Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.)
Abstract
:1. Introduction
2. Results
2.1. Variation of Height Growth and Dry Weight of Leaves, Stem, Root and Total Biomass between the Treatments
2.2. Seasonal Variations in Photosynthesis Rate and Stomatal Conductance
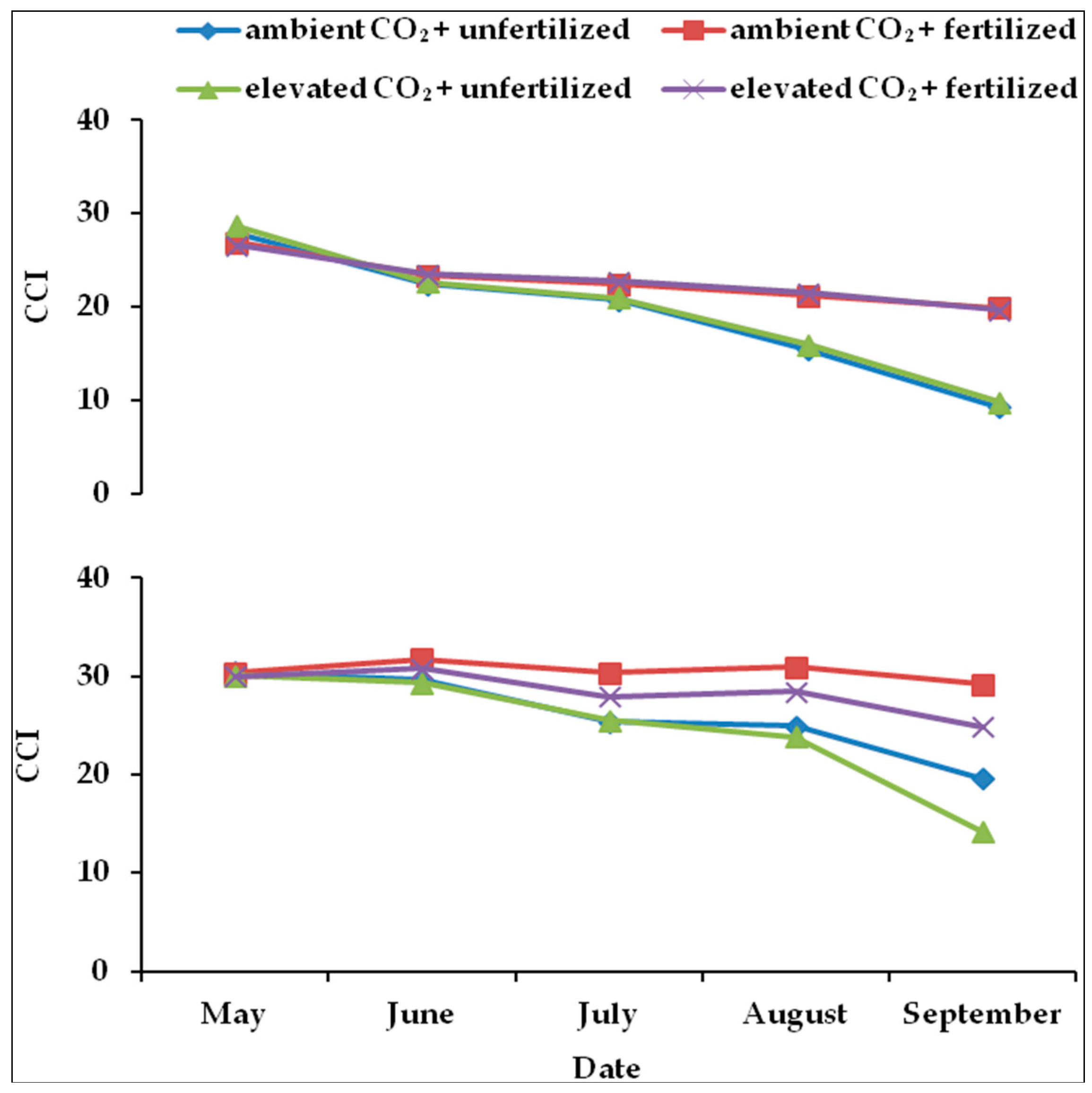
2.3. Chlorophyll Concentration Index (CCI) in Leaves
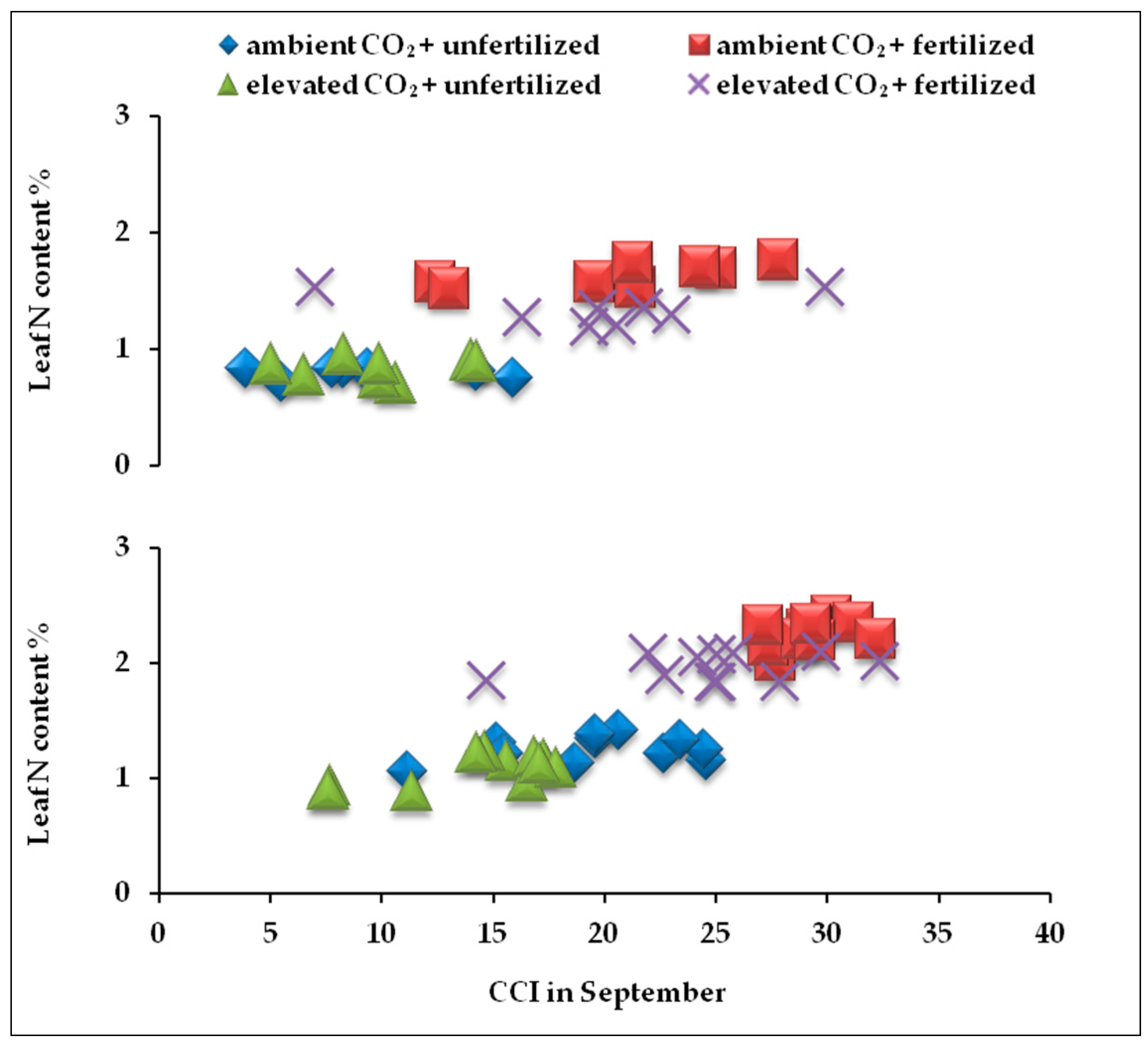
2.4. Correlation between Nitrogen Content and CCI at the end of Growing Seasons
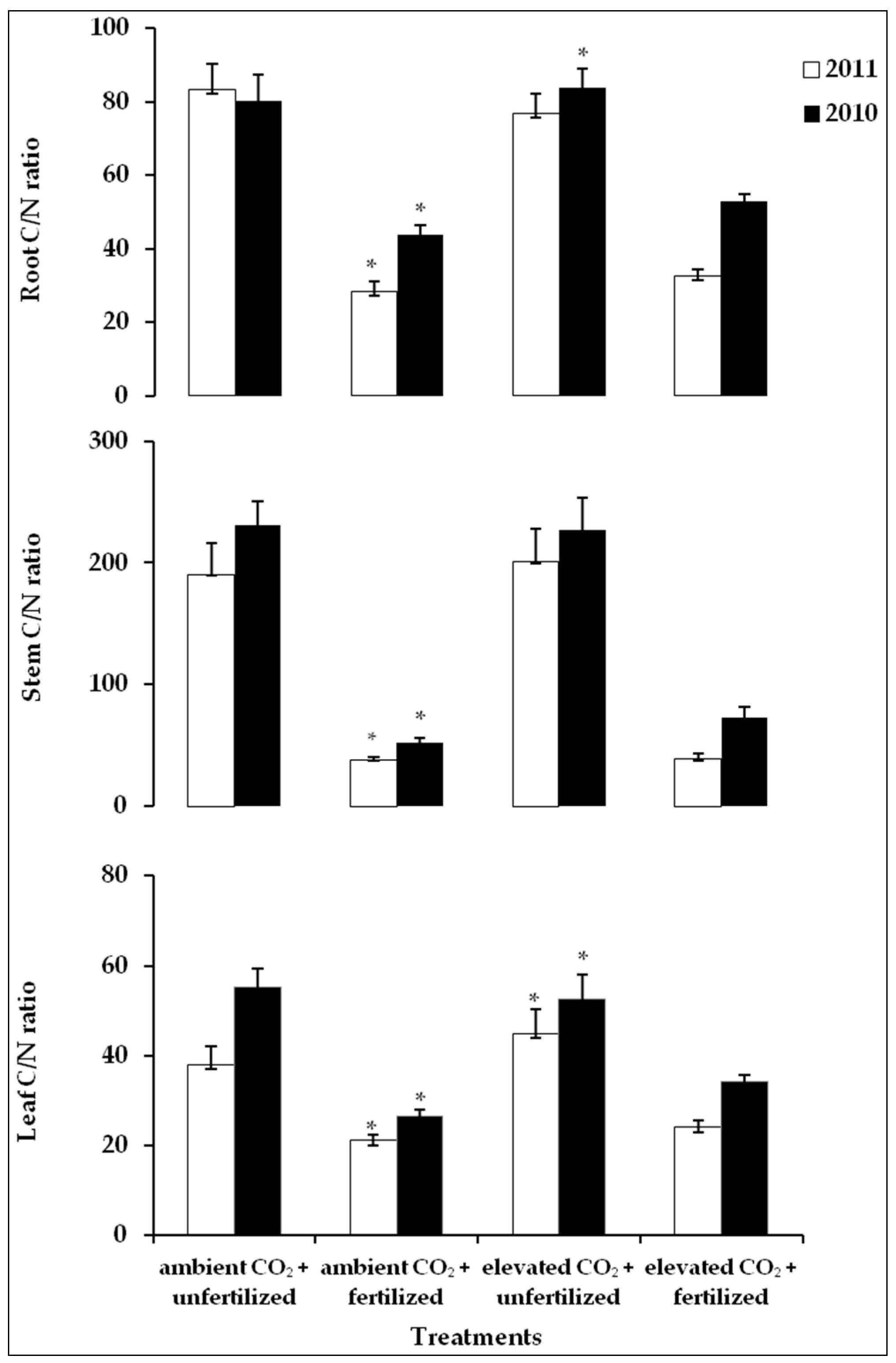
2.5. C/N Ratio in Plants Tissues
3. Discussion
3.1. Phenological Responses to Elevated CO2
3.2. Physiological Responses to Elevated CO2
3.3. Biochemical Responses to Elevated CO2
4. Materials and Methods
4.1. Growth Conditions and Experimental Design
4.2. Seasonal Gas Exchange Measurements
4.3. Growth Measurements
4.4. C/N Ratio
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burschel, P.; Weber, M. Der Treibhauseffekt. Bedrohung und Aufgabe für die Forstwirtschaft. Allg. Forstz. 1988, 43, 1010–1016. [Google Scholar]
- Matyssek, R.; Fromm, J.; Rennenberg, H.; Roloff, A. Biologie der Bäume; Ulmer-Verlag: Stuttgart, Germany, 2010. [Google Scholar]
- Cure, J.D.; Acock, B. Crop responses to CO2 doubling: A literature survey. Agric. For. Meteorol. 1986, 38, 127–145. [Google Scholar] [CrossRef]
- Sionit, N.; Kramer, P.J. Woody plants reactions to CO2 enrichment. In CO2 Enrichment and Greenhouse Crops; Enoch, H.Z., Timball, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1986; Volume II, pp. 69–85. [Google Scholar]
- Strain, B.R. Direct effects of increasing atmospheric CO2 on plants and ecosystems. Trends Ecol. 1987, 2, 18–21. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Evol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Miao, S.L. Successional status, seed size, and responses of tree seedlings to CO2, light, and nutrients. Ecology 1993, 74, 104–112. [Google Scholar] [CrossRef]
- Ceulemans, R.; Mousseau, M. Tansley Review No. 71. Effects of elevated atmospheric CO2 on woody plants. New Phythol. 1994, 127, 425–446. [Google Scholar] [CrossRef]
