Understanding Plant Nitrogen Metabolism through Metabolomics and Computational Approaches
Abstract
:1. Introduction
2. Discussion
2.1. Role of Metabolism in NUE
2.2. Effect of Transgene Expression on Nitrogen Metabolism
2.3. Metabolomics Technology
2.4. Metabolic Flux Analysis
2.5. Modeling Fluxes in NUE
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hawkesford, M.J.; Barraclough, P. An overview of nutrient use efficiency and strategies for crop improvement. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops, 1st ed.; Hawkesford, M.J., Barraclough, P., Eds.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2011. [Google Scholar]
- Good, A.G.; Beatty, P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Franzluebbers, A.J.; Weyers, S.L.; Reicosky, D.C. Agricultural opportunities to mitigate greenhouse gas emissions. Environ. Pollut. 2007, 150, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Montzka, S.; Dlugokencky, E.; Butler, J. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Doney, S. The growing human footprint on coastal and open-ocean biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Drinkwater, L. The fate of nitrogen in grain cropping systems: A meta-analysis of 15N field experiments. Ecol. Appl. 2009, 19, 2167–2184. [Google Scholar] [CrossRef] [PubMed]
- Antman, A.; Brubaek, S.; Andersen, B.H.; Lindqvist, K.; Markus-Johansson, M.; Sorensen, J.; Teerikangas, J. Nordic Agriculture Air and Climate; Nordic Council of Ministers: Copenhagen, Denmark, 2015. [Google Scholar]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. Bioengineering nitrogen acquisition in rice: Can novel initiatives in rice genomics and physiology contribute to global food security? BioEssays 2004, 26, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.R.; Lochab, S.; Raghuram, N. Improving plant nitrogen-use efficiency. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 209–218. [Google Scholar]
- Amiour, N.; Imbaud, S.; Clément, G.; Agier, N.; Zivy, M.; Valot, B.; Balliau, T.; Armengaud, P.; Quilleré, I.; Cañas, R.; et al. The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps in the control of nitrogen metabolism in crops such as maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef] [PubMed]
- Shrawat, A.K.; Carroll, R.T.; DePauw, M.; Taylor, G.J.; Good, A.G. Genetic engineering of improved nitrogen use efficiency in rice by the tissue—specific expression of alanine aminotransferase. Plant Biotechnol. J. 2008, 6, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzandonakes, N.; Alston, J.; Bradford, K. Compliance costs for regulatory approval of new biotech crops. Nat. Biotechnol. 2007, 25, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.J.; Bi, Y.-M.; Coneva, V.; Han, M.; Good, A.G. The challenges of commercializing second-generation transgenic crop traits necessitate the development of international public sector research infrastructure. J. Exp. Bot. 2014. [Google Scholar] [CrossRef] [PubMed]
- Simo, C.; Ibanez, C.; Valdes, A.; Cifuentes, A.; Garcia-Canas, V. Metabolomics of genetically modified crops. Int. J. Mol. Sci. 2014, 15, 18941–18966. [Google Scholar] [CrossRef] [PubMed]
