Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts
Abstract
1. Introduction
2. Methods Used for Bioactive Compound Extraction, Isolation, and Purification
2.1. Extraction of Phenolic Compounds Using Solvents
2.2. Microwave-Assisted Extraction (MAE)
2.3. Ultrasonic-Assisted Extraction
2.4. Techniques of Isolation and Purification of Bioactive Molecules from Plants
2.5. Purification of the Bioactive Molecule
2.6. Structural Clarification of the Bioactive Molecules
2.7. UV-Visible Spectroscopy
2.8. Infrared Spectroscopy
2.9. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.10. Mass Spectrometry for Chemical Compounds Identification
3. Lipid Oxidation
- (1)
- Initiation:RH + initiator → RROOH + initiator → ROO•
- (2)
- Propagation:R + O2 → ROOROO + RH → ROOH + R•
- (3)
- Termination:R + R → R-RROO• + R → ROOH
4. Plants as a Source of Antioxidants
4.1. Presence of Antioxidant in Red Algae
4.2. Antioxidants from Monocots
4.3. Antioxidants from Vegetables
4.4. Antioxidants from Fruits
4.5. Cooking Herbs as an Important Source of Antioxidants
4.6. Antioxidant from Legumes
4.7. Antioxidants from Trees
4.8. Antioxidant from Shrubs
4.9. Characterization of Antioxidants from Other Eudicots
5. Plants Vitamins and Phenolic Compounds as Antioxidants
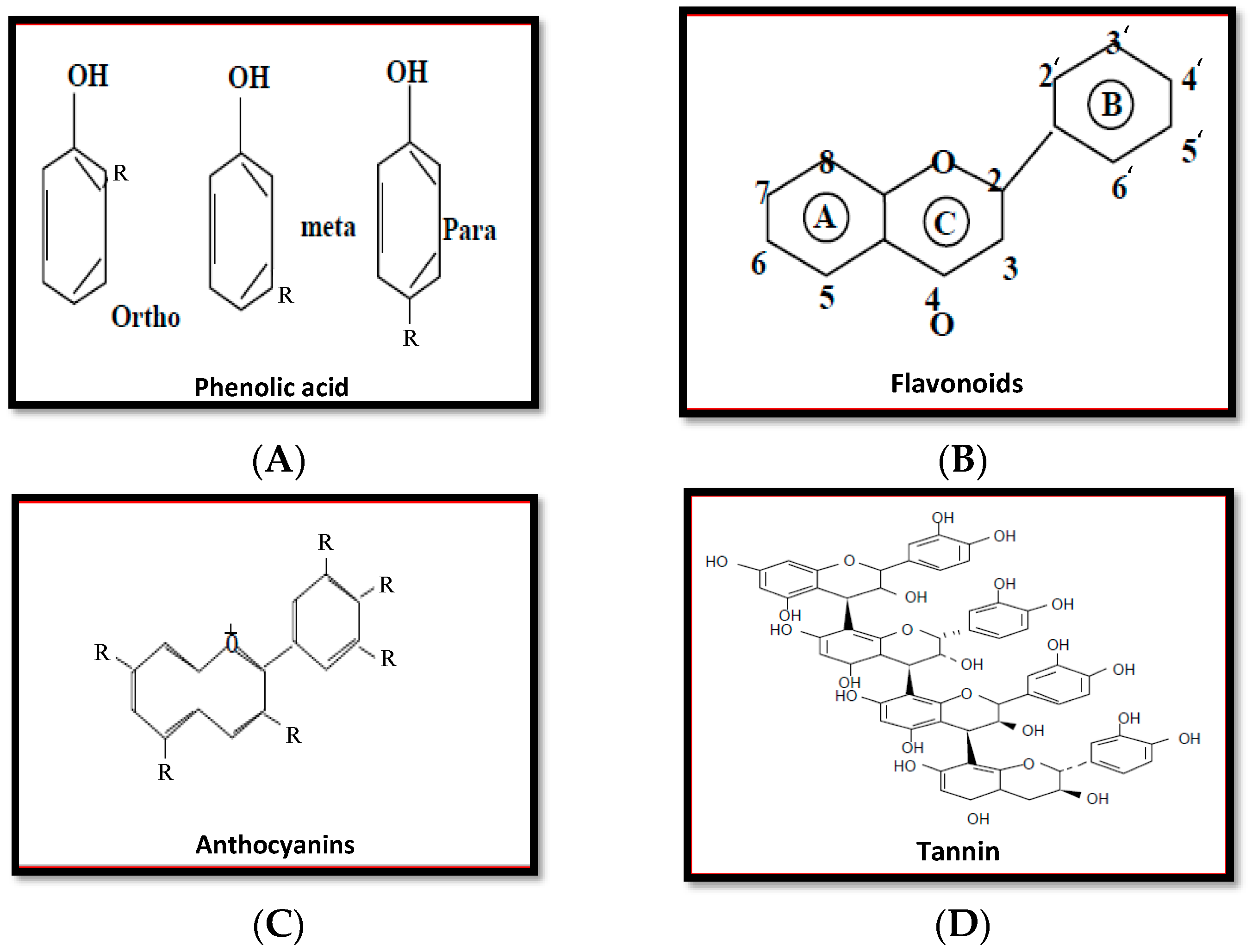
5.1. Phenolic Compounds
5.1.1. Phenols and Phenolic Acid
5.1.2. Flavonoids
5.1.3. Anthocyanins
5.1.4. Tannins
5.2. Vitamins Role in Cancer Prevention
6. Plants as an Antimicrobial Source
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jakubowski, W.; Bartosz, G. Estimation of oxidative stress in saccharomyces cerevisae with fluorescent probes. Int. J. Biochem. Cell Biol. 1997, 29, 1297–1301. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, I.B.; Sader, H.S.; Gonçalves, A.G.; Reis, A.O.; Gales, A.C.; Varella, A.D.; Younes, R.N. Screening of antibacterial extracts from plants native to the brazilian amazon rain forest and atlantic forest. Braz. J. Med. Biol. Res. 2004, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Ma, A.; Ross, M.A.; Collins, A.R. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996, 56, 1291–1295. [Google Scholar] [PubMed]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (petroselinum crispum) and cilantro (coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Ruan, Z.P.; Zhang, L.L.; Lin, Y.M. Evaluation of the antioxidant activity of syzygium cumini leaves. Molecules 2008, 13, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty three ivorian plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Anokwuru, C.P.; Anyasor, G.N.; Ajibaye, O.; Fakoya, O.; Okebugwu, P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nat. Sci. 2011, 9, 53–61. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Kingston, H.M.; Jessie, L.B. Introduction to Microwave Sample Preparation; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar]
- Suzara, S.; Costa, D.A.; Gariepyb, Y.; Rochaa, S.C.S.; Raghavanb, V. Spilanthol extraction using microwave: Calibration curve for gas chromatography. Chem. Eng. Trans. 2013, 32, 1783–1788. [Google Scholar]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Tsubaki, S.; Sakamoto, M.; Azuma, J. Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions. Food Chem. 2000, 123, 1255–1258. [Google Scholar] [CrossRef]