- Körner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phythol. 2006, 172, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.S. A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 1996, 19, 127–137. [Google Scholar] [CrossRef]
- Saxe, H.; Ellsworth, D.S.; Heath, D.S. Tansley Review no. 98. Tree and forest functioning in an enriched CO2 atmosphere. New Phythol. 1998, 139, 395–436. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Badeck, F.W.; DePury, D.G.G.; Barton, C.V.M.; Broadmeadow, M.; Ceulemans, R.; De Angelis, P.; Forstreuter, M.; Jach, M.E.; Kellomaki, S.; et al. Effects of elevated CO2 on photosynthesis in European forest species: A meta-analysis of model parameters. Plant Cell Environ. 1999, 22, 1475–1495. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2—Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef]
- Taub, D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Educ. Knowl. 2010, 1, Article 21. [Google Scholar]
- Long, S.P.; Drake, B.G. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In Crop Photosynthesis: Spatial and Temporal Determinants; Baker, N.R., Thomas, H., Eds.; Elsevier: New York, NY, USA, 1992; pp. 69–107. [Google Scholar]
- Ceulemans, R.; Janssens, I.A.; Jach, M.E. Effects of CO2 enrichment on trees and forests: Lessons to be learned in view of future ecosystem studies. Ann. Bot. 1999, 84, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- EL Kohen, A.; Venet, L.; Mousseau, M. Growth and photosynthesis of two deciduous forest species at elevated carbon dioxide. Funct. Ecol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Woodward, F.I. Potential impacts of global elevated CO2 concentrations on plants. Curr. Opin. Plant Biol. 2002, 5, 207–211. [Google Scholar] [CrossRef]
- Koike, T.; Watanabe, M.; Watanabe, Y.; Agathokleous, E.; Eguchi, N.; Takagi, K.; Satoh, F.; Kitaoka, S.; Funada, R. Ecophysiology of deciduous trees native to Northeast Asia grown under FACE (Free Air CO2 Enrichment). J. Agric. Meteorol. 2015, 71, 174–184. [Google Scholar] [CrossRef]