- Takehisa, H.; Sato, Y.; Antonio, B.A.; Nagamura, Y. Global transcriptome profile of rice root in response to essential macronutrient deficiency. Plant Signal. Behav. 2013, 8, e24409. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Lu, Y.; Xie, W.; Zhu, T.; Lian, X. Transcriptome response to nitrogen starvation in rice. J. Biosci. 2012, 37, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Humbert, S.; Subedi, S.; Cohn, J.; Zeng, B.; Bi, Y.M.; Chen, X.; Zhu, T.; McNicholas, P.D.; Rothstein, S.J. Genome-wide expression profiling of maize in response to individual and combined water and nitrogen stresses. BMC Genom. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, S.A.; Lewis, D.C.; Kennedy, G.; Furbank, R.T.; Jenkins, C.L.; Tabe, L.M. Large scale transcriptome analysis of the effects of nitrogen nutrition on accumulation of stem carbohydrate reserves in reproductive stage wheat. Plant Mol. Biol. 2008, 66, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, R.; Tong, Y.; Zhao, H.; Xie, Q.; Liu, D.; Zhang, A.; Li, B.; Xu, H.; An, D. Mapping QTLs for yield and nitrogen-related traits in wheat: Influence of nitrogen and phosphorus fertilization on QTL expression. Theor. Appl. Genet. 2014, 127, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Senthilvel, S.; Vinod, K.K.; Malarvizhi, P.; Maheswaran, M. QTL and QTL × environment effects on agronomic and nitrogen acquisition traits in rice. J. Integr. Plant Biol. 2008, 50, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Coque, M.; Gallais, A. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization in maize. Theor. Appl. Genet. 2006, 112, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Genome-enabled plant metabolomics. J. Chromatogr. B 2014, 966, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Grafahrend-Belau, E.; Weise, S.; Koschutzki, D.; Scholz, U.; Junker, B.H.; Schreiber, F. Metacrop: A detailed database of crop plant metabolism. Nucl. Acids Res. 2008, 36, D954–D958. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control of phosphate homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Weckwerth, W. Deciphering metabolic networks. Eur. J. Biochem. 2003, 270, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Edison, A.S.; Hall, R.D.; Junot, C.; Karp, P.D.; Kurland, I.J.; Mistrik, R.; Reed, L.K.; Saito, K.; Salek, R.M.; Steinbeck, C.; et al. The time is right to focus on model organism metabolomes. Metabolites 2016. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kusano, M. Recent progress in the development of metabolome databases for plant systems biology. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vander Gheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Oikawa, A.; Shirai, T.; Kuwahara, A.; Iijima, H.; Tanaka, K.; Hirai, M.Y. Capillary electrophoresis-mass spectrometry reveals the distribution of carbon metabolites during nitrogen starvation in Synechocstis sp. PCC 6803. Environ. Microbiol. 2014, 16, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Bölling, C.; Fiehn, O. Metabolite profiling of Chlamydomonas reinhardtii under nutrient deprivation. Plant Physiol. 2005, 139, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Wase, N.; Black, P.N.; Stanley, B.A.; DiRusso, C.C. Integrated quantitative analysis of nitrogen stress response in Chlamydomonas reinhardtii using metabolite and protein profiling. J. Proteome Res. 2014, 13, 1373–1396. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.C.; Steinfath, M.; Lisec, J.; Becher, M.; Witucka-Wall, H.; Torjek, O.; Fiehn, O.; Eckardt, A.; Willmitzer, L.; Selbig, J.; et al. The metabolic signature related to high plant growth rate in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 4759–4764. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, K.; El-kereamy, A.; Gidda, S.; Bi, Y.M.; Rothstein, S.J. AMT1; 1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. J. Exp. Bot. 2014, 65, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Paez-Valencia, J.; Sanchez-Lares, J.; Marsh, E.; Dorneles, L.T.; Santos, M.P.; Sanchez, D.; Winter, A.; Murphy, S.; Cox, J.; Trzaska, M.; et al. Enhanced proton translocating pyrophosphatase activity improves nitrogen use efficiency in Romaine lettuce. Plant Physiol. 