Elucidating Scent and Color Variation in White and Pink-Flowered Hydrangea arborescens ‘Annabelle’ Through Multi-Omics Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. RNA Extraction and RNA-Sequencing
2.3. UPLC-MS/MS Metabolite Analysis
2.4. Analysis of Floral Volatiles and Volatile Identification
2.5. Quantitative Real-Time PCR
2.6. Data Analysis
3. Results
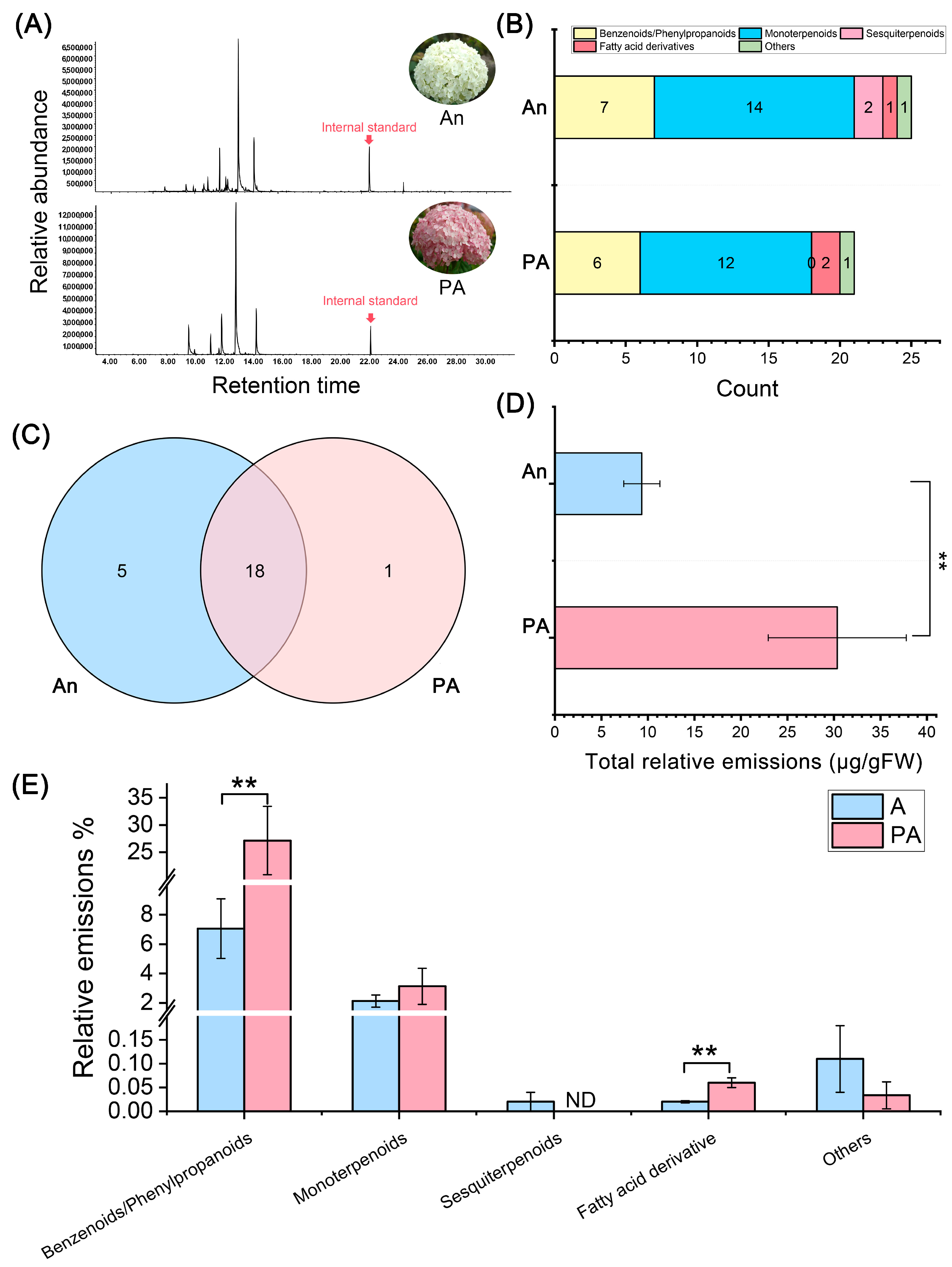
3.1. Volatile Organic Compound (VOC) Profiling Between ‘An’ and ‘PA’ Varieties
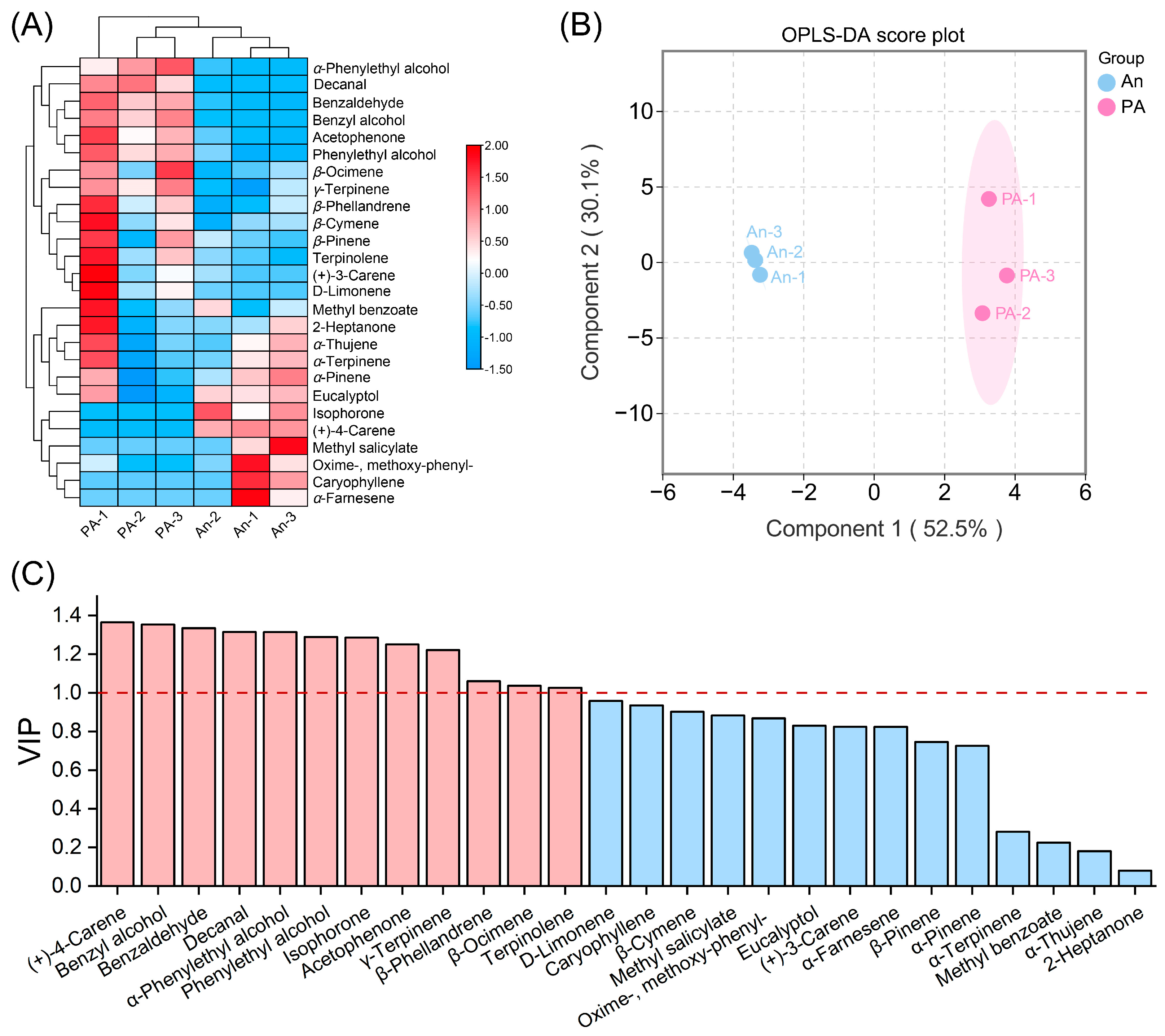
3.2. Multivariate and Differential Metabolite Analysis
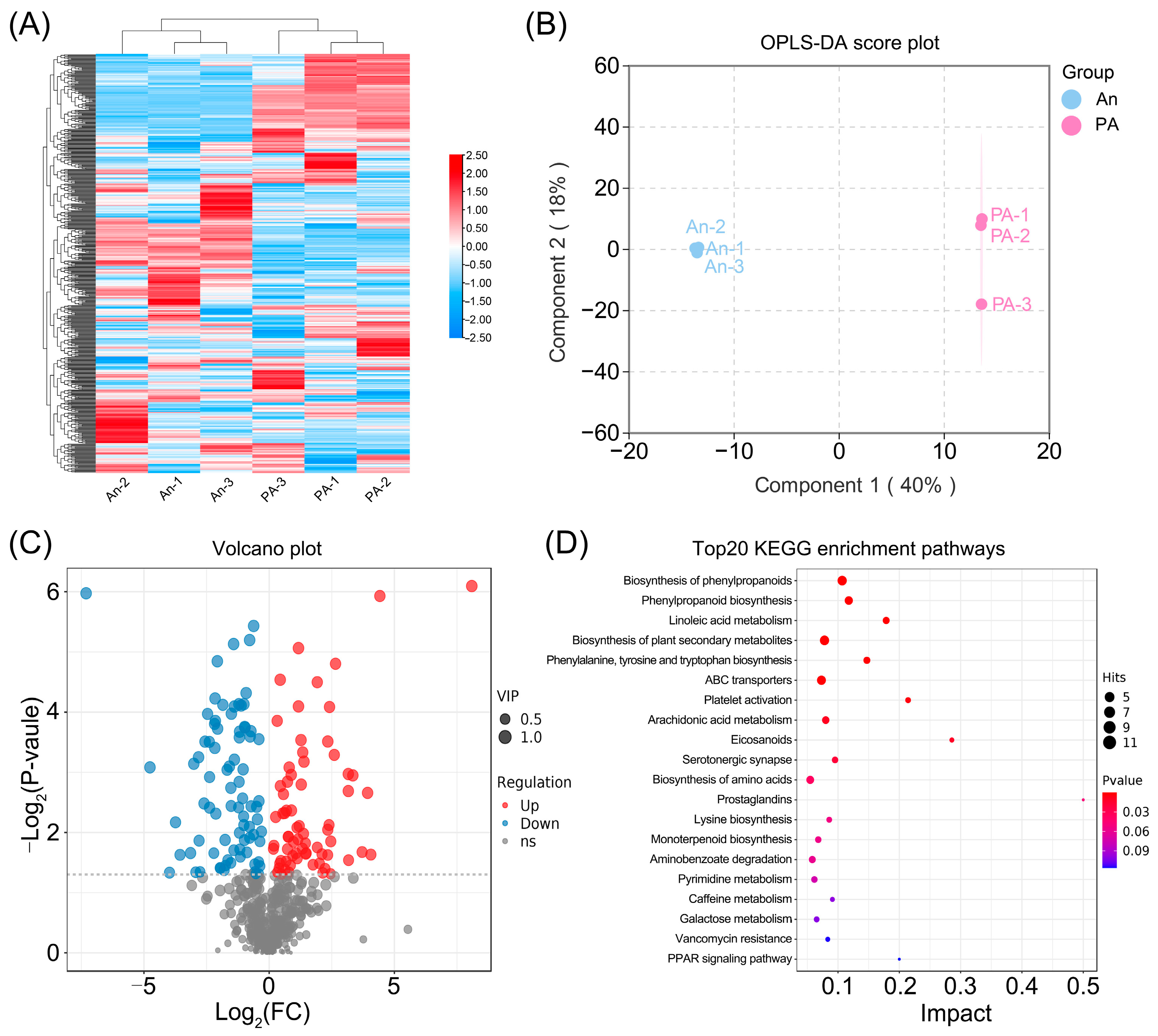
3.3. Comparative Metabolomic Analysis of ‘An’ and ‘PA’ Flowers
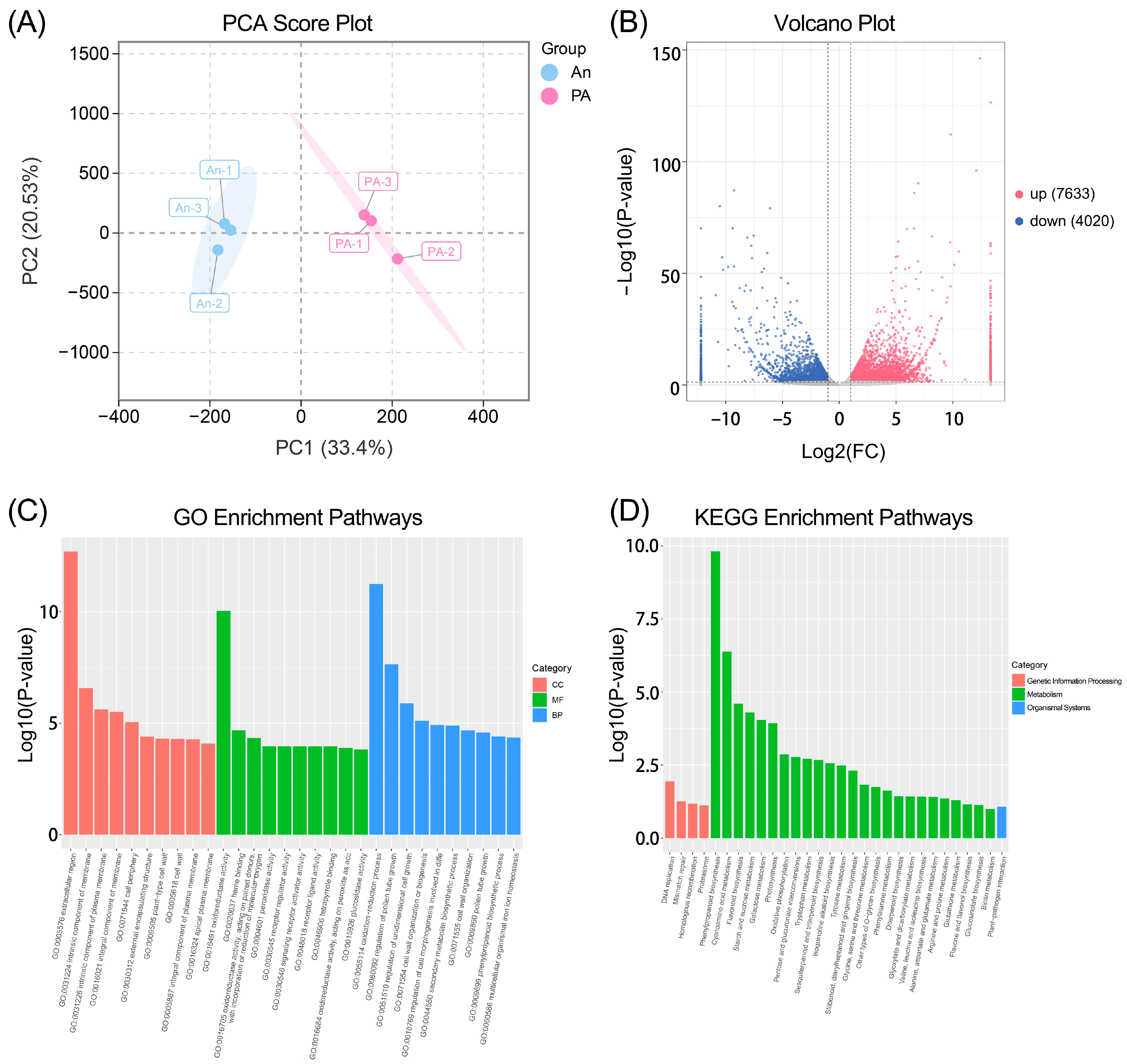
3.4. Transcriptome Analysis of ‘An’ and ‘PA’ Flowers
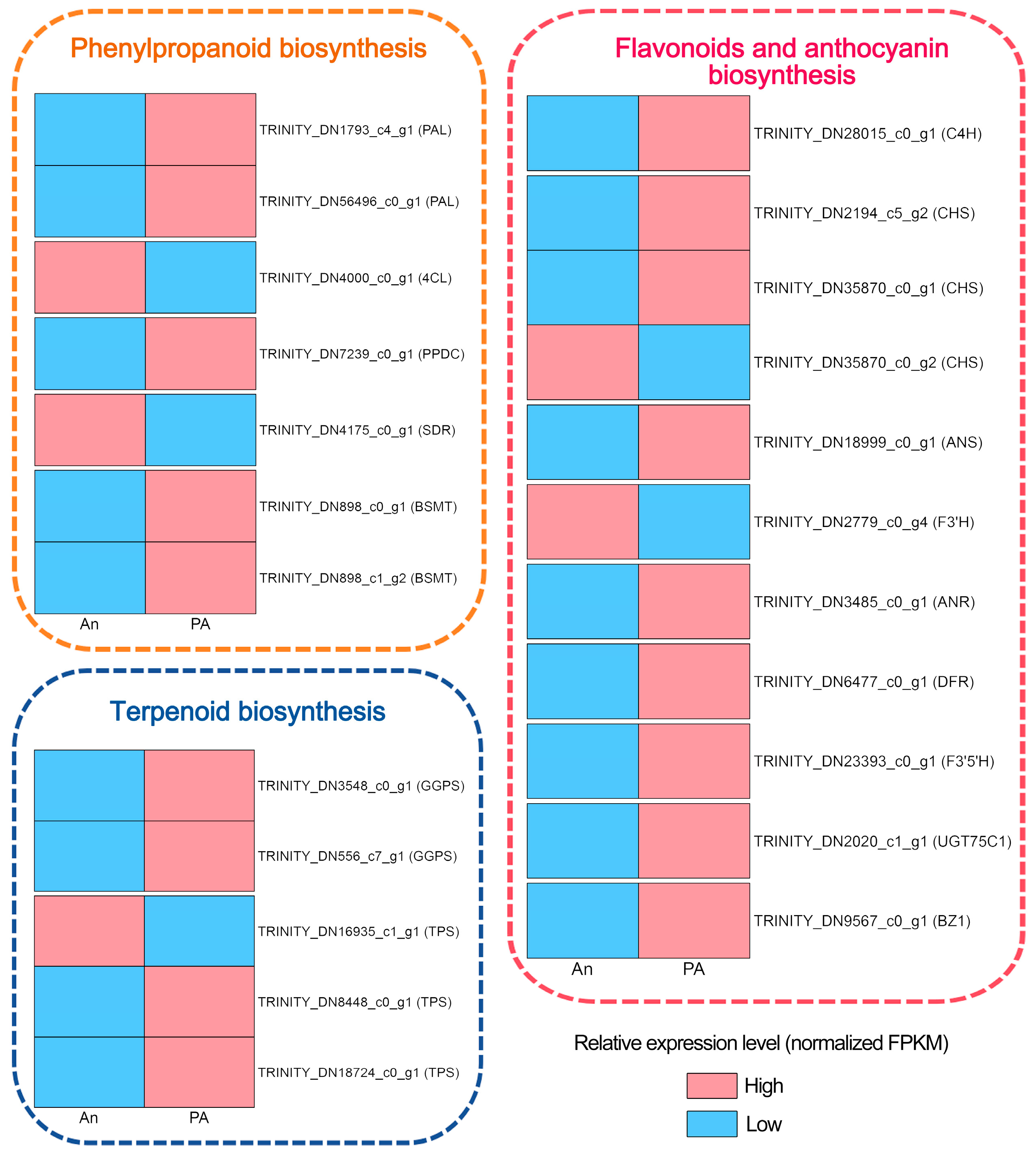
3.5. Identification of Structural Biosynthesis DEGs Associated with Secondary Metabolic Pathways
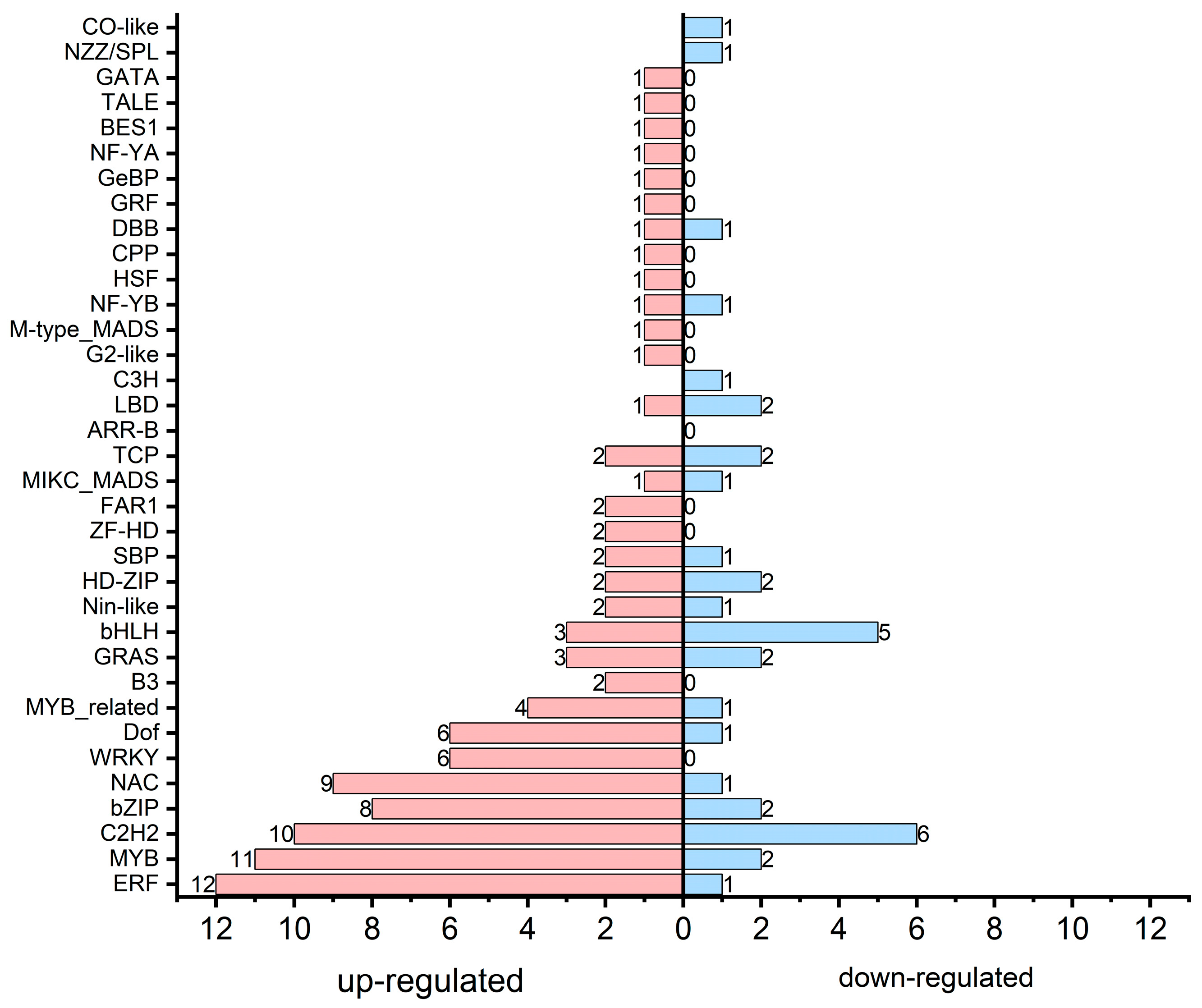
3.6. Transcription Factor Associated with Secondary Metabolism
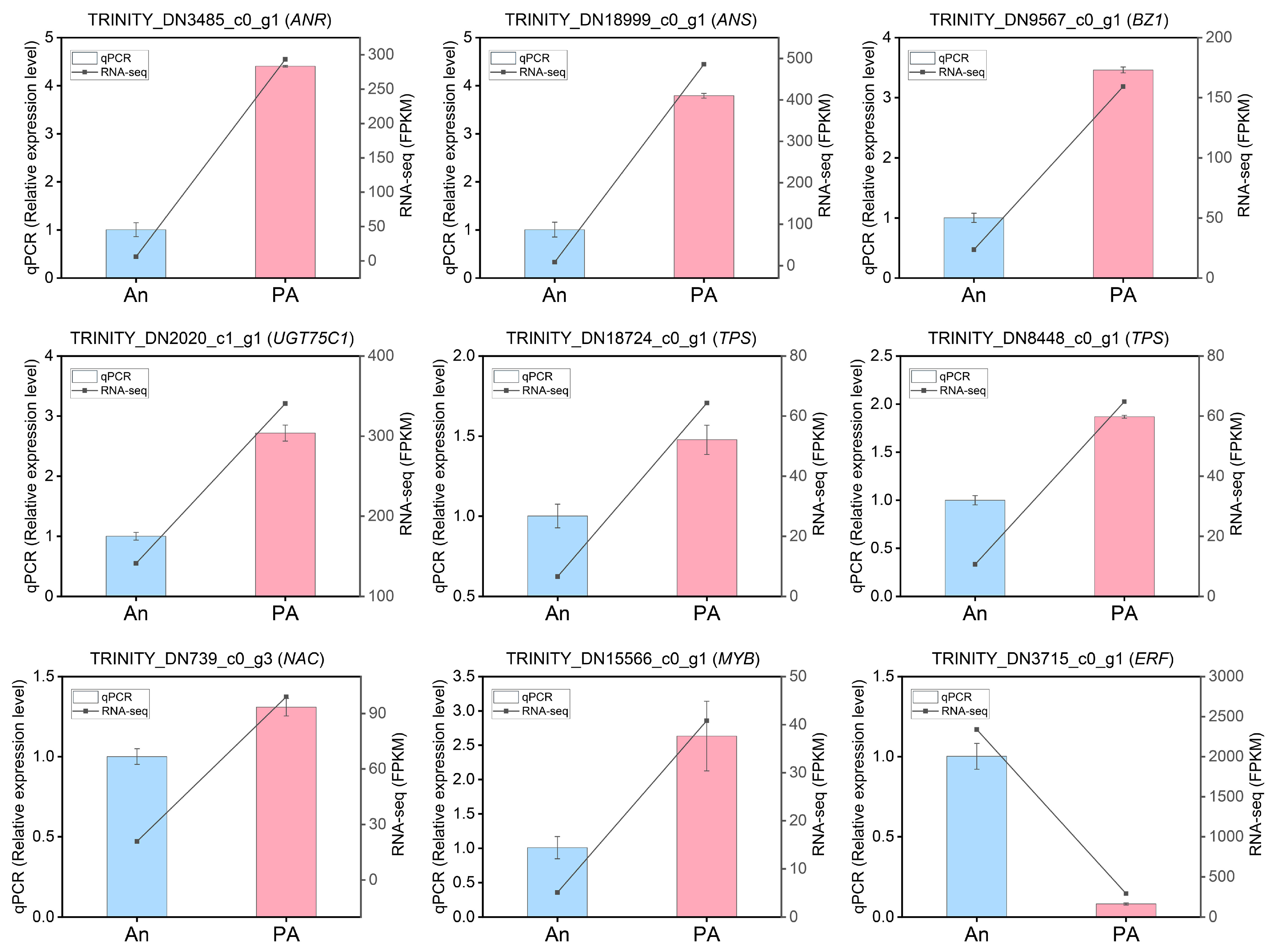
3.7. Verification of DEGs Profiling by qRT-PCR
4. Discussion
4.1. Versatile Pathways of Floral Volatiles Biosynthesis in ‘An’ and ‘PA’
4.2. Metabolomic Reconfiguration and Secondary Metabolite Enrichment
4.3. Transcriptomic Regulation of Pigment and Volatile Organic Compound Biosynthesis
4.4. Coordinated Color–Scent Metabolic Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Relationships among floral VOC emissions, floral rewards and visits of pollinators in five plant species of a Mediterranean shrubland. Plant Ecol. Evol. 2015, 148, 90–99. [Google Scholar] [CrossRef]
- Kessler, A.; Chautá, A. The ecological consequences of herbivore-induced plant responses on plant–pollinator interactions. Emerg. Top. Life Sci. 2020, 4, 33–43. [Google Scholar]