- Pritchard, S.; Rogers, H.; Prior, S.; Peterson, C. Elevated CO2 and Plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef]
- Poorter, H.; van Berkel, Y.; Baxter, R.; den Hertog, J.; Dijkstra, P.; Gifford, R.M.; Griffin, K.L.; Roy, R.J.; Wong, S.C. The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ. 1997, 20, 472–482. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phythol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Reekie, E.G.; Bazzaz, F.A. Competition patterns of resource use among seedlings of five tropical trees grown at ambient and elevated CO2. Oecologia 1989, 79, 212–222. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Garbutt, K. The response of annuals in competitive neighborhoods: Effects of elevated CO2. Ecology 1998, 69, 937–946. [Google Scholar] [CrossRef]
- Sionit, N.; Hellmers, H.; Strain, B.R. Interaction of atmospheric CO2 enrichment and irradiance on plant growth. Agron. J. 1982, 74, 721–725. [Google Scholar] [CrossRef]
- Garbutt, K.; Williams, W.E.; Bazzaz, F.A. Analysis of the differential response of five annuals to elevated CO2 during growth. Ecology 1990, 71, 1185–1194. [Google Scholar] [CrossRef]
- Bloom, A.J. As carbon dioxide rises, food quality will decline without careful nitrogen management. Calif. Agric. 2009, 63, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Norby, R.J.; Gunderson, C.A.; Wullschleger, S.D.; O’Neill, E.G.; McCracken, M.K. Productivity and compensatory response of yellow poplar trees in elevated CO2. Nature 1992, 357, 322–324. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Miglietta, F.; Raschi, A.; Körner, C. Thirty years of in situ growth under elevated CO2: A model for future forest responses? Glob. Chang. Biol. 1997, 3, 463–471. [Google Scholar] [CrossRef]
- Poorter, H.; Navas, M.L. Plant growth and competition at elevated CO2: On winners, losers and functional groups. New Phythol. 2003, 157, 175–198. [Google Scholar] [CrossRef]