2013, 161, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Xia, K.; Yang, X.; Grotemeyer, M.S.; Meier, S.; Rentsch, D.; Xu, X.; Zhang, M. Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol. J. 2013, 11, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.K.; Rochon, A.; Bi, Y.M.; Bozzo, G.G.; Rothstein, S.J.; Shelp, B.J. Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase 1. Physiol. Plant. 2011, 141, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Zhao, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. Accumulated expression level of cytosolic glutamine synthetase 1gene (OsGS1; 1 or OsGS1; 2) alter plant development and the carbon-nitrogen metabolic status in rice. PLoS ONE 2014, 9, e95581. [Google Scholar] [CrossRef]
- Habash, D.Z.; Massiah, A.J.; Rong, H.L.; Wallsgrove, R.M.; Leigh, R.A. The role of cytosolic glutamine synthetase in wheat. Ann. Appl. Biol. 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, B.; Pan, L.; Chen, L.; Fu, X.; Li, K. Overexpression of Arabidopsis Dof1, GS1 and GS2 Enhanced Nitrogen Assimilation in Transgenic Tobacco Grown Under Low-Nitrogen Conditions. Plant Mol. Biol. Rep. 2013, 31, 886–900. [Google Scholar] [CrossRef]
- Chickova, S.; Arellano, J.; Vance, C.P.; Hernandez, G. Transgenic tobacco plants the overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J. Exp. Bot. 2001, 52, 2079–2087. [Google Scholar]
- Ameziane, R.K.; Bernhard, R.B.; Lightfoot, D. Expression of the bacterial gdhA gene encoding NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 2000, 221, 47–57. [Google Scholar] [CrossRef]
- Brears, T.; Liu, C.; Knight, T.J.; Coruzzi, G.M. Ectopic overexpression of asparagine synthetase in transgenic tobacco. Plant Physiol. 1993, 103, 1285–1290. [Google Scholar] [PubMed]
- Xia, T.; Xiao, D.; Liu, D.; Chai, W.; Gong, Q.; Wang, N.N. Heterologous expression of ATG8c from soybean confers tolerance to nitrogen deficiency and increases yield in Arabidopsis. PLoS ONE 2012, 7, e37217. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Akiyama, A.; Kisaka, H.; Uchimiya, H.; Miwa, T. Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 7833–7888. [Google Scholar] [CrossRef] [PubMed]
- Kurai, T.; Wakayama, M.; Abiko, T.; Yanagisawa, S.; Aoki, N.; Ohsugi, R. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotech. J. 2011, 9, 826–837. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liang, G.; Li, Y.; Wang, F.; Yu, D. Two young microRNAs originating from target duplication mediate nitrogen starvation adaptation via regulation of glucosinolate synthesis in Arabidopsis thaliana. Plant Physiol. 2014, 164, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, H.; Hamera, S.; Chen, X.; Fang, R. miR444a has multiple functions in the rice nitrate-signaling pathway. Plant J. Cell Mol. Biol. 2014, 78, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-M.; Kant, S.; Clarke, J.; Clark, J.; Gidda, S.; Ming, F.; Rothstein, S.J. Increased nitrogen-use efficiency in transgenic rice plants over-expressing a nitrogen-responsive early nodulin gene identified from rice expression profiling. Plant Cell Environ. 