- Christophoridou, S.; Dais, P.; Tseng, L.H.; Spraul, M. Separation and identification of phenolic compounds in olive oil by coupling high-performance liquid chromatography with postcolumn solid-phase extraction to nuclear magnetic resonance spectroscopy (lc-spe-nmr). J. Agric. Food Chem. 2005, 53, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.J.; Raghavan, G.S.V.; Orsat, V.; Dai, J. Microwave-assisted extraction of capsaicinoids from capsicum fruit. J. Food Biochem. 2004, 28, 113–122. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the use of ultrasound in the extraction of antioxidants from rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.I.; Choi, J.B.; Lee, K.; Chung, M.S.; Pyun, Y.R. Antimicrobial activity of torilin isolated from torilis japonica fruit against bacillus subtilis. J. Food Sci. 2008, 73, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Luque-Garcı́a, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Mulinacci, N.; Prucher, D.; Peruzzi, M.; Romani, A.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Commercial and laboratory extracts from artichoke leaves: Estimation of caffeoyl esters and flavonoidic compounds content. J. Pharm. Biomed. Anal. 2004, 34, 349–357. [Google Scholar] [CrossRef]
- Altemimi, A.W.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a tlc-densitometric method. Chem. Cent. J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lightfoot, D.A.; Kinsel, M.; Watson, D.G. Employing response surface methodology for the optimization of ultrasound assisted extraction of lutein and β-carotene from spinach. Molecules 2015, 20, 6611–6625. [Google Scholar] [CrossRef] [PubMed]
- Sarajlija, H.; Čkelj, N.; Novotni, D.; Mršić, G.; Ćurić, M.; Brncic, M.; Curic, D. Preparation of flaxseed for lignan determination by gas chromatography-mass spectrometry method. Czech J. Food Sci. 2012, 30, 45–52. [Google Scholar]
- Popova, I.E.; Hall, C.; Kubátová, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Kemp, W. Energy and electromagnetic spectrum. In Organic Spectroscopy; Kemp, W., Ed.; Macmillan Press: London, UK, 1991; pp. 1–7. [Google Scholar]
- Kemp, W. Infrared spectroscopy. In Organic Spectroscopy; Macmillan Press Ltd.: London, UK, 1991; pp. 19–56. [Google Scholar]
- Urbano, M.; Luque de Castro, M.D.; Pérez, P.M.; García-Olmo, J.; Gómez-Nieto, M.A. Ultraviolet–visible spectroscopy and pattern recognition methods for differentiation and classification of wines. Food Chem. 2006, 97, 166–175. [Google Scholar] [CrossRef]
- Cherkaoui, A.; Hibbs, J.; Emonet, S.; Tangomo, M.; Girard, M.; Francois, P.; Schrenzel, J. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J. Clin. Microbiol. 2010, 48, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Beckey, H.D. 1–Theory of Field Ionization (FI) and Field Emission (FE); Pergamon: Bergama, Turkey, 1971. [Google Scholar]
- Beckey, H.D. 2–Field Ionization Sources; Pergamon: Bergama, Turkey, 1971. [Google Scholar]
- Beckey, H.D. 3–Application of the FI Mass Spectrometer to Physico-Chemical Problems; Pergamon: Bergama, Turkey, 1971. [Google Scholar]
- Beckey, H.D. 4–Qualitative Analyses with the Fi Mass Spectrometer; Pergamon: Bergama, Turkey, 1971. [Google Scholar]
- Kemp, W. Nuclear magnetic resonance spectroscopy. In Organic Spectroscopy; Kemp, W., Ed.; Macmillan Press: London, UK, 1991; pp. 101–240. [Google Scholar]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Clemente, T.E.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Lakhssassi, N.; Zhou, Z.; Liu, S.; Colantonio, V.; AbuGhazaleh, A.; Meksem, K. Characterization of the fad2 gene family in soybean reveals the limitations of gel-based tilling in genes with high copy number. Front. Plant Sci. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Yu, S.; Etherton, T.D.; Morgan, R.; Moriarty, K.; Shaffer, D. Fatty acids and progression of coronary artery disease. Am. J. Clin. Nutr. 1997, 65, 1088–1090. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Yu, S. Individual fatty acid effects on plasma lipids and lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1628–1644. [Google Scholar]