- Roberntz, P.; Stockfors, J. Effects of elevated CO2 and nitrogen on net photosynthesis, stomatal conductance and needle respiration of field-grown Norway spruce trees. Tree Physiol. 1998, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2879. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. Concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Beerling, D.J.; Heath, J.; Woodward, F.I.; Mansfield, T.A. Drought—CO2 interactions in trees observations and mechanisms. New Phythol. 1996, 134, 235–242. [Google Scholar] [CrossRef]
- Overdieck, D.; Ziche, D.; Böttcher-Jungclaus, K. Temperature responses of growth and wood anatomy in European beech saplings grown in different carbon dioxide concentrations. Tree Physiol. 2007, 27, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Grams, T.E.E.; Anegg, S.; Häberle, K.H.; Langebartels, C.; Matyssek, R. Interactions of chronic exposure to elevated CO2 and O3 levels in the photosynthetic light and dark reactions of European beech (Fagus sylvatica L.). New Phythol. 1999, 144, 95–107. [Google Scholar] [CrossRef]
- Lütz, C.; Anegg, S.; Gerant, D.; Slaoui-Sosse, B.; Gerard, J.; Dizengremel, P. Beech trees exposed to high CO2 and to simulated summer ozone levels: Effects on photosynthesis, chloroplast components and leaf enzyme activity. Physiol. Plant. 2000, 109, 252–259. [Google Scholar] [CrossRef]
- Liu, X.; Kozovits, A.R.; Grams, T.E.E.; Blaschke, H.; Rennenberg, H.; Matyssek, R. Competition modifies effects of enhanced ozone/carbon dioxide concentrations on carbohydrate and biomass accumulation in juvenile Norway spruce and European beech. Tree Physiol. 2004, 24, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.A.D.; Approbato, A.U.; Legracie, J.R.; Martinez, C.A. Soil-nutrient availability modifies the response of young pioneer and late successional trees to elevated carbon dioxide in a Brazilian tropical environment. Environ. Exp. Bot. 2012, 77, 53–62. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.; Lambie, S.M. Re-analysis of plant CO2 responses during the exponential growth phase: Interactions with light, temperature, nutrients and water availability. Funct. Plant Biol. 2015, 42, 989–1000. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Yin, X. Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: A retrospective synthesis across 62 species. Glob. Chang. Biol. 2002, 8, 631–642. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant. Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Spiecker, H.; Mieläikinen, K.; Köhl, M.; Skovsgaard, J.P. Discussion. In Growth Trends in European Forests; Springer: Berlin/Heidelberg, Germany, 1996; pp. 355–367. [Google Scholar]
- Lotfiomran, N.; Fromm, J.; Luinstra, G.A. Effects of elevated CO2 and different nutrient supplies on wood structure of European beech (Fagus sylvatica) and gray poplar (Populus× canescens). IAWA J. 2015, 36, 84–97. [Google Scholar]
- Overdieck, D. Effects of atmospheric CO2 enrichment on CO2 exchange rates of beech stands in small model ecosystems. Water Air Soil Pollut. 1993, 70, 259–277. [Google Scholar] [CrossRef]
- Epron, D.; Liozon, R.; Mousseau, M. Effects of elevated CO2 concentration on leaf characteristics and photosynthetic capacity of beech (Fagus sylvatica) during the growing season. Tree Physiol. 1996, 16, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.; Kerstiens, G. Effects of elevated CO2 on leaf gas exchange in beech and oak at two levels of nutrient supply: Consequences for sensitivity to drought in beech. Plant Cell Environ. 1997, 20, 57–67. [Google Scholar] [CrossRef]
- Leverenz, J.W.; Bruhn, D.; Saxe, H. Responses of two provenances of Fagus sylvatica seedlings to a combination of four temperature and two CO2 treatments during their first growing season: Gas exchange of leaves and roots. New Phythol. 1999, 144, 437–454. [Google Scholar] [CrossRef]