2009, 32, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Minocha, R.; Long, S.; Minocha, S.C. Transgenic manipulation of a single polyamine in poplar cells affects the accumulation of all amino acids. Amino Acids 2010, 38, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Pracharoenwattana, I.; Zhou, W.; Keech, O.; Francisco, P.B.; Udomchalothorn, T.; Tschoep, H.; Stitt, M.; Gibon, Y.; Smith, S.M. Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J. Cell Mol. Boil. 2010, 62, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.J.; Beatty, P.H.; Good, A.G.; Muench, D.G. Review: Manipulation of microRNA Expression to Improve Nitrogen Use Efficiency. Plant Sci. 2013, 210, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Radchuk, R.; Radchuk, V.; Götz, K.P.; Weichert, H.; Richter, A.; Emery, R.J.; Weschke, W.; Weber, H. Ectopic expression of phosphoenolpyruvate carboxylase in Vicia narbonensis seeds: Effects of improved nutrient status on seed maturation and transcriptional regulatory networks. Plant J. Cell Mol. Boil. 2007, 51, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Shao, H.; Teixeira da Silva, J.A. A critical review on the improvement of photosynthetic carbon assimilation in C3 plants using genetic engineering. Crit. Rev. Biotech. 2012, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Work, V.; D’Adamo, S.; Radakovits, R.; Jinkerson, R.E.; Posewitz, M.C. Improving photosynthesis and metabolic networks for the competitive production of phototroph-derived biofuels. Curr. Opin. Biotech. 2012, 23, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, M.; O’Neill, M. Plant nucleotide-sugar formation, interconversion, and salvage by sugar recycling. Ann. Rev. Plant Biol. 2011, 62, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, R.L.; Zhu, T.; Rothstein, S.J. Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genom. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.A.; Stokes, T.L.; Thum, K.; Xu, X.; Obertello, M.; Katari, M.S.; Tanurdzic, M.; Dean, A.; Nero, D.C.; McClung, C.R. Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc. Natl. Acad. Sci. USA 2008, 105, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, K.; Kuroda, M.; Terauchi, K.; Hoshi, M.; Ikenaga, S.; Ishimaru, Y.; Abe, K.; Asakura, T. Additional nitrogen fertilization at heading time of rice down-regulated cellulose synthesis in seed endosperm. PLoS ONE 2014, 9, e98738. [Google Scholar] [CrossRef] [PubMed]
- Guevara, D.; El-Kereamy, A.; Yaish, M.W.; Mei-Bi, Y.; Rothstein, S.J. Functional Characterization of the Rice UDP-glucose 4-epimerase 1, OsUGE1: A Potential role in cell wall carbohydrate partitioning during limiting nitrogen conditions. PLoS ONE 2014, 9, e96158. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dai, X.Y.; Xu, Y.Y.; Chong, K. Over-expression of OsUGE-1 altered raffinose level and tolerance to abiotic stress but not morphology in Arabidopsis. J. Plant Physiol. 2007, 164, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, L.; Zhang, W.; Takechi, K.; Takano, H.; Lin, X. Overexpression of UDP-glucose pyrophosphorylase from Larix gmelinii enhances vegetative growth in transgenic Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Fang, G.; Zhao, Q.; Zeng, Q.; Li, X.; Gong, H.; Li, Y. Nitrite promotes the growth and decreases the lignin content of indica rice calli: A comprehensive transcriptome analysis of nitrite-responsive genes during in vitro culture of rice. PLoS ONE 2014, 9, e95105. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotech. 2011, 29, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, F.; Ratcliffe, R.G. NMR analysis of plant nitrogen metabolism. Photosynth. Res. 2005, 83, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, W.; Klein, M.S.; Kaspar, H.; Fagerer, S.R.; Nürnberger, N.; Dettmer, K. Urinary metabolite quantification employing 2D NMR spectroscopy. Anal. Chem. 2008, 23, 9288–9297. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.A.; Schommer, S.C.; Hodis, B.; Robb, K.A.; Tonelli, M.; Westler, W.M. Method for determining molar concentrations of metabolites in complex solutions from two-dimensional 1H-13C NMR spectra. Anal. Chem. 2007, 79, 9385–9390. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Quantitative metabolomics using NMR. Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Fan, T.W.; Lane, A.N.; Higashi, R.M. In Vivo and In Vitro Metabolomic Analysis of Anaerobic Rice Coleoptiles Revealed Unexpected Pathways. Russ. J. Plant Physiol. 2003, 50, 787–793. [Google Scholar] [CrossRef]
- Hinse, C.; Richter, C.; Provenzani, A.; Stöckigt, J. In vivo monitoring of alkaloid metabolism in hybrid plant cell cultures by 2D cryo-NMR without labelling. Bioorg. Med. Chem. 2003, 11, 3913–3919. [Google Scholar] [CrossRef]
- Aubert, S.; Hennion, F.; Bouchereau, A.; Gout, E.; Bligny, R.; Dorne, A.-J. Subcellular compartmentation of proline in the leaves of the subantarctic Kerguelen cabbage Pringlea antiscorbutica R. Br. In vivo 13C-NMR study. Plant Cell Environ. 1999, 22, 255–259. [Google Scholar] [CrossRef]
- Chiwocha, S.D.; Abrams, S.R.; Ambrose, S.J.; Cutler, A.J.; Loewen, M.; Ross, A.R.; Kermode, A.R. A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: An analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J. 2003, 35, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.; Rantalainen, M.; Li, J.V.; Maher, A.D.; Malmodin, D.; Ahmadi, K.R.; Faber, J.H.; Barrett, A.; Min, J.L.; Rayner, N.W.; et al. A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS Genet. 2011, 7, e1002270. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.; Higashi, R.M. Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Biophys. Tools Biol. 2008, 84, 541–588. [Google Scholar]
- Nelson, C.J.; Alexova, R.; Jacoby, R.P.; Millar, A.H. Proteins with High Turnover Rate in Barley Leaves Estimated by Proteome Analysis Combined with in Planta Isotope Labeling. Plant Physiol. 2014, 166, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.-M.; Lane, A.N. NMR-based stable isotope resolved metabolomics in systems biochemistry. J. Biomol. NMR 2011, 49, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Gianchandani, E.P.; Papin, J.A. Flux balance analysis in the era of metabolomics. Brief Bioinform. 2006, 7, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.S.; Palsson, B.O. Metabolic flux balance analysis and the in silico analysis of Escherichia coli K-12 gene deletions. BMC Bioinform. 2000, 1. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Lewis, I.A.; Morrisey, J.M.; McLean, K.J.; Ganesan, S.M.; Painter, H.J.; Mather, M.W.; Jacobs-Lorena, M.; Llinas, M.; Vaidya, A.B. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015, 11, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Fowler, W.U.; Kimball, E.; Lu, W.; Rabinowitz, J.D. Kinetic flux profiling of nitrogen assimilation in Escherichia coli. Natl. Chem. Biol. 2006, 2, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Grafahrend-Belau, E.; Junker, A.; Eschenroder, A.; Muller, J.; Schreiber, F.; Junker, B.H. Multiscale Metabolic Modeling: Dynamic Flux Balance Analysis on a Whole-Plant Scale. Plant Physiol. 2013, 163, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Resendis-Antonio, O.; Reed, J.L.; Encarnacion, S.; Collado-Vides, J.; Palsson, B. Metabolic reconstruction and modeling of nitrogen fixation in Rhizobium etli. PLoS Comput. Biol. 