- Byfield, G.E.; Xue, H.; Upchurch, R.G. Two genes from soybean encoding soluble δ9 stearoyl-acp desaturases. Crop Sci. 2006, 46, 840–846. [Google Scholar] [CrossRef]
- Lakhssassi, N.; Colantonio, V.; Flowers, N.D.; Zhou, Z.; Henry, J.S.; Liu, S.; Meksem, K. Stearoyl-acyl carrier protein desaturase mutations uncover an impact of stearic acid in leaf and nodule structure. Plant Physiol. 2017, 174, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- St Angelo, A.J. Lipid oxidation on foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 175–224. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of iranian ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Negi, P.S.; Chauhan, A.S.; Sadia, G.A.; Rohinishree, Y.S.; Ramteke, R.S. Antioxidant and antibacterial activities of various seabuckthorn (hippophae rhamnoides l.) seed extracts. Food Chem. 2005, 92, 119–124. [Google Scholar] [CrossRef]
- Baharlouei, A.; Sharifi-Sirchi, G.R.; Bonjar, G.H.S. Identification of an antifungal chitinase from a potential biocontrol agent, streptomyces plicatus strain 101, and its new antagonistic spectrum of activity. Philipp. Agric. Sci. 2010, 93, 439–445. [Google Scholar]
- Baharlouei, A.; Sharifi-Sirchi, G.R.; Bonjar, G.H.S. Biological control of sclerotinia sclerotiorum (oilseed rape isolate) by an effective antagonist streptomyces. Afr. J. Biotechnol. 2011, 10, 5785–5794. [Google Scholar]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of foeniculum vulgare and crithmum maritimum essential oils. Planta Medica 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, N.J. Implications for the evolution of sex, multicellularity, and the mesoproterozoic/neoproterozoic radiation of eukaryotes. Paleobiology 2000, 26, 386–404. [Google Scholar] [CrossRef]
- Ashawat, M.S.; Shailendra, S.; Swarnlata, S. In vitro antioxidant activity of ethanolic extracts of centella asiatica, punica granatum, glycyrrhiza glabra and areca catechu. Res. J. Med. Plants 2007, 1, 13–16. [Google Scholar]
- Londonkar, R.; Kamble, A. Evaluation of free radical scavenging activity of pandanus odoratissimus. Int. J. Pharmacol. 2009, 5, 377–380. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Ahmad, I. The in vitro antioxidant activity and total phenolic content of four indian medicinal plants. J. Appl. Biol. Sci. 2007, 1, 87–90. [Google Scholar]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.E.; Watts, B.M. The antioxidant activity of vegetable extracts i. Flavone aglyconesa. J. Food Sci. 1964, 29, 27–33. [Google Scholar] [CrossRef]
- Plumb, G.W.; García-Conesa, M.T.; Kroon, P.A.; Rhodes, M.; Ridley, S.; Williamson, G. Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora. J. Sci. Food Agric. 1999, 79, 390–392. [Google Scholar] [CrossRef]
- Frankel, E.N.M.; Meyer, A. Antioxidants in grapes and grape juices and their potential health effects. Pharm. Biol. 1998, 36, 1–7. [Google Scholar] [CrossRef]
