Biochemical Diversity and Nutraceutical Potential of Medicinal Plant-Based Herbal Teas from Southwestern Türkiye
Abstract
1. Introduction
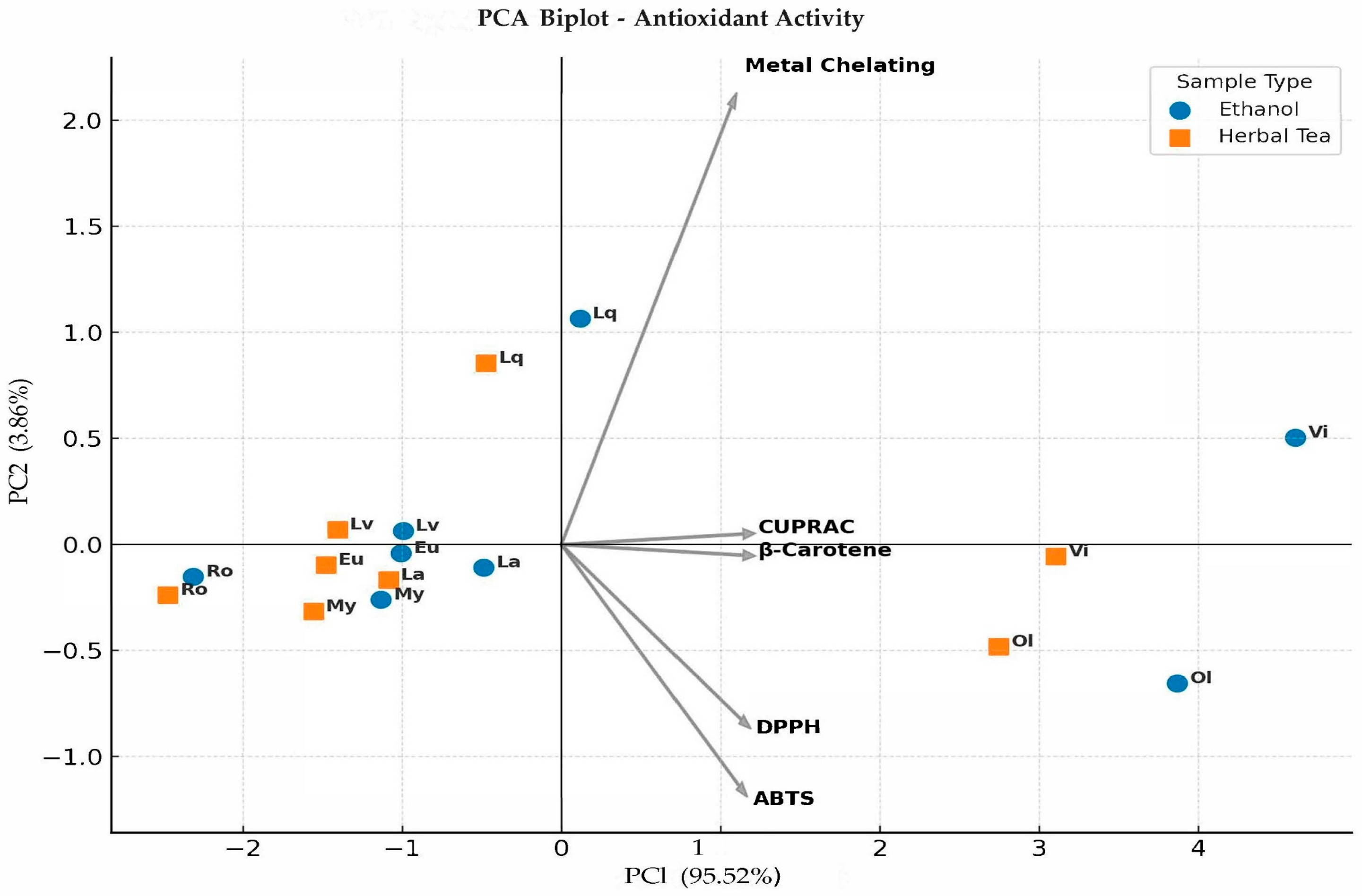
2. Results and Discussion
2.1. Antioxidant Activity Determination
2.1.1. Total Antioxidant Activity: β-Carotene Bleaching Method
2.1.2. DPPH Free Radical Scavenging Activity Method
2.1.3. ABTS+ Cation Radical Scavenging Activity Method
2.1.4. CUPRAC Method
2.1.5. Bipyridine Metal Chelating Activity
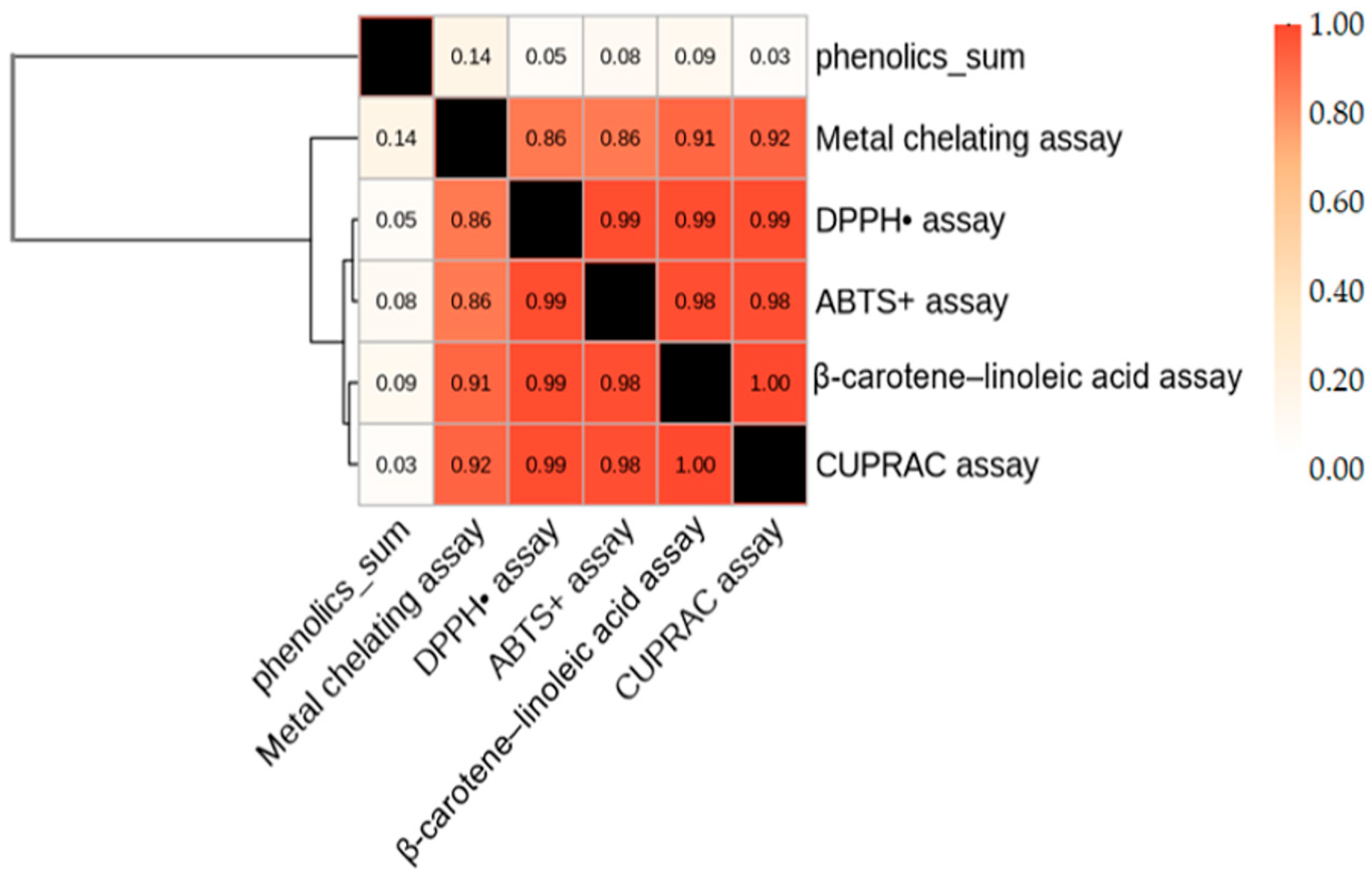
2.2. Identification of Phenolic Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Obtaining Teas and Extracts
3.3. Determination of Antioxidant Activity
3.3.1. Total Antioxidant Activity: β-Carotene Bleaching
3.3.2. DPPH Free Radical Scavenging Activity
3.3.3. ABTS+ Cation Radical Scavenging Activity
3.3.4. CUPRAC Method (Copper Reducing Antioxidant Capacity)
3.3.5. Bipyridine Metal Chelating Activity
3.4. Identification of Phenolic Compounds
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirezer, L.Ö. Our Responsibilities on the Use of Plants in Medicine; Herbal Treatment Symposium: İstanbul, Turkey, 2010; pp. 87–88. [Google Scholar]