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Kundu, S. Flux balance analysis of genome-scale metabolic model of rice (Oryza sativa): Aiming to increase biomass. J. Biosci. 2015, 40, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Ratcliffe, R.G. Flux-Balance Modeling of Plant Metabolism. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Dal’Molin, C.G.; Quek, L.E.; Palfreyman, R.W.; Brumbley, S.M.; Nielsen, L.K. C4gem, a genome-scale metabolic model to study c4 plant metabolism. Plant Physiol. 2010, 154, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Saha, R.; Guillard, L.; Clement, G.; Armengaud, P.; Canas, R.; Maranas, C.D.; Lea, P.J.; Hirel, B. Nitrogen-use efficiency in maize (Zea mays l.): From ‘omics’ studies to metabolic modelling. J. Exp. Bot. 2014, 65, 5657–5671. [Google Scholar] [CrossRef] [PubMed]
- Braune, H.; Müller, J.; Diepenbrock, W. Measurement and Modelling Awn Photosynthesis of Barley (Hordeum vulgare L.) for Virtual Crop Models. Pflanzenbauwissenschaften 2007, 11, 10–15. [Google Scholar]
- Good, A.G.; Beatty, P.H. Biotechnological Approaches to Improving Nitrogen Use Efficiency in Plants: Alanine Aminotransferase as a Case Study. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops, 1st ed.; Hawkesford, M.J., Barraclough, P., Eds.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2011. [Google Scholar]

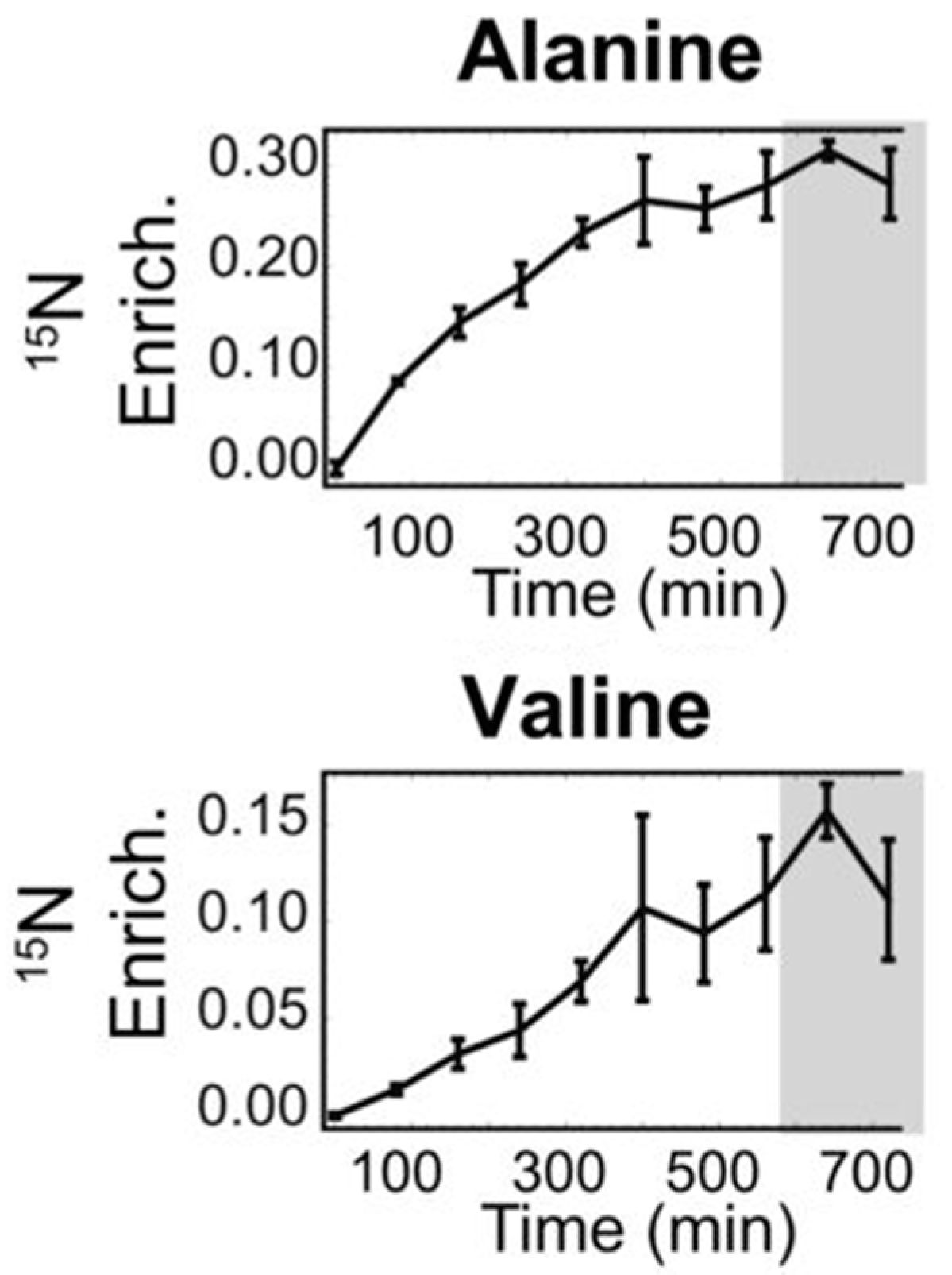
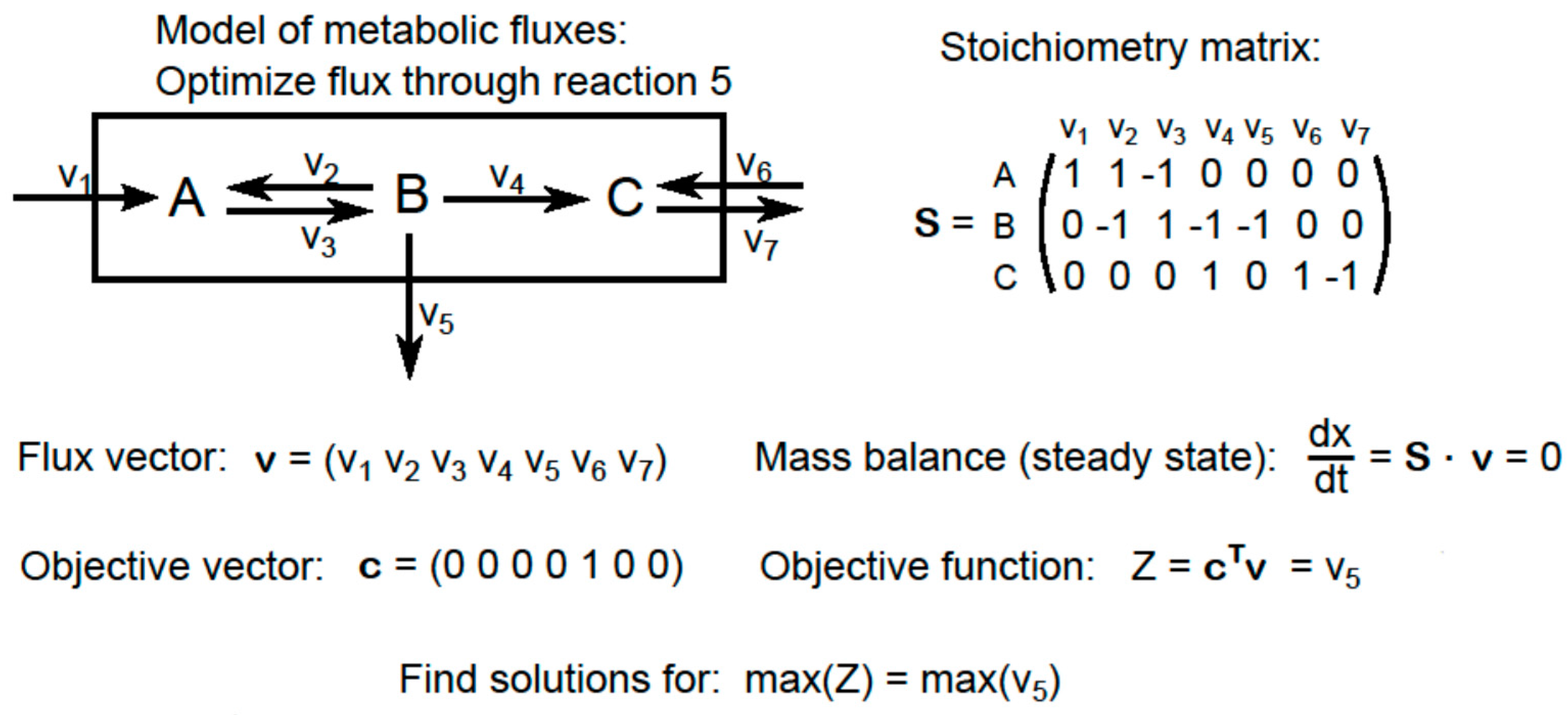
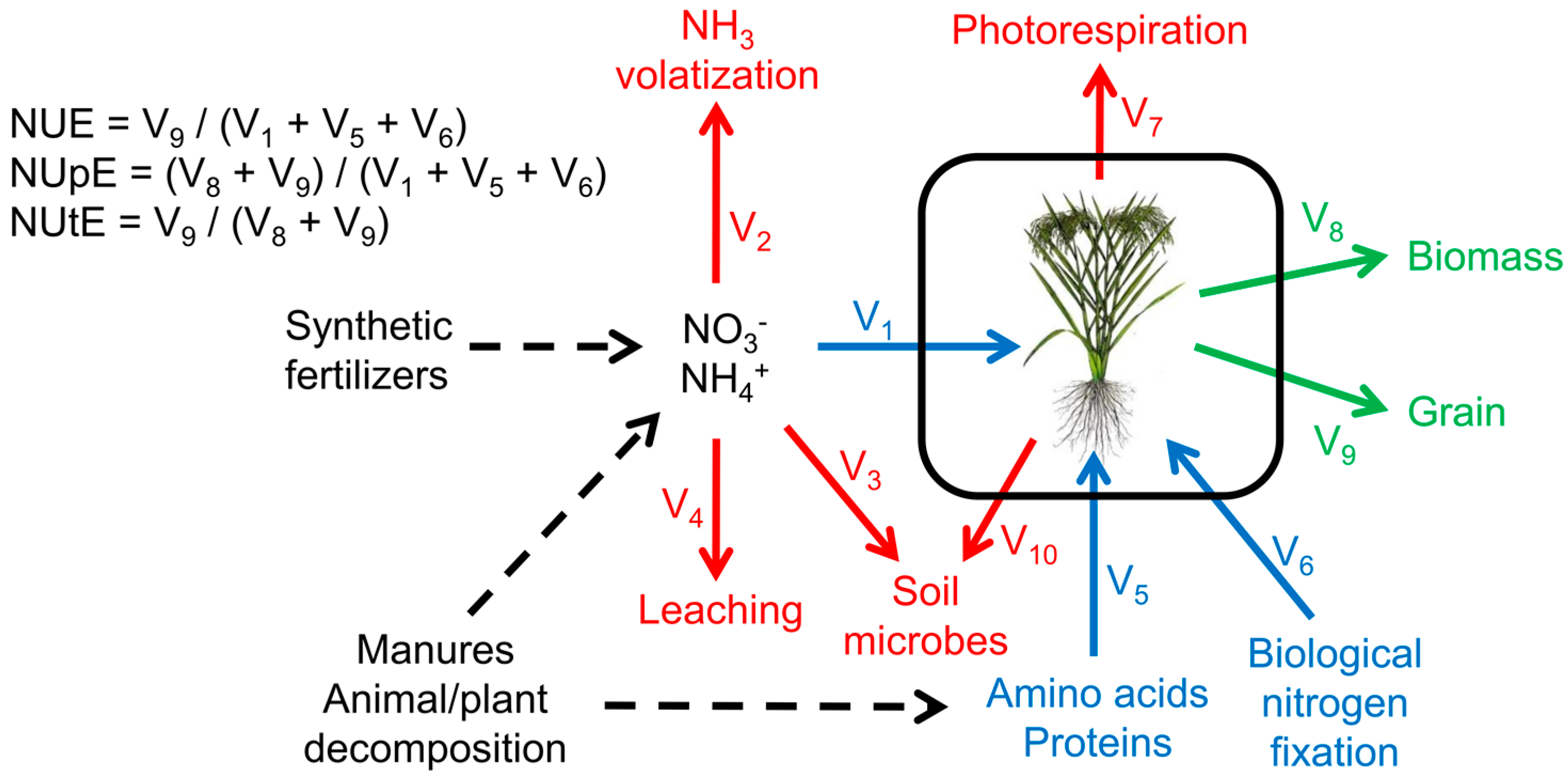
| Species | Synechocystis sp. PC 6803 | Chlamydomos reinhardtii | Chlamydomonas reinhardtii | Arabidopsis thaliana | Zea mays | Zea mays | |||
|---|---|---|---|---|---|---|---|---|---|
| N depletion condition: length of time and concentration shift | 4 h; 5 to 0 mM NH4+ | 24 h; 7 to 0 mM NH4+ | 1, 2, 6 days; 7.48 to 0 mM NH4+ | 0, 2, 10 days; 6 to 0 mM NO3−) | Inbred lines A188, B73; 0.15 or 15 mM NO3− | Inbred line B73 0.1 or 10 mM NO3− | |||
| Technique | CE-MS, LC-MS/MS | GC-TOF-MS | GC-MS | GC-MS, anion HPLC | GC-MS | GC-MS | |||
| Reference | [32] | [33] | [34] | [35] | [26] | [11] | |||
| Metabolite | 1–2–6d | Shoot | Root | A188 | B73 | Veg. | Mat. | ||
| Amino Acids (percentages, %) | |||||||||
| Alanine | 200 | 6 | 44–87–64 | 66–27 | 86–84 | 11–15 | 28–40 | 18 | 18 |
| Arginine | 48 | 129–97–65 | 14–51 | 97–82 | 26 | ||||
| Asparagine | 55 | 18 | 64–20 | 163–24 | 11–85 | 32–78 | 1 | ||
| Aspartate | 21 | 63 | 21–17–14 | 44–27 | 48–32 | 28–52 | 45–163 | 4 | |
| Cysteine | 135 | 58–57 | 72–83 | ||||||
| Glutamate | 88 | 18 | 21–72–57 | 68–54 | 82–29 | 27–50 | 57–62 | 12 | |
| Glutamine | 43 | 10 | 58–50–56 | 25–96 | 3 | ||||
| Glycine | 262 | 35 | 76–161–86 | 56–22 | 178–706 | 8–38 | 21–131 | 3 | |
| Histidine | 136 | 55 | |||||||
| Isoleucine | 576 | 250 | 38–42–29 | 116–67 | 103–90 | 48–40 | 61–49 | 14 | |
| Leucine | 688 | 59 | 25–21–14 | 139–70 | 97–78 | 56–38 | 84–34 | 40 | |
| Lysine | 124 | 42 | 28–62–58 | 84–37 | 106–72 | 77–84 | 198–91 | 26 | 54 |
| Methionine | 241 | 18 | 146–201–215 | 32 | |||||
| Phenylalanine | 433 | 44 | 16–25–11 | 129–66 | 73–44 | 49–42 | 64–40 | 19 | |
| Proline | 198 | 34 | 71–49–95 | 72–23 | 73–38 | 40–57 | 70–65 | 8 | |
| Serine | 472 | 14 | 21–61–42 | 134–75 | 86–57 | 11–31 | 25–86 | 3 | |
| Threonine | 349 | 246 | 38–187–95 | 81–46 | 97–46 | 25–28 | 47–74 | 6 | 26 |
| Tryptophan | 83 | 495 | 8–24–6 | 88–53 | 74–52 | 62 | |||
| Tyrosine | 1284 | 111 | 24–51–26 | 171–92 | 103–90 | 40–54 | 71–61 | 21 | 40 |
| Valine | 235 | 33 | 40–57–50 | 91–66 | 103–90 | 44–47 | 58–61 | 10 | |