- Wangensteen, H.; Miron, A.; Alamgir, M.; Rajia, S.; Samuelsen, A.B.; Malterud, K.E. Antioxidant and 15-lipoxygenase inhibitory activity of rotenoids, isoflavones and phenolic glycosides from sarcolobus globosus. Fitoterapia 2006, 77, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomeä, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from vitis vinifera l. Cv. Graciano, tempranillo, and cabernet sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Beekwilder, J.; Schaart, J.G.; Mumm, R.; Soriano, J.M.; Jacobsen, E.; Schouten, H.J. Differences in acidity of apples are probably mainly caused by a malic acid transporter gene on lg16. Tree Genet. Genomes 2013, 9, 475–487. [Google Scholar] [CrossRef]
- Jugde, H.; Nguy, D.; Moller, I.; Cooney, J.M.; Atkinson, R.G. Isolation and characterization of a novel glycosyltransferase that converts phloretin to phlorizin, a potent antioxidant in apple. FEBS J. 2008, 275, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Doligez, A.; Fournier-Level, A.; Le Cunff, L.; Bertrand, Y.; Canaguier, A.; Morel, C.; Miralles, V.; Veran, F.; Souquet, J.M.; et al. Dissecting genetic architecture of grape proanthocyanidin composition through quantitative trait locus mapping. BMC Plant Biol. 2012, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Ubi, B.E.; Robinson, S.P.; Walker, A.R. Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin. Plant Sci. 2006, 170, 487–499. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Abdille, M.H.; Singh, R.P.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant activity of the extracts from dillenia indica fruits. Food Chem. 2005, 90, 891–896. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of holy basil and galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F.; Alasalvar, C. Antioxidant activity of cherry laurel fruit (laurocerasus officinalis roem.) and its concentrated juice. Food Chem. 2006, 99, 121–128. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Naz, Q.; Ejaz, A.; Yilmaz, G.; Kan, Y.; Konuklugil, B.; Şener, B.; Iqbal Choudhary, M. Antioxidant and anticholinesterase evaluation of selected turkish salvia species. Food Chem. 2007, 103, 1247–1254. [Google Scholar] [CrossRef]
- Rathee, J.S.; Hassarajani, S.A.; Chattopadhyay, S. Antioxidant activity of mammea longifolia bud extracts. Food Chem. 2006, 99, 436–443. [Google Scholar] [CrossRef]
- Baskar, R.; Rajeswari, V.; Kumar, T.S. In vitro antioxidant studies in leaves of annona species. Indian J. Exp. Biol. 2007, 45, 480–485. [Google Scholar] [PubMed]
- Lu, Y.; Yeap, F.L. Antioxidant activities of polyphenols from sage (salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Zhao, G.R.; Xiang, Z.J.; Ye, T.X.; Yuan, Y.J.; Guo, Z.X. Antioxidant activities of salvia miltiorrhiza and panax notoginseng. Food Chem. 2006, 99, 767–774. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Manian, S. The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (macrotyloma uniflorum (lam.) verdc.) seeds. Food Chem. 2007, 105, 950–958. [Google Scholar] [CrossRef]
- Samak, G.; Shenoy, R.P.; Manjunatha, S.M.; Vinayak, K.S. Superoxide and hydroxyl radical scavenging actions of botanical extracts of wagatea spicata. Food Chem. 2009, 115, 631–634. [Google Scholar] [CrossRef]