- Acıbuca, V.; Budak, D.B. Place and Importance of Medicinal and Aromatic Plants in the World and Turkey. Cukurova J. Agric. Food Sci. 2018, 33, 37–44. [Google Scholar]
- Göktaş, Ö.; Gıdık, B. Usage Areas of Medicinal and Aromatic Plants. Bayburt Univ. J. Sci. Technol. 2019, 2, 145–151. [Google Scholar] [CrossRef]
- Titz, A. Policy, Research and Development and Commercialisation Strategies. In Proceedings of the Medicinal Herbs and Plants: Scope for Diversified and Sustainable Extraction, Bangalore, India, 22–26 July 2004. [Google Scholar]
- Legislation Information System—Legislation No. 11908. 2024. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=11908&MevzuatTur=7&MevzuatTertip=5 (accessed on 26 January 2024).
- Okatan, V. Phenolic Compounds and Phytochemicals in Fruits of Black Mulberry (Morus nigra L.) Genotypes from the Aegean Region in Turkey. Folia Hortic. 2018, 30, 93. [Google Scholar] [CrossRef]
- Tamer, C.E.; Temel, Ş.G.; Suna, S.; Karabacak, A.Ö.; Özcan, T.; Ersan, L.Y.; Kaya, B.T.; Çopur, Ö.U. Evaluation of Bioaccessibility and Functional Properties of Kombucha Beverages Fortified with Different Medicinal Plant Extracts. Turk. J. Agric. For. 2021, 45, 13–32. [Google Scholar] [CrossRef]