- Whitehead, M.R.; Gaskett, A.C.; Johnson, S.D. Floral community predicts pollinators’ color preference: Implications for Batesian floral mimicry. Behav. Ecol. 2019, 30, 213–222. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Abbas, F.; O’Neill Rothenberg, D.; Zhou, Y.; Ke, Y.; Wang, H.C. Volatile Organic Compounds as Mediators of Plant Communication and Adaptation to Climate Change. Physiol. Plant. 2022, 174, e13840. [Google Scholar] [CrossRef]
- Abbas, F.; Yu, Y.; Bendahmane, M.; Wang, H.C. Plant volatiles and color compounds: From biosynthesis to function. Physiol. Plant. 2023, 175, e13947. [Google Scholar] [CrossRef]
- Bashandy, H.; Pietiäinen, M.; Carvalho, E.; Lim, K.-J.; Elomaa, P.; Martens, S.; Teeri, T.H. Anthocyanin biosynthesis in gerbera cultivar ‘Estelle’and its acyanic sport ‘Ivory’. Planta 2015, 242, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, J.; Yao, Y.; Cao, J.; Yang, W.; Long, Y.; Liu, J.; Yang, W. PhAAT1, encoding an anthocyanin acyltransferase, is transcriptionally regulated by PhAN2 in petunia. Physiol. Plant. 2023, 175, e13851. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Martín, D.; Piulachs, M.-D.; Cunillera, N.; Ferrer, A.; Bellés, X. Mitochondrial targeting of farnesyl diphosphate synthase is a widespread phenomenon in eukaryotes. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.E.; Shrestha, M.; Howard, S.R.; Petersen, P.; Dyer, A.G. Signal or cue: The role of structural colors in flower pollination. Curr. Zool. 2019, 65, 467–481. [Google Scholar] [CrossRef]
- Keting, H.; Ke, H.; Silan, D. Flower color breeding by molecular design in ornamentals. Mol. Plant Breed. 2008, 6, 16–24. [Google Scholar]
- Li, Y.; Liu, X.; Cai, X.; Shan, X.; Gao, R.; Yang, S.; Han, T.; Wang, S.; Wang, L.; Gao, X. Dihydroflavonol 4-reductase genes from Freesia hybrida play important and partially overlapping roles in the biosynthesis of flavonoids. Front. Plant Sci. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zhao, M.; Hu, Y.; Meng, F.; Song, X.; Tigabu, M.; Chiang, V.L.; Sederoff, R.; Ma, W. Molecular and metabolic insights into anthocyanin biosynthesis for leaf color change in chokecherry (Padus virginiana). Int. J. Mol. Sciences 2021, 22, 10697. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, C.; Hui, J. Effect of Light Intensity on Anthocyanin Synthesis Assessed Using Leaves of Aglaonema commutatum. Genes 2025, 16, 375. [Google Scholar] [CrossRef]
- Wong, D.C.; Perkins, J.; Peakall, R. Anthocyanin and flavonol glycoside metabolic pathways underpin floral color mimicry and contrast in a sexually deceptive orchid. Front. Plant Sci. 2022, 13, 860997. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, D.; Shinkai, Y.; Kondo, T. Change of color and components in sepals of chameleon hydrangea during maturation and senescence. Phytochemistry 2008, 69, 3159–3165. [Google Scholar] [CrossRef]
- Ke, Y.; Zhou, Y.; Lv, Y.; Qi, Y.; Wei, H.; Lei, Y.; Huang, F.; Abbas, F. Integrated metabolome and transcriptome analysis provides insights on the floral scent formation in Hydrangea arborescens. Physiol. Plant. 2023, 175, e13914. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ashraf, U.; Wang, D.; Hassan, W.; Zou, Y.; Qi, Y.; Zhou, Y.; Abbas, F. Function of Anthocyanin and Chlorophyll Metabolic Pathways in the Floral Sepals Color Formation in Different Hydrangea Cultivars. Plants 2025, 14, 742. [Google Scholar] [CrossRef]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Bennewitz, S.; Bergau, N.; Athmer, B.; Henning, A.; Majovsky, P.; Jiménez-Gómez, J.M.; Hoehenwarter, W.; Tissier, A. Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell 2017, 29, 960–983. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Verdonk, J.C.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 2005, 17, 1612–1624. [Google Scholar] [CrossRef]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 2004, 135, 1993–2011. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Z.; Yang, L.; Chen, Q.; Shao, L.; Liu, J.; Yu, Y. PhERF6, interacting with EOBI, negatively regulates fragrance biosynthesis in petunia flowers. New Phytol. 