- Barla, A.; Öztürk, M.; Kültür, Ş.; Öksüz, S. Screening of antioxidant activity of three euphorbia species from turkey. Fitoterapia 2007, 78, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (prunus amygdalus) and pine sawdust (pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Seedi, H.R.; Mohammed, M.M. Phytochemical investigation and hepatoprotective activity of cupressus sempervirens l. Leaves growing in egypt. Nat. Prod. Res. 2007, 21, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Thind, T.S.; Singh, B.; Arora, S. Inhibition of lipid peroxidation by extracts/subfractions of chickrassy (chukrasia tabularis a. Juss.). Die Naturwissenschaften 2009, 96, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C. Chemical constituents and antimicrobial and antioxidant potentials of essential oil and acetone extract of nigella sativa seeds. J. Sci. Food Agric. 2005, 85, 2297–2306. [Google Scholar] [CrossRef]
- Tiwari, O.P.; Tripathi, Y.B. Antioxidant properties of different fractions of vitex negundo linn. Food Chem. 2007, 100, 1170–1176. [Google Scholar] [CrossRef]
- Zin, Z.M.; Abdul-Hamid, A.; Osman, A. Antioxidative activity of extracts from mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002, 78, 227–231. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Saavedra, G.; Murcia, M.A.; Jimenez, A.M.; Codina, C. Investigation of bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci. 2003, 73, 1667–1681. [Google Scholar] [CrossRef]
- Harish, R.; Shivanandappa, T. Antioxidant activity and hepatoprotective potential of phyllanthus niruri. Food Chem. 2006, 95, 180–185. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (psidium guajava l.) leaves. Food Chem. 2007, 101, 686–694. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Damien Dorman, H.J.; Koşar, M.; Hiltunen, R. Chemical composition and in vitro antioxidant evaluation of a water-soluble moldavian balm (dracocephalum moldavica l.) extract. LWT Food Sci. Technol. 2007, 40, 239–248. [Google Scholar] [CrossRef]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (morus indica l.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Ayaz, S.; Alpay-Karaoglu, S.; Gruz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. Var. Acephala dc.) extracts and their antioxidant and antibacterial activities. Food Chem. 1976, 7, 3. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (trigonella foenum graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Pandey, N.; Tripathi, Y.B. Antioxidant activity of tuberosin isolated from pueraria tuberose linn. J. Inflamm. 2010, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Q.; Ke, L.; Jiang, J.; Ying, T. Antioxidant activities of various extracts of lotus (nelumbo nuficera gaertn) rhizome. Asia Pac. J. Clin. Nutr. 2007, 16, 158–163. [Google Scholar] [PubMed]
- Meot-Duros, L.; Magne, C. Antioxidant activity and phenol content of crithmum maritimum l. Leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Sakat, S.; Juvekar, R.A.; Gambhire, M.N.; Juvekar, M.; Wankhede, S. In vitro antioxidant and anti-inflammatory activity of methanol extract of oxalis corniculata linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Jain, S.; Jain, A.; Jain, N.; Jain, D.K.; Balekar, N. Phytochemical investigation and evaluation of in vitro free radical scavenging activity of tabernaemontana divaricata linn. Nat. Prod. Res. 2010, 24, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Laitonjam, W.S.; Kongbrailatpam, B.D. Studies on the chemical constituents and antioxidant activities of extracts from the roots of smilax lanceaefolia roxb. Nat. Prod. Res. 2010, 24, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gandul-Rojas, B.; Cepero, M.R.; Minguez-Mosquera, M.I. Chlorophyll and carotenoid patterns in olive fruits, olea europaea cv. Arbequina. J. Agric. Food Chem. 1999, 47, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhang, S. Separation and identification of the yellow carotenoids in Potamogeton crispus L. Food Chem. 2008, 106, 410–414. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Abu-Ghazaleh, A.; Lightfoot, D.A. Evaluation of the antimicrobial activities of ultrasonicated spinach leaf extracts using rapd markers and electron microscopy. Arch. Microbiol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry Book; Springer: Dordrecht, The Netherland, 2006. [Google Scholar]