- Barut, M.; Nadeem, M.A.; Akgür, Ö.; Tansi, L.S.; Aasim, M.; Altaf, M.T.; Baloch, F.S. Medicinal and Aromatic Plants in the Omics Era: Application of Plant Breeding and Biotechnology for Plant Secondary Metabolite Production. Turk. J. Agric. For. 2022, 46, 182–203. [Google Scholar] [CrossRef]
- Aslam, J.; Shahzad, M.I.; Ali, H.M.; Ramzan, M.; Ahmad, F.U.D.; Aleem, M.T.; Minhas, A.; Hirad, A.H.; Alarfaj, A.A.A. Multidirectional Phytochemical Profiling, Antimicrobial, Antioxidant and Toxicity Studies of Neurada procumbens L.: A Desert Medicinal Plant. J. King Saud Univ. Sci. 2023, 35, 102862. [Google Scholar] [CrossRef]
- Kurt, O.; Çelik, N.; Göre, M.; Kurt, H. Threats to Biodiversity: Bio-trafficking in Turkey. Turk. J. Agric. Food Sci. Technol. 2019, 7, 46–51. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Forestry—Nuh’un Gemisi (Noah’s Ark) National Biodiversity Database. 2024. Available online: https://nuhungemisi.tarimorman.gov.tr/public/istatistik (accessed on 26 January 2024).