| Organic Acids (percentages, %) | |||||||||
| Aconitate | 115 | 55–30 | 102–65 | 45 | 45 | ||||
| Benzoate | 73 | 43–114–102 | 92–85 | 64–90 | |||||
| Citrate | 59 | 56 | 133–1478–1134 | 59–25 | 156–45 | 886 | |||
| Erythonate | 134–242 | 113–120 | 50 | 48 | |||||
| Fumarate | 1015 | 16 | 33–125–95 | 442–397 | 94–67 | 41–78 | 51–68 | 52 | 135 |
| Glycerate | 141 | 58 | 40–48–46 | 172–67 | 1209–5301 | ||||
| 2-oxoglutarate | 360 | 46 | 95–45–114 | 105–86 | 152–97 | 80–95 | 108–45 | ||
| Lactate | 39–63–54 | 109–130 | 22–52 | ||||||
| Malate | 876 | 26 | 34–87–82 | 113–97 | 993–461 | 34–20 | 23–23 | 61 | |
| Maleate | 28–56–148 | 157–182 | 84–85 | 900 | |||||
| Oxaloacetate | 94 | 1–36–1 | 160–83 | 125–192 | |||||
| Pyruvate | 334 | 25 | 21–27–100 | 81–75 | 71–106 | 75–50 | 81–34 | 11 | |
| Shikimate | 168 | 40 | 103–77 | 160–53 | 170–216 | 131–312 | 26 | ||
| Succinate | 398 | 81 | 97–216–167 | 178–346 | 114–67 | ||||
| Threonate | 39 | 96–131–111 | 99–132 | 267–238 | |||||
| Alcohols and Sugars (percentages, %) | |||||||||
| Glycerol | 77 | 1.6–0.9–0.5 | 100–93 | 64–70 | |||||
| Inositol | 13 | 30–52–84 | 97–74 | 177–258 | 67 | ||||
| Fructose | 312–202–97 | 462–218 | 687–277 | 44–34 | 29–31 | 11 | |||
| Galactose | 17–7–7 | 343–487 | 208–225 | 30 | 15 | ||||
| Glucose | 27–10–3 | 405–545 | 413–515 | 30–21 | 29–24 | 6 | |||
| Maltose | 62 | 95–93 | 79–86 | 121 | |||||
| Mannose | 223–184 | 132–362 | 16 | 24 | |||||
| Raffinose | 183 | 973–7981 | 198–313 | 275–268 | 270–159 | 333 | |||
| Sucrose | 89–89 | 99–110 | 71 | ||||||
| Xylose | 67–195–231 | 119–149 | 271–357 | ||||||
| Phosphorylated Compounds (percentages, %) | |||||||||
| 6-phosphogluconic acid | 136 | 6 | 120–177–171 | ||||||
| Fructose-6P: Fru-6P | 148 | 64 | 20–44–73 | 65–55 | 76–65 | 72–296 | 137–526 | 21 | |
| Fructose-1,6-bisP | 82 | 67 | |||||||
| Glucose-1-P | 119 | 61 | |||||||
| Glucose-6-P | 148 | 89 | 53–99–44 | 70–57 | 88–84 | 77–356 | 131–559 | 14 | |
| Glycerate-3P | 139 | 161 | 52–230–83 | 11 | 150 | ||||
| myo-inositol-P | 108–83–71 | 148–77 | 66–70 | ||||||
| Phosphoenol-pyruvate | 104 | 19 | |||||||
| Ribulose-5P | 127 | 99 | 69–174 | 100–333 | |||||
| Nitrogenous Compounds (percentages, %) | |||||||||
| γ-aminobutyric acid | 536 | 167–114–43 | 204–138 | 217–96 | 29–25 | 38–39 | 8 | ||
| Adenine | 100 | 9 | 24–52–56 | ||||||
| Citrulline | 23 | 23 | 11–73 | 23–138 | |||||
| Hydroxylamine | 139 | 114–81–72 | 59–8 | 19–34 | |||||
| Ornithine | 21 | 6 | 127–87–59 | 10–72 | 48–94 | ||||
| Putrescine | 9 | 11–13–8 | 12 | 9 | |||||
| Uracil | 10 | 13–17–18 | |||||||
| Genetic Construct | Conditions | Technique | Core Metabolomic Results (Compared to WT) | References |
|---|---|---|---|---|
| N metabolism | ||||
| Oryza sativa GS1;1 and GS1;2 overexpressed in Oryza sativa cv. Zhonghua 11 under the control of the CaMV 35S promoter | Metabolic analysis done on tillering stage roots and shoots of plants growth with Low N and Moderate N | GC-TOF-MS | Low N: GS1;1 and GS1; 2 increased sugars, organic acids, free amino acids in shoots and decreased in roots. Moderate N: same results for both lines in shoots as for low N, in roots GS1;1 increased sugars, organic acids and free amino acids GS1;2 roots had decreased metabolites. | [41] |
| Pisum sativum AS1 overexpressed in Nicotiana tabacum under the control of the CaMV 35S promoter | 16 h light/8 h dark, 21 day old plants grown in sand, fertilized with Hoagland solution with 10 mM NO3− | HPLC | 10–100 fold increased Asn. Decreased Gln, Asp. No change in Glu. | [46] |
