leukotrienes Production in IgE-Antigen Complex-Stimulated RBL-2H3 Cells
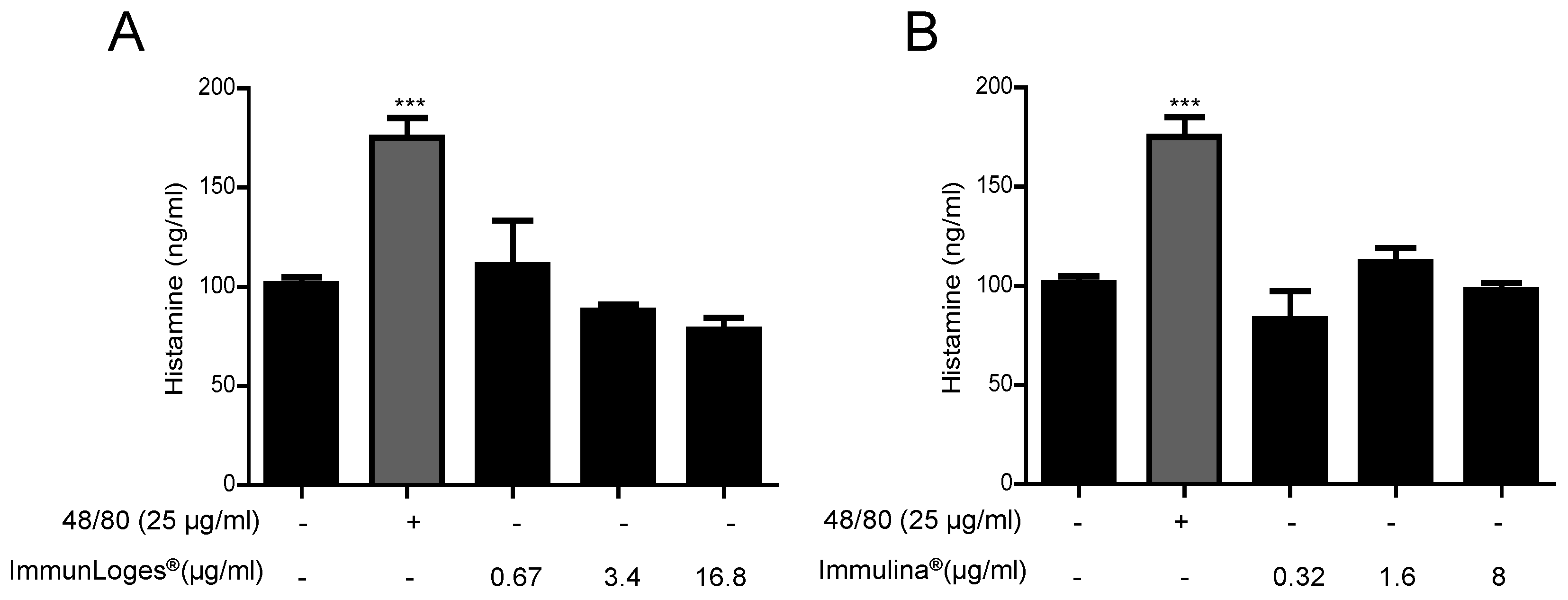
2.7. Effect on the Release of Histamine in IgE-Antigen Complex-Stimulated RBL-2H3 Cells
3. Discussion
4. Materials and Methods
4.1. Test items
4.2. Cell Culture
4.3. Cytotoxicity
4.4. NF-ĸB Activation in Raw264.7-KBF-Luc Cells
4.5. IL-17-Induced M1 Polarization
4.6. IL-4-Induced M2 Polarization
4.7. Detection of Leukotrienes and Cytokines in RBL-2H3 Cells
4.8. Detection of Histamine Release in RBL-2H3 Cells
4.9. RBL-2H3 Mast Cells Stabilization
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid. Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Lee, E.H.; Kim, H.M. Spirulina platensis inhibits anaphylactic reaction. Life Sci. 1997, 61, 1237–1244. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, E.H.; Cho, H.H.; Moon, Y.H. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by spirulina. Biochem. Pharmacol. 1998, 55, 1071–1076. [Google Scholar] [CrossRef]
- Mao, T.K.; Van de Water, J.; Gershwin, M.E. Effects of a spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J. Med. Food 2005, 8, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sayin, I.; Cingi, C.; Oghan, F.; Baykal, B.; Ulusoy, S. Complementary therapies in allergic rhinitis. ISRN Allergy 2013, 2013, 938751. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Pugh, N.D.; Ma, G.; Pasco, D.S. Toll-like receptor 2-dependent activation of monocytes by spirulina polysaccharide and its immune enhancing action in mice. Int. Immunopharmacol. 2006, 6, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from spirulina platensis, aphanizomenon flos-aquae and chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Polotsky, A.; Phan, P.V.; Pugh, N.; Pasco, D.; Frondoza, C.G. Immolina, a high-molecular-weight polysaccharide fraction of spirulina, enhances chemokine expression in human monocytic thp-1 cells. J. Altern. Complement. Med. 2006, 12, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lobner, M.; Walsted, A.; Larsen, R.; Bendtzen, K.; Nielsen, C.H. Enhancement of human adaptive immune responses by administration of a high-molecular-weight polysaccharide extract from the cyanobacterium arthrospira platensis. J. Med. Food 2008, 11, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.H.; Balachandran, P.; Christensen, O.; Pugh, N.D.; Tamta, H.; Sufka, K.J.; Wu, X.; Walsted, A.; Schjorring-Thyssen, M.; Enevold, C.; et al. Enhancement of natural killer cell activity in healthy subjects by immulina(r), a spirulina extract enriched for braun-type lipoproteins. Planta Med. 2010, 76, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.D.; Edwall, D.; Lindmark, L.; Kousoulas, K.G.; Iyer, A.V.; Haron, M.H.; Pasco, D.S. Oral administration of a spirulina extract enriched for braun-type lipoproteins protects mice against influenza a (h1n1) virus infection. Phytomedicine 2015, 22, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and m2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Hamdaoui, N.; Bizy, A.; Zoltani, K.; Souktani, R.; Zafrani, E.S.; Mallat, A.; Lotersztajn, S.; Lafdil, F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 2014, 59, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Quinnell, R.J.; Britton, J. Do helminth parasites protect against atopy and allergic disease? Clin. Exp. Allergy 2009, 39, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.J. Can helminths or helminth-derived products be used in humans to prevent or treat allergic diseases? Trends Immunol. 2009, 30, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S.; Schleimer, R.P. Mast cells, basophils, and eosinophils: Distinct but overlapping pathways for recruitment. Immunol. Rev. 2001, 179, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. New concepts about the mast cell. N. Engl. J. Med. 1993, 328, 257–265. [Google Scholar] [CrossRef]