2017, 215, 1490–1502. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Mei, X.; Zhou, Y.; Du, B.; Watanabe, N.; Yang, Z. Regulation of biosynthesis and emission of volatile phenylpropanoids/benzenoids in petunia × hybrida flowers by multi-factors of circadian clock, light, and temperature. Plant Physiol. Biochem. 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Helsper, J.P.; Davies, J.A.; Bouwmeester, H.J.; Krol, A.F.; van Kampen, M.H. Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 1998, 207, 88–95. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Fan, Y. Functional characterization and expression analysis of two terpene synthases involved in floral scent formation in Lilium ‘Siberia’. Planta 2019, 249, 71–93. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Zhou, Y.; Ashraf, U.; Li, X.; Yu, Y.; Yue, Y.; Ahmad, K.W.; Yu, R.; Fan, Y. Molecular cloning, characterization and expression analysis of LoTPS2 and LoTPS4 involved in floral scent formation in oriental hybrid Lilium variety ‘Siberia’. Phytochemistry 2020, 173, 112294. [Google Scholar] [CrossRef]
- Abbas, F.; Nian, X.; Zhou, Y.; Ke, Y.; Liu, L.; Yu, R.; Fan, Y. Putative regulatory role of hexokinase and fructokinase in terpenoid aroma biosynthesis in Lilium ‘Siberia’. Plant Physiol. Biochem. 2021, 167, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Obando, W.; Hirsch, G.N.; Wetzstein, H.Y. Genotypic variation in flower induction and development in Hydrangea macrophylla. HortScience 2005, 40, 1695–1698. [Google Scholar] [CrossRef]
- Yoshida, K.; Toyama-Kato, Y.; Kameda, K.; Kondo, T. Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol. 2003, 44, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, K.; Luo, L.; Pan, H.-t.; Zhang, Q.-x.; Yang, Y.-y. Production of interspecific hybrids between Hydrangea macrophylla and Hydrangea arborescens via ovary culture. HortScience 2015, 50, 1765–1769. [Google Scholar] [CrossRef]
