Interaction of 4-Aminobutyrate (GABA) with the Tricarboxylic Acid Cycle in Plants Under Salinity Stress
Abstract
1. Introduction