- Nguyen, M.; Pace, A.J.; Koller, B.H. Age-induced reprogramming of mast cell degranulation. J. Immunol. 2005, 175, 5701–5707. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Levi-Schaffer, F. The human mast cell. J. Allergy Clin. Immunol. 1997, 99, 155–160. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Kaliner, M.; Donlon, M.A. The mast cell. Crit. Rev. Immunol. 1981, 3, 23–74. [Google Scholar] [PubMed]
- Yano, O.; Kanellopoulos, J.; Kieran, M.; Le Bail, O.; Israel, A.; Kourilsky, P. Purification of kbf1, a common factor binding to both h-2 and beta 2-microglobulin enhancers. EMBO J. 1987, 6, 3317–3324. [Google Scholar] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage polarization comes of age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Csoka, B.; Nemeth, Z.H.; Selmeczy, Z.; Koscso, B.; Pacher, P.; Vizi, E.S.; Deitch, E.A.; Hasko, G. Role of A2A adenosine receptors in regulation of opsonized E. coli-induced macrophage function. Purinergic Signal. 2007, 3, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Desnues, B.; Mege, J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Salinas, R.; Serafin-Lopez, J.; Ramos-Payan, R.; Mendez-Aragon, P.; Hernandez-Pando, R.; Van Soolingen, D.; Flores-Romo, L.; Estrada-Parra, S.; Estrada-Garcia, I. Differential pattern of cytokine expression by macrophages infected in vitro with different mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 2005, 140, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, M.E.; Gigliotti-Rothfuchs, A.; Wigzell, H. The role of ifn-gamma in the outcome of chlamydial infection. Curr. Opin. Immunol. 2002, 14, 444–451. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells–key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; St John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Friedman, S.; Seaf, M.; Levi-Schaffer, F. Mast cells and eosinophils in allergy: Close friends or just neighbors. Eur. J. Pharmacol. 2016, 778, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The role of IL-33 and mast cells in allergy and inflammation. Clin. Transl. Allergy 2015, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiang, Z. Roles and relevance of mast cells in infection and vaccination. J. Biomed. Res. 2016, 30, 253–263. [Google Scholar] [PubMed]
- Ferrer, M. Immunological events in chronic spontaneous urticaria. Clin. Transl. Allergy 2015, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.P.; Bot, I.; Kovanen, P.T. Mast cells in human and experimental cardiometabolic diseases. Nat. Rev. Cardiol. 2015, 12, 643–658. [Google Scholar] [CrossRef] [PubMed]

| Composition | Per Capsule |
|---|---|
| Immulina® | 200 mg |
| Betox 93® | 60 mg |
| Vineatrol® | 0.5 mg |
| Vitamin C | 40 mg |
| Vitamin D | 10 µg |
| Selenite | 50 µg |
| Zinc | 5 mg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appel, K.; Munoz, E.; Navarrete, C.; Cruz-Teno, C.; Biller, A.; Thiemann, E. Immunomodulatory and Inhibitory Effect of Immulina®, and Immunloges® in the Ig-E Mediated Activation of RBL-2H3 Cells. A New Role in Allergic Inflammatory Responses. Plants 2018, 7, 13. https://doi.org/10.3390/plants7010013
Appel K, Munoz E, Navarrete C, Cruz-Teno C, Biller A, Thiemann E. Immunomodulatory and Inhibitory Effect of Immulina®, and Immunloges® in the Ig-E Mediated Activation of RBL-2H3 Cells. A New Role in Allergic Inflammatory Responses. Plants. 2018; 7(1):13. https://doi.org/10.3390/plants7010013
Chicago/Turabian StyleAppel, Kurt, Eduardo Munoz, Carmen Navarrete, Cristina Cruz-Teno, Andreas Biller, and Eva Thiemann. 2018. "Immunomodulatory and Inhibitory Effect of Immulina®, and Immunloges® in the Ig-E Mediated Activation of RBL-2H3 Cells. A New Role in Allergic Inflammatory Responses" Plants 7, no. 1: 13. https://doi.org/10.3390/plants7010013
APA StyleAppel, K., Munoz, E., Navarrete, C., Cruz-Teno, C., Biller, A., & Thiemann, E. (2018). Immunomodulatory and Inhibitory Effect of Immulina®, and Immunloges® in the Ig-E Mediated Activation of RBL-2H3 Cells. A New Role in Allergic Inflammatory Responses. Plants, 7(1), 13. https://doi.org/10.3390/plants7010013