- Liozon, R.; Badeck, F.W.; Genty, B.; Meyer, S.; Saugier, B. Leaf photosynthetic characteristics of beech (Fagus sylvatica L.) saplings during three years of exposure to elevated CO2 concentration. Tree Physiol. 2000, 20, 239–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mousseau, M.; Dufrene, E.; El Kohen, A.; Epron, D.; Godard, D.; Liozon, R.; Pontailler, J.Y.; Saugier, B. Growth Strategy and Tree Response to Elevated CO2: A Comparison of Beech (Fagus sylvatica) and Sweet Chestnut. In Carbon Dioxide and Terrestrial Ecosystems; Academic Press: San Diego, CA, USA, 1996; p. 71. [Google Scholar]
- Gifford, R.M.; Barrett, D.J.; Lutze, J.L. The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 2000, 224, 1–14. [Google Scholar] [CrossRef]
- De Graaff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Van Vuuren, M.M.I.; Robinson, D.; Fitter, A.H.; Chasalow, S.D.; Williamson, L.; Raven, J.A. Effects of elevated atmospheric CO2 and soil water availability on root biomass, root length and N, P and K uptake by wheat. New Phytol. 1997, 135, 455–465. [Google Scholar] [CrossRef]
- McDonald, E.P.; Erickson, J.E.; Kruger, E.L. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 2002, 29, 1115–1120. [Google Scholar] [CrossRef]
- Del Pozo, A.; Pérez, P.; Gutiérrez, D.; Alonso, A.; Morcuende, R.; Martínez-Carrasco, R. Gas exchange acclimation to elevated CO2 in upper-sunlit and lower-shaded canopy leaves in relation to nitrogen acquisition and partitioning in wheat grown in field chambers. Environ. Exp. Bot. 2007, 59, 371–380. [Google Scholar] [CrossRef]


| Height | Leaf | Stem | Root | Total biomass | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p-value | F | p-value | F | p-value | F | p-value | F | p-value | ||
| CO2 concentration | 2010 | 2.88 | 0.10 | 3.27 | 0.08 | 5.10 * | 0.03 | 0.02 | 0.88 | 2.18 | 0.15 |
| 2011 | 0.00 | 0.98 | 6.55 ** | 0.01 | 4.36 * | 0.04 | 2.32 | 0.14 | 1.76 | 0.19 | |
| Fertilization | 2010 | 1.16 | 0.29 | 1.35 | 0.25 | 0.15 | 0.70 | 1.53 | 0.22 | 1.05 | 0.31 |
| 2011 | 0.69 | 0.41 | 1.70 | 2.00 | 0.16 | 0.90 | 0.42 | 0.52 | 0.02 | 0.88 | |
| CO2 × Fertilization | 2010 | 1.65 | 0.21 | 0.87 | 0.36 | 1.30 | 0.26 | 0.14 | 0.70 | 0.25 | 0.62 |
| 2011 | 1.13 | 0.30 | 15.2 *** | 0.00 | 0.00 | 0.95 | 0.58 | 0.45 | 2.18 | 0.15 | |
| Source | Ac | gs | ||||
|---|---|---|---|---|---|---|