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and Herbal Infusions: Their Antioxidant Activity and Phenolic Profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Joubert, E.; Gelderblom, W.C.A.; Louw, A.; de Beer, D. South African Herbal Teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—A Review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef]
- Toker, R.; Gölükcü, M.; Tokgöz, H. Uses of Medicinal and Aromatic Plants in Food Industry. J. Turk. Seed Grow. Assoc. 2015, 4, 54–59. [Google Scholar]
- Liu, Y.; Guo, C.; Zang, E.; Shi, R.; Liu, Q.; Zhang, M.; Zhang, K.; Li, M. Review on Herbal Tea as a Functional Food: Classification, Active Compounds, Biological Activity, and Industrial Status. J. Future Foods 2023, 3, 206–219. [Google Scholar] [CrossRef]
- Shopova, E.; Ivanov, S.; Brankova, L.; Moyankova, D.; Georgieva, D.; Polizoev, D.; Djilianov, D. Antioxidant Capacity of Herbal Teas from Bulgarian Market. Bulg. J. Agric. Sci. 2023, 29, 1159–1164. [Google Scholar]
- Danesi, F.; Saha, S.; Kroon, P.A.; Glibetić, M.; Konić-Ristić, A.; D’Antuono, L.F.; Bordoni, A. Bioactive-Rich Sideritis scardica Tea (Mountain Tea) Is as Potent as Camellia sinensis Tea at Inducing Cellular Antioxidant Defences and Preventing Oxidative Stress. Sci. Food Agric. 2013, 93, 3558–3564. [Google Scholar] [CrossRef]
- Saleh, I.G.; Ali, Z.; Abe, N.; Wilson, F.D.; Hamada, F.M.; Abd-Ellah, M.F.; Walker, L.A.; Khan, I.A.; Ashfaq, M.K. Effect of green tea and its polyphenols on mouse liver. Fitoterapia 2013, 90, 51–159. [Google Scholar] [CrossRef]
- Picerno, P.; Mencherini, T.; Loggia, R.D.; Meloni, M.; Sanogo, R.; Aquino, R.P. An extract of Lannea microcarpa: Composition, activity and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Pharm. Pharmacol. 2006, 58, 981–988. [Google Scholar] [CrossRef]
- Hernández, V.; Malafronte, N.; Mora, F.; Pesca, M.S.; Aquino, R.P.; Mencherini, T. Antioxidant and antiangiogenic activity of Astronium graveolens Jacq. leaves. Nat. Prod. Res. 2014, 28, 917–922. [Google Scholar] [CrossRef]
- Taamalli, A.; Iswaldi, I.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. UPLC–QTOF/MS for a rapid characterisation of phenolic compounds from leaves of Myrtus communis L. Phytochem. Anal. 2014, 25, 89–96. [Google Scholar] [CrossRef]
- Cavusoglu, S.; Yilmaz, N.; Islek, F.; Tekin, O.; Sagbas, H.I.; Ercisli, S.; Rampáčková, E.; Nečas, T. Effect of methyl jasmonate, cytokinin, and lavender oil on antioxidant enzyme system of apricot fruit (Prunus armeniaca L.). Sustainability 2021, 13, 8565. [Google Scholar] [CrossRef]
- Kaygısız, A. Determination of the Antioxidant Activities of Plantain, Yarrow, and Rosemary Extracts. Master’s Thesis, Istanbul Sabahattin Zaim Univ., İstanbul, Turkey, 2021. [Google Scholar]
- Yazıcı, S.Ö.; Aşkın, B.; Kaynarca, G.B. Determination of Antioxidant Properties and Composition of Rosemary and Thyme Essential Oils. Turk. J. Agric. Food Sci. Technol. 2020, 8, 2105–2112. [Google Scholar] [CrossRef]