| Hordeum vulgare AlaAT overexpressed in Oryza sativa under the control of the root-specific OsANT1 promoter | 14 h light/10 h dark, 45 day old plants grown hydroponically in 0.5, 2.0, and 5.0 mM NH4+ | HPLC | Increased Gln, Glu, Asn, Asp, and Arg in roots and shoots. | [12] |
| N recycling/protein degradation/C:N balance | ||||
| Mus musculus ODC overexpressed in Populus nigra under the control of a 2X CaMV 35S promoter | Cell cultures grown in MS media | HPLC | Increased Ala, Thr, Val, Ile, and GABA. Decreased Gln, Glu, Orn, Arg, His, Ser, Gly, Cys, Phe, Trp, Asp, Lys, Leu, Met. | [53] |
| Arabidopsis FUM2 overexpressed in Arabidopsis under the control of a 2X CaMV 35S promoter | 8 h light/16 h dark, plants grown for 42 days with 1.25 mg (low) or 31.5 mg (high) inorganic nitrogen | GC-MS | Increased starch, FUM2 knockouts reduced fumarate levels, varied amino acid levels according to light cycle. | [54] |
| Regulatory transgenes | ||||
| Zea mays Dof1 expressed in Arabidopsis under the control of the CaMV 35S promoter; also expressed in potato | Constant light, plants grown on modified MS medium; low N = 1 mM NH4NO3/1 mM KNO3; high N = 10 mM NH4NO3/10 mM KNO3 | Hitachi amino acid analyzer; enzymatic assay | Increased total [amino acid], NH4+ Decreased glucose, malate No change in sucrose, citrate, or 2-OG Similar to transgenic potato | [48] |
| Zea mays Dof1 expressed in Oryza sativa under the control of the CaMV 35S promoter | 14 h day/10 h night, hydroponic growth at 360 (high) or 90 µM (low) NH4+ | CE-MS/MS | Increased concentration of some amino acids under high and low [N] | [49] |
| N-responsive transgenes | ||||
| Oryza sativa ENOD93 expressed in Oryza sativa under the control of the 35S C4PDK promoter | 16 h day/8 h night for 4 weeks then 10 h day/14 h night for 1 week for flowering, soil growth at 1 mM (low), 5 mM (median) or 10 mM (high) nitrate | Biochemical assays | Increased total amino acids in OsENOD93-ox line roots in all N levels but more so under N stress. No increase in amino acid levels in shoots. Higher biomass in OsENOD93-ox. | [52] |
| Co-expressed N metabolism and Regulatory transgenes | ||||
| Arabidopsis Dof1, GS1, GS2 expressed in tobacco under the control of the leaf specific rbcS promoter from tomato | Growth in perlite and low N nutrient solution for 60 and 90 days | RP-HPLC and biochemical assays | Transgenic tobacco co-expressing Dof1, GS1, GS2 had increased amino acids, glucose, sucrose and decreased nitrate, malic acid, citric acid and showed growth advantages | [43] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beatty, P.H.; Klein, M.S.; Fischer, J.J.; Lewis, I.A.; Muench, D.G.; Good, A.G. Understanding Plant Nitrogen Metabolism through Metabolomics and Computational Approaches. Plants 2016, 5, 39. https://doi.org/10.3390/plants5040039
Beatty PH, Klein MS, Fischer JJ, Lewis IA, Muench DG, Good AG. Understanding Plant Nitrogen Metabolism through Metabolomics and Computational Approaches. Plants. 2016; 5(4):39. https://doi.org/10.3390/plants5040039
Chicago/Turabian StyleBeatty, Perrin H., Matthias S. Klein, Jeffrey J. Fischer, Ian A. Lewis, Douglas G. Muench, and Allen G. Good. 2016. "Understanding Plant Nitrogen Metabolism through Metabolomics and Computational Approaches" Plants 5, no. 4: 39. https://doi.org/10.3390/plants5040039
APA StyleBeatty, P. H., Klein, M. S., Fischer, J. J., Lewis, I. A., Muench, D. G., & Good, A. G. (2016). Understanding Plant Nitrogen Metabolism through Metabolomics and Computational Approaches. Plants, 5(4), 39. https://doi.org/10.3390/plants5040039