| MS | F | p-value | MS | F | p-value | |
| CO2 concentration | 28.02 | 5.54 * | 0.021 | 0.490 | 397.5 *** | 0.000 |
| Fertilization | 439.24 | 86.83 *** | 0.000 | 0.036 | 29.6 *** | 0.000 |
| CO2 × Fertilization | 3.22 | 0.636 | 0.428 | 0.008 | 6.86 * | 0.011 |
| Date | 620.55 | 165.25 *** | 0.000 | 0.097 | 61.394 *** | 0.000 |
| Date × CO2 | 6.49 | 1.73 | 0.193 | 0.014 | 8.592 *** | 0.000 |
| Date × Fertilization | 5.43 | 1.44 | 0.233 | 0.015 | 9.228 *** | 0.000 |
| CO2 concentration | Fertilization | CO2 × Fertilization | Leaf nitrogen content | ||
|---|---|---|---|---|---|
| CCI | p-value | p-value | p-value | values of Pearson correlation | |
| 2010 | 0.95 | 0.000 *** | 0.83 | 0.74 ** | |
| 2011 | 0.000 *** | 0.000 *** | 0.67 | 0.85 ** |
| Treatments | C/N ratio | ||||||
|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | |||||
| F | p-value | F | p-value | F | p-value | ||
| CO2 concentration | 2010 | 10.26 ** | 0.003 | 3.02 | 0.09 | 6.83 * | 0.012 |
| 2011 | 38.67 *** | 0.000 | 1.84 | 0,18 | 1.23 | 0.27 | |
| Fertilization | 2010 | 588.55 *** | 0.000 | 1122 *** | 0.000 | 201.5 *** | 0.000 |
| 2011 | 574.55 *** | 0.000 | 1378 *** | 0.000 | 2155 *** | 0.000 | |
| CO2 × Fertilization | 2010 | 20.49 *** | 0.000 | 3.90 | 0.12 | 1.43 | 0.24 |
| 2011 | 6.10 * | 0.016 | 1.33 | 0.25 | 24.5 *** | 0.000 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotfiomran, N.; Köhl, M.; Fromm, J. Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.). Plants 2016, 5, 38. https://doi.org/10.3390/plants5030038
Lotfiomran N, Köhl M, Fromm J. Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.). Plants. 2016; 5(3):38. https://doi.org/10.3390/plants5030038
Chicago/Turabian StyleLotfiomran, Neda, Michael Köhl, and Jörg Fromm. 2016. "Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.)" Plants 5, no. 3: 38. https://doi.org/10.3390/plants5030038
APA StyleLotfiomran, N., Köhl, M., & Fromm, J. (2016). Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.). Plants, 5(3), 38. https://doi.org/10.3390/plants5030038