- Ranjbar Nedamani, E.; Sadeghi Mahoonak, A.; Ghorbani, M.; Kashaninejad, M. Evaluation of Antioxidant Interactions in Combined Extracts of Green Tea (Camellia sinensis), Rosemary (Rosmarinus officinalis) and Oak Fruit (Quercus brantii). J. Food Sci. Technol. 2015, 52, 4565–4571. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Upadhyay, P. GC–MS Chemical Profile, Antioxidant Activity, and Sun Protection Factor of Essential Oil of Tea Tree (Melaleuca alternifolia) and Rosemary (Rosmarinus officinalis L.). Orient. J. Chem. 2022, 38, 1266–1275. [Google Scholar] [CrossRef]
- Rezanejad, R.; Heidarieh, M.; Ojagh, S.M.; Rezaei, M.; Raeisi, M.; Alishahi, A. Values of Antioxidant Activities (ABTS and DPPH) and Ferric Reducing and Chelating Powers of Gamma-Irradiated Rosemary Extract. Radiochim. Acta 2020, 108, 477–482. [Google Scholar] [CrossRef]
- Francolino, R.; Martino, M.; Caputo, L.; Amato, G.; Chianese, G.; Gargiulo, E.; Formisano, C.; Romano, B.; Ercolano, G.; Ianaro, A.; et al. Phytochemical Constituents and Biological Activity of Wild and Cultivated Rosmarinus officinalis Hydroalcoholic Extracts. Antioxidants 2023, 12, 1633. [Google Scholar] [CrossRef]
- Karadağ, A. Antioxidant Potential and Phenolic Composition of Some Aromatic and Medicinal Herbs in Turkey. Eur. J. Sci. Technol. 2019, 16, 631–637. [Google Scholar] [CrossRef]
- Karataş, G. Some Physical and Phytochemical Properties of Bay Leaves from Different Provinces in Turkey. J. New Results Eng. Nat. Sci. 2024, 19, 41–53. [Google Scholar]
- Kıvrak, Ş.; Göktürk, T.; Kıvrak, İ. Assessment of Volatile Oil Composition, Phenolics and Antioxidant Activity of Bay (Laurus nobilis) Leaf and Usage in Cosmetic Applications. Int. J. Second. Metab. 2017, 4, 148–161. [Google Scholar] [CrossRef]
- Karagözler, A.A.; Yavaşera, R.; Sunnaa, Ç. Investigation of Some Antioxidant Properties of Lavender Plant. In Proceedings of the 3rd Congress on Cosmetic Chemistry, Production and Standardization, Antalya, Türkiye, 15–17 February 2013. [Google Scholar]
- Messaoud, C.; Chograni, H.; Boussaid, M. Chemical Composition and Antioxidant Activities of Essential Oils and Methanol Extracts of Three Wild Lavandula L. Species. Nat. Prod. Res. 2012, 26, 1976–1984. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Abbasi-Ahd, A.; Behpour, M.; Batooli, H. Antioxidant Activity of the Essential Oil and Methanolic Extract of Eucalyptus largiflorens and Eucalyptus intertexta from Central Iran. J. Essent. Oil Bear. Plants 2010, 13, 377–384. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Investigation of Phytochemicals and Antioxidant Capacity of Selected Eucalyptus Species Using Conventional Extraction. Chem. Pap. 2016, 70, 567–575. [Google Scholar] [CrossRef]
- Wang, K.; Pan, Y.; Wang, H.; Zhang, Y.; Lei, Q.; Zhu, Z.; Li, H.; Liang, M. Antioxidant Activities of Liquidambar formosana Hance Leaf Extracts. Med. Chem. Res. 2010, 19, 166–176. [Google Scholar] [CrossRef]