- Hursting, S.D.; Slaga, T.J.; Fischer, S.M.; DiGiovanni, J.; Phang, J.M. Mechanism-based cancer prevention approaches: Targets, examples, and the use of transgenic mice. J. Natl. Cancer Inst. 1999, 91, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.; Cheng, H.M.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radhakrishnan, A.K. Rind of the rambutan, nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nassr-allah, A.A.; Aboul-enein, K.M.; Lightfoot, D.A.; Cocchetto, A.; El-shemy, H.A. Anti-cancer and anti-oxidant activity of some egyptian medicinal plants. J. Med. Plants Res. 2009, 3, 799–808. [Google Scholar]
- El-Shemy, H.A.; Aboul-Enein, K.M.; Lightfoot, D.A. Predicting in silico which mixtures of the natural products of plants might most effectively kill human leukemia cells? Evid.-Based Complement. Altern. Med. 2013, 2013, 801501. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow leaves’ extracts contain anti-tumor agents effective against three cell types. PLoS ONE 2007, 2, e178. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.B. A study of the protective properties of iraqi olive leaves against oxidation and pathogenic bacteria in food applications. Antioxidants 2017, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin c contents of nectarine, peach, and plum cultivars from california. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Shariff, N.; Sudarshana, M.S.; Umesha, S.; Hariprasad, P. Antimicrobial activity of rauvolfia tetraphylla and physalis minima leaf and callus extracts. Afr. J. Biotechnol. 2006, 5, 946–950. [Google Scholar]
- Devi, P.U.; Murugan, S.; Suja, S.; Selvi, S.; Chinnaswamy, P.; Vijayanand, E. Antibacterial, in vitro lipid per oxidation and phytochemical observation on achyranthes bidentata blume. Pak. J. Nutr. 2007, 6, 447–451. [Google Scholar] [CrossRef]
- Dabur, R.; Gupta, A.; Mandal, T.K.; Singh, D.D.; Bajpai, V.; Gurav, A.M.; Lavekar, G.S. Antimicrobial activity of some indian medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.M.; Rajaguru, P.; Latha, M.; Ananthan, R. Ethanol extract of gymnema montanum leaves reduces glycoprotein components in experimental diabetes. Nutr. Res. 2007, 27, 97–103. [Google Scholar] [CrossRef]
- Zakaria, Z.; Sreenivasan, S.; Mohamad, M. Antimicrobial activity of piper ribesoides root extract against staphylococcus aureus. J. Appl. Biol. Sci. 2007, 1, 87–90. [Google Scholar]
- Ghosh, S.; Subudhi, E.; Nayak, S. Antimicrobial assay of stevia rebaudiana bertoni leaf extracts against 10 pathogens. Int. J. Integr. Biol. 2008, 2, 1–5. [Google Scholar]
- Mahesh, B.; Satish, S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J. Agric. Sci. 2008, 4, 839–843. [Google Scholar]
- Ehsan, B.R. Antimicrobial activity of the ethanolic extract of bryonopsis laciniosa leaf, stem, fruit and seed. Afr. J. Biotechnol. 2009, 8, 565–3567. [Google Scholar]
- Khond, M.; Bhosale, J.D.; Tasleem, A.; Mandal, T.; Padhi, M.M.; Dabur, R. Screening of some selected medicinal plants extracts for in-vitro antimicrobial activity. Middle-East J. Sci. Res. 2009, 4, 271–278. [Google Scholar]