- Ito, D.; Shinkai, Y.; Kato, Y.; Kondo, T.; Yoshida, K. Chemical studies on different color development in blue-and red-colored sepal cells of Hydrangea macrophylla. Biosci. Biotechnol. Biochem. 2009, 73, 1054–1059. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Fan, Y. Auxin-responsive R2R3-MYB transcription factors HcMYB1 and HcMYB2 activate volatile biosynthesis in Hedychium coronarium flowers. Front. Plant Sci. 2021, 12, 710826. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Yue, Y.; Li, X.; Yu, Y.; Fan, Y. Genome-Wide Analysis and Characterization of the Aux/IAA Family Genes Related to Floral Scent Formation in Hedychium coronarium. Int. J. Mol. Sci. 2019, 20, 3235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Abbas, F.; He, J.; Yan, F.; Wang, Q.; Yu, Y.; Yu, R.; Fan, Y. Floral volatile chemical diversity in Hedychium F1 hybrid population. Ind. Crops Prod. 2022, 184, 115032. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.-G.; Wang, T.-Y.; Huang, X.-M.; Hu, G.-B.; Zhou, B.-Y.; Wang, H.-C.; Abbas, F. ERF transcription factors govern anthocyanin biosynthesis in litchi pericarp by modulating the expression of anthocyanin biosynthesis genes. Sci. Hortic. 2024, 337, 113464. [Google Scholar] [CrossRef]
- Zou, S.-C.; Zhuo, M.-G.; Abbas, F.; Hu, G.-B.; Wang, H.-C.; Huang, X.-M. Transcription factor LcNAC002 coregulates chlorophyll degradation and anthocyanin biosynthesis in litchi. Plant Physiol. 2023, 192, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Haig, T.; Hatfield, P. On-site field sampling and analysis of fragrance from living Lavender (Lavandula angustifolia L.) flowers by solid-phase microextraction coupled to gas chromatography and ion-trap mass spectrometry. J. Chromatogr. A 2001, 917, 245–250. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, L.; Wang, Y.; Ma, L.; Yang, Y.; Zhou, X.; Wang, L.; Yu, X.; Li, S. Floral volatile benzenoids/phenylpropanoids: Biosynthetic pathway, regulation and ecological value. Hortic. Res. 2024, 11, uhae220. [Google Scholar] [CrossRef]