- Ulusoy, H.; Ceylan, Ş.; Peker, H. Determination of Antioxidant and Antimicrobial Activity of Sweetgum (Liquidambar orientalis) Leaf, a Medicinal Plant. Polímeros 2021, 31, e2021015. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A Comprehensive Study on the Phenolic Profile of Widely Used Culinary Herbs and Spices: Rosemary, Thyme, Oregano, Cinnamon, Cumin and Bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Elez Garofulić, I.; Balbino, S.; Pedisić, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Konovalov, D.A.; Alieva, N.M. Phenolic Compounds of Laurus nobilis. Pharm. Pharmacol. 2019, 7, 244–259. [Google Scholar] [CrossRef]
- Sriti, J.; Fares, N.; Msaada, K.; Zarroug, Y.; Boulares, M.; Djebbi, S.; Selmi, S.; Limam, F. Phenological Stage Effect on Phenolic Composition, Antioxidant, and Antibacterial Activity of Lavandula stoechas Extract. Riv. Ital. Sostanze Grasse 2022, 99, 225–234. [Google Scholar]
- Mykhailenko, O.; Hurina, V.; Ivanauskas, L.; Marksa, M.; Skybitska, M.; Kovalenko, O.; Lytkin, D.; Vladymyrova, I.; Georgiyants, V. Lavandula angustifolia Herb from Ukraine: Comparative Chemical Profile and In Vitro Antioxidant Activity. Chem. Biodivers. 2024, 21, e202400640. [Google Scholar] [CrossRef]
- Karan, T. Metabolic Profile and Biological Activities of Lavandula stoechas L. Cell. Mol. Biol. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.J.; Quílez, M.; Ortiz de Elguea-Culebras, G.; Melero-Bravo, E.; Sánchez-Vioque, R.; Jordán, M.J. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants 2023, 12, 1986. [Google Scholar] [CrossRef]
- Dezsi, Ș.; Bădărău, A.S.; Bischin, C.; Vodnar, D.C.; Silaghi-Dumitrescu, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L. Antimicrobial and Antioxidant Activities and Phenolic Profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johnson Leaves. Molecules 2015, 20, 4720–4734. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, R.A.; Mahmood, A.; ud Din, M. Chemical Composition and In Vitro Antioxidant and Antitumor Activities of Eucalyptus camaldulensis Dehn. Leaves. Ind. Crop. Prod. 2015, 74, 241–248. [Google Scholar] [CrossRef]
- Özbek, A.G.; Bilek, S.E. Properties and Usage of Liquidambar orientalis. Clin. Nutr. Metab. 2018, 1, 1–3. [Google Scholar] [CrossRef]
- Taş Küçükaydın, M. Preparation and Characterization of Biopolymer-Based Wound Dressing Materials Containing Plant Resin and Bioactive Compounds. Ph.D. Thesis, Muğla Sıtkı Koçman Univ., Muğla, Turkey, 2023. [Google Scholar]
- Değirmencioğlu, N.; Gürbüz, O.; Karatepe, G.E.; Irkin, R. Influence of Hot Air Drying on Phenolic Compounds and Antioxidant Capacity of Blueberry (Vaccinium myrtillus) Fruit and Leaf. J. Appl. Bot. Food Qual. 2017, 90, 115–125. [Google Scholar] [CrossRef]
- Mechi, D.; Baccouri, B.; Martín-Vertedor, D.; Abaza, L. Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts. Molecules 2023, 28, 707. [Google Scholar] [CrossRef] [PubMed]
- Borghini, F.; Tamasi, G.; Loiselle, S.A.; Baglioni, M.; Ferrari, S.; Bisozzi, F.; Costantini, S.; Tozzi, C.; Riccaboni, A.; Rossi, C. Phenolic Profiles in Olive Leaves from Different Cultivars in Tuscany and Their Use as a Marker of Varietal and Geographical Origin on a Small Scale. Molecules 2024, 29, 3617. [Google Scholar] [CrossRef]