- Pavithra, P.S.; Sreevidya, N.; Verma, R.S. Antibacterial and antioxidant activity of methanol extract of evolvulus nummularius. Indian J. Pharmacol. 2009, 41, 233–236. [Google Scholar] [PubMed]
- Patra, A.; Jha, S.; Murthy, P.N.; Vaibhav, A.D.; Chattopadhyay, P.; Panigrahi, G.; Roy, D. Anti-inflammatory and antipyretic activities of hygrophila spinosa t. Anders leaves (acanthaceae). Trop. J. Pharm. Res. 2009, 8, 133–137. [Google Scholar] [CrossRef]
- Akroum, S. Antimicrobial activity of some alimentary and medicinal plants. Afr. J. Microbiol. Res. 2012, 6, 1860–1864. [Google Scholar]
- Bajpai, V.K.; Rahman, A.; Shukla, S.; Mehta, A.; Shukla, S.; Arafat, S.M.Y.; Rahman, M.M.; Ferdousi, Z. Antibacterial activity of leaf extracts of pongamia pinnata from india. Pharm. Biol. 2009, 47, 1162–1167. [Google Scholar] [CrossRef]
- Bansal, S. Anti-bacterial efficacy of some plants used in folkaric medicines in arid zone. J. Pharm. Res. 2010, 3, 2640–2642. [Google Scholar]
- Vinothkumar, P.S.K.; Ahmed, P.; Sivamani, K.; Senthilkumar, B. Evaluation of antibacterial activities of andrographis paniculata leaf extract against gram positive and gram negative species by in vitro methods. J. Pharm. Res. 2010, 3, 1513–1515. [Google Scholar]
- Kumar, K.H.; Hullatti, K.K.; Sharanappa, P.; Sharma, P. Computative antimicrobial activity and tlcbioautographic analysis of root and aerial parts of andrographis serpyllifolia. Int. J. Pharm. Pharm. Sci. 2010, 2, 52–54. [Google Scholar]
- Jamuna, B.A.; Rai, V.R.; Samaga, P.V. Evaluation of the antimicrobial activity of three medicinal plants of south india. Malays. J. Microbiol. 2011, 7, 14–18. [Google Scholar] [CrossRef]
- Khanahmadi, M.; Rezazadeh, S.; Taran, M. In vitro antimicrobial and antioxidant properties of smyrnum cordifolium Boiss. (Umbelliferae) extract. Asian J. Plant Sci. 2010, 9, 99–103. [Google Scholar]
- Koperuncholan, M.; Kumar, P.S.; Sathiyanarayanan, G.; Vivek, G. Phytochemical screening and antimicrobial studies of some ethnomedicinal plants in south-eastern slope of western ghats. Int. J. Med. Res. 2010, 1, 48–58. [Google Scholar]
- Niranjan, M.H.; Kavitha, H.U.; Sreedharamurthy, S.; Sudarshana, M.S. Antibacterial activity of schrebera swietenioides roxb. Against some human pathogenic bacteria. J. Pharm. Res. 2010, 3, 1779–1781. [Google Scholar]
- Naveen, S.M.T. Evaluation of antibacterial activity of flower extracts of cassia auriculata. Ethnobot. Leafl. 2010, 14, 8–20. [Google Scholar]
| Antioxidant Assay | Mechanism | Endpoint | Quantification | Lipophilic and Hydrophilic AOC |
|---|---|---|---|---|
| ORAC | HAT | Fixed time | AUC | Yes |
| TRAP | HAT | Lag phase | IC50 lag time | No |
| FRAP | SET | Time varies | ∆OD fixed time | No |
| TEAC | SET | Time varies | ∆OD fixed time | Yes |
| DPPH | SET | IC50 | ∆OD fixed time | No |
| LDL oxidation | SET | Lag phase | Lag time | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. https://doi.org/10.3390/plants6040042
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017; 6(4):42. https://doi.org/10.3390/plants6040042
Chicago/Turabian StyleAltemimi, Ammar, Naoufal Lakhssassi, Azam Baharlouei, Dennis G. Watson, and David A. Lightfoot. 2017. "Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts" Plants 6, no. 4: 42. https://doi.org/10.3390/plants6040042
APA StyleAltemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G., & Lightfoot, D. A. (2017). Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants, 6(4), 42. https://doi.org/10.3390/plants6040042