- Zan, W.; Wu, Q.; Dou, S.; Wang, Y.; Zhu, Z.; Xing, S.; Yu, Y. Analysis of flower color diversity revealed the co-regulation of cyanidin and peonidin in the red petals coloration of Rosa rugosa. Plant Physiol. Biochem. 2024, 216, 109126. [Google Scholar] [CrossRef]
- Shen, Y.; Rao, Y.; Ma, M.; Li, Y.; He, Y.; Wang, Z.; Liang, M.; Ning, G. Coordination among flower pigments, scents and pollinators in ornamental plants. Hortic. Adv. 2024, 2, 6. [Google Scholar] [CrossRef]
- Stephane, F.F.Y.; Juleshttps, B. Terpenoids as important bioactive constituents of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Wang, J.; Dudareva, N.; Bhakta, S.; Raguso, R.A.; Pichersky, E. Floral Scent Production in Clarkia breweri (Onagraceae) (II. Localization and Developmental Modulation of the Enzyme S-Adenosyl-L-Methionine:(Iso) Eugenol O-Methyltransferase and Phenylpropanoid Emission). Plant Physiol. 1997, 114, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of Isoprenoids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 63–106. [Google Scholar]
- Zhou, Y.; Peng, Q.; Zeng, L.; Tang, J.; Li, J.; Dong, F.; Yang, Z. Study of the biochemical formation pathway of aroma compound 1-phenylethanol in tea (Camellia sinensis (L.) O. Kuntze) flowers and other plants. Food Chem. 2018, 258, 352–358. [Google Scholar] [CrossRef]
- Du, H.; Lai, L.; Wang, F.; Sun, W.; Zhang, L.; Li, X.; Wang, L.; Jiang, L.; Zheng, Y. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biol. 2018, 20, 121–129. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, G.; Li, D.; Zhang, D.; Chen, Z.; Yang, Z.; Yang, K.; Xie, X.; Wu, Y. Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl. Horticulturae 2024, 10, 959. [Google Scholar] [CrossRef]