- Siamandoura, P.; Tzia, C. Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules 2023, 28, 353. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, A.; Işık, M.; Dikici, E.; Yüksel, M. Anticholinergic, Antioxidant, and Antibacterial Properties of Vitex agnus-castus L. Seed Extract: Assessment of Its Phenolic Content by LC/MS/MS. Chem. Biodivers. 2022, 19, e202200143. [Google Scholar] [CrossRef]
- Berrani, A.; Marmouzi, I.; Bouyahya, A.; Kharbach, M.; El Hamdani, M.; El Jemli, M.; Lrhorfi, A.; Zouarhi, M.; Faouzi’m, E.A.; Bengueddour, R. Phenolic Compound Analysis and Pharmacological Screening of Vitex agnus-castus Functional Parts. Biomed. Res. Int. 2021, 2021, 6695311. [Google Scholar] [CrossRef]
- Malayoğlu, H.B. The antioxidant effect of rosemary (Rosmarinus officinalis L.). Anim. Prod. 2010, 51, 59–67. [Google Scholar]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Gölükçü, M.; Toker, R.; Tokgöz, H. The effect of brewing at different temperatures and times on some quality characteristics of mountain tea (Sideritis congesta L.). J. West Mediterr. Agric. Res. Inst. 2013, 39, 155–162. [Google Scholar]
- Dönmez, M.F.; Uysal Şahin, B.; Usanmaz Bozhüyük, A. Antibacterial effect of essential oil and extracts from Satureja species against bacterial pathogens in beans. J. Agric. 2020, 3, 57–70. [Google Scholar] [CrossRef]
- Öztürk, H.; Kolak, U.; Meriç, C. Antioxidant, anticholinesterase and antibacterial activities of Jurinea consanguinea DC. Rec. Nat. Prod. 2011, 5, 43. [Google Scholar]
- Djebili, S.; Taş, M.; Bouguerra, A.; Kucukaydin, S.; Ceylan, O.; Duru, M.E.; Barkat, M. Volatile compound profile and essential oil composition of three wild Algerian aromatic plants with their antioxidant and antibiofilm activities. J. Food Meas. Charact. 2022, 16, 987–999. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Sujayev, A.; Garibov, E.; Nazarov, N.; Huyut, Z.; Alwasel, S.H.; Gulçin, İ. Synthesis of New Cyclic Thioureas and Evaluation of Their Metal-Chelating Activity, Acetylcholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Profiles. J. Biochem. Mol. Toxicol. 2017, 31, e21897. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. HPLC-DAD Phytochemical Profiles of Thymus cariensis and T. cilicicus with Antioxidant, Cytotoxic, Anticholinesterase, Anti-urease, Anti-tyrosinase, and Antidiabetic Activities. S. Afr. J. Bot. 2021, 143, 155–163. [Google Scholar] [CrossRef]
- Blunch, N.J. Introduction to Structural Equation Modeling Using IBM SPSS Statistics and AMOS; Sage: Newcastle upon Tyne, UK, 2012. [Google Scholar]

| β-Carotene–Linoleic Acid Assay | DPPH• Assay | ABTS+ Assay | CUPRAC Assay | Metal Chelating Assay | ||
|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | A0.50 (µg/mL) | IC50 (µg/mL) | ||
| Ethanol | Rosemary | 3.55 ± 0.17a | 6.12 ± 0.25a | 4.80 ± 0.36a | 5.24 ± 0.33a | 14.71 ± 0.45a |
| Herbal Tea | 2.90 ± 0.11a | 4.76 ± 0.18a | 3.42 ± 0.15a | 3.88 ± 0.17a | 9.22 ± 0.13a | |