- Dudareva, N.; Martin, D.; Kish, C.M.; Kolosova, N.; Gorenstein, N.; Fäldt, J.; Miller, B.; Bohlmann, J. (E)-β-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: Function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 2003, 15, 1227–1241. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Ma, B.; Leng, P.; Wu, J.; Hu, Z. SoNAC72-SoMYB44/SobHLH130 module contributes to flower color fading via regulating anthocyanin biosynthesis by directly binding to the SoUFGT1 promoter in lilac (Syringa oblata). Hortic. Res. 2024, 12, uhae326. [Google Scholar] [CrossRef]
- An, X.-H.; Tian, Y.; Chen, K.-Q.; Liu, X.-J.; Liu, D.-D.; Xie, X.-B.; Cheng, C.-G.; Cong, P.-H.; Hao, Y.-J. MdMYB9 and MdMYB11 are Involved in the Regulation of the JA-Induced Biosynthesis of Anthocyanin and Proanthocyanidin in Apples. Plant Cell Physiol. 2014, 56, 650–662. [Google Scholar] [CrossRef]
- Ning, Z.; Hu, K.; Zhou, Z.; Zhao, D.; Tang, J.; Wang, H.; Li, L.; Ding, C.; Chen, X.; Yao, G. IbERF71, with IbMYB340 and IbbHLH2, coregulates anthocyanin accumulation by binding to the IbANS1 promoter in purple-fleshed sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 157–169. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Wu, D.; Yan, J.; Qi, Y.; Song, S.; Xie, D. Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 2014, 26, 1118–1133. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid–and auxin-mediated signaling in jasmonic acid–induced leaf senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Abbas, F.; Zhou, Y.; O’Neill Rothenberg, D.; Alam, I.; Ke, Y.; Wang, H.-C. Aroma Components in Horticultural Crops: Chemical Diversity and Usage of Metabolic Engineering for Industrial Applications. Plants 2023, 12, 1748. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Lackus, N.D.; Schmidt, A.; Gershenzon, J.; Köllner, T.G. A peroxisomal β-oxidative pathway contributes to the formation of C6–C1 aromatic volatiles in poplar. Plant Physiol. 2021, 186, 891–909. [Google Scholar] [CrossRef]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef]
- Rasouli, O.; Ahmadi, N.; Monfared, S.R. Molecular characterization and expression pattern of RhPAR, RhMYB1 and RhANS genes involving in scent and color production in Rosa damascena. Sci. Hortic. 2020, 272, 109399. [Google Scholar] [CrossRef]
- Kiani, H.S.; Noudehi, M.S.; Shokrpour, M.; Zargar, M.; Naghavi, M.R. Investigation of genes involved in scent and color production in Rosa damascena Mill. Sci. Rep. 2024, 14, 20576. [Google Scholar] [CrossRef]
- Martínez-Harms, J.; Warskulat, A.C.; Dudek, B.; Kunert, G.; Lorenz, S.; Hansson, B.S.; Schneider, B. Biosynthetic and Functional Color–Scent Associations in Flowers of Papaver nudicaule and Their Impact on Pollinators. ChemBioChem 2018, 19, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Wang, Y.; Peng, T.; Wang, J. Differences in Anthocyanin Biosynthesis Drive Flower Coloration Variations in Pansy (Viola × Wittrockiana Gams). Physiol. Plant. 2025, 177, e70594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ke, Y.; Wang, D.; Fang, Z.; Zou, Y.; Hussain, Z.; Iqbal, S.; Zhou, Y.; Abbas, F. Elucidating Scent and Color Variation in White and Pink-Flowered Hydrangea arborescens ‘Annabelle’ Through Multi-Omics Profiling. Plants 2026, 15, 155. https://doi.org/10.3390/plants15010155
Ke Y, Wang D, Fang Z, Zou Y, Hussain Z, Iqbal S, Zhou Y, Abbas F. Elucidating Scent and Color Variation in White and Pink-Flowered Hydrangea arborescens ‘Annabelle’ Through Multi-Omics Profiling. Plants. 2026; 15(1):155. https://doi.org/10.3390/plants15010155
Chicago/Turabian StyleKe, Yanguo, Dongdong Wang, Zhongjian Fang, Ying Zou, Zahoor Hussain, Shahid Iqbal, Yiwei Zhou, and Farhat Abbas. 2026. "Elucidating Scent and Color Variation in White and Pink-Flowered Hydrangea arborescens ‘Annabelle’ Through Multi-Omics Profiling" Plants 15, no. 1: 155. https://doi.org/10.3390/plants15010155
APA StyleKe, Y., Wang, D., Fang, Z., Zou, Y., Hussain, Z., Iqbal, S., Zhou, Y., & Abbas, F. (2026). Elucidating Scent and Color Variation in White and Pink-Flowered Hydrangea arborescens ‘Annabelle’ Through Multi-Omics Profiling. Plants, 15(1), 155. https://doi.org/10.3390/plants15010155