| Ethanol | Laurel | 18.21 ± 0.31c | 27.45 ± 0.36d | 21.68 ± 0.56d | 28.37 ± 0.14d | 42.29 ± 0.35c |
| Herbal Tea | 15.37 ± 0.26c | 22.54 ± 0.42c | 14.80 ± 0.63c | 19.11 ± 0.35c | 31.57 ± 0.44b | |
| Ethanol | Lavender | 18.27 ± 0.38c | 22.98 ± 0.51c | 13.59 ± 0.33b | 15.68 ± 0.39b | 40.69 ± 0.27c |
| Herbal Tea | 11.30 ± 0.24b | 17.61 ± 0.64b | 10.85 ± 0.27b | 13.27 ± 0.20b | 35.41 ± 0.71b | |
| Ethanol | Eucalyptus | 16.79 ± 0.29c | 22.65 ± 0.52c | 14.56 ± 0.90c | 18.50 ± 0.31c | 36.78 ± 0.60b |
| Herbal Tea | 8.97 ± 0.12b | 18.10 ± 0.47b | 11.05 ± 0.23b | 15.66 ± 0.54b | 28.78 ± 0.68b | |
| Ethanol | Liquidambar | 24.80 ± 0.44d | 21.34 ± 0.58c | 19.22 ± 0.69c | 32.77 ± 0.35d | 85.90 ± 0.66d |
| Herbal Tea | 19.97 ± 0.23c | 17.82 ± 0.39b | 14.56 ± 0.42b | 25.08 ± 0.18c | 71.30 ± 0.88c | |
| Ethanol | Myrtle | 16.19 ± 0.26c | 18.21 ± 0.44b | 17.54 ± 0.48c | 19.60 ± 0.56c | 27.67 ± 0.22b |
| Herbal Tea | 12.20 ± 0.14b | 16.80 ± 0.38b | 10.95 ± 0.27b | 15.69 ± 0.40b | 18.95 ± 0.31a | |
| Ethanol | Olive | 62.78 ± 0.80e | 77.56 ± 0.67e | 75.09 ± 0.93e | 70.28 ± 0.55e | 92.80 ± 0.66e |
| Herbal Tea | 51.47 ± 0.95e | 65.20 ± 0.84e | 61.04 ± 0.58e | 58.20 ± 0.63e | 81.17 ± 0.73d | |
| Ethanol | Vitex | 73.91 ± 0.25e | 81.61 ± 0.76e | 61.90 ± 0.60e | 80.96 ± 0.87e | 132.1 ± 0.86e |
| Herbal Tea | 55.36 ± 0.85e | 69.94 ± 0.88e | 54.23 ± 0.58e | 65.80 ± 0.73e | 95.72 ± 0.95e | |
| α-tocopherol | 2.10 ± 0.05 | 38.20 ± 0.50 | 35.50 ± 0.55 | 60.20 ± 0.45 | - | |
| BHA | 1.50 ± 0.03 | 19.50 ± 0.30 | 12.70 ± 0.10 | 25.40 ± 0.38 | - | |
| EDTA | - | - | - | - | 5.50 ± 0.35 |
| Phenolic Compound | Antioxidant Assay Showing Highest Correlation | Pearson r |
|---|---|---|
| Rosmarinic acid | ABTS | 0.982 |
| Quercetin | CUPRAC | 0.955 |
| Ferulic acid | DPPH | 0.912 |
| Caffeic acid | ABTS | 0.889 |
| Ellagic acid | Metal chelation | 0.721 |
| p-Hydroxybenzoic acid | CUPRAC | 0.684 |
| Syringic acid | Metal chelation | 0.641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sagbas, H.I.; Kordali, S.; Sahin, S.; Küçükaydın, S.; Uyduran, E. Biochemical Diversity and Nutraceutical Potential of Medicinal Plant-Based Herbal Teas from Southwestern Türkiye. Plants 2026, 15, 125. https://doi.org/10.3390/plants15010125
Sagbas HI, Kordali S, Sahin S, Küçükaydın S, Uyduran E. Biochemical Diversity and Nutraceutical Potential of Medicinal Plant-Based Herbal Teas from Southwestern Türkiye. Plants. 2026; 15(1):125. https://doi.org/10.3390/plants15010125
Chicago/Turabian StyleSagbas, Halil Ibrahim, Saban Kordali, Sena Sahin, Selçuk Küçükaydın, and Elif Uyduran. 2026. "Biochemical Diversity and Nutraceutical Potential of Medicinal Plant-Based Herbal Teas from Southwestern Türkiye" Plants 15, no. 1: 125. https://doi.org/10.3390/plants15010125
APA StyleSagbas, H. I., Kordali, S., Sahin, S., Küçükaydın, S., & Uyduran, E. (2026). Biochemical Diversity and Nutraceutical Potential of Medicinal Plant-Based Herbal Teas from Southwestern Türkiye. Plants, 15(1), 125. https://doi.org/10.3390/plants15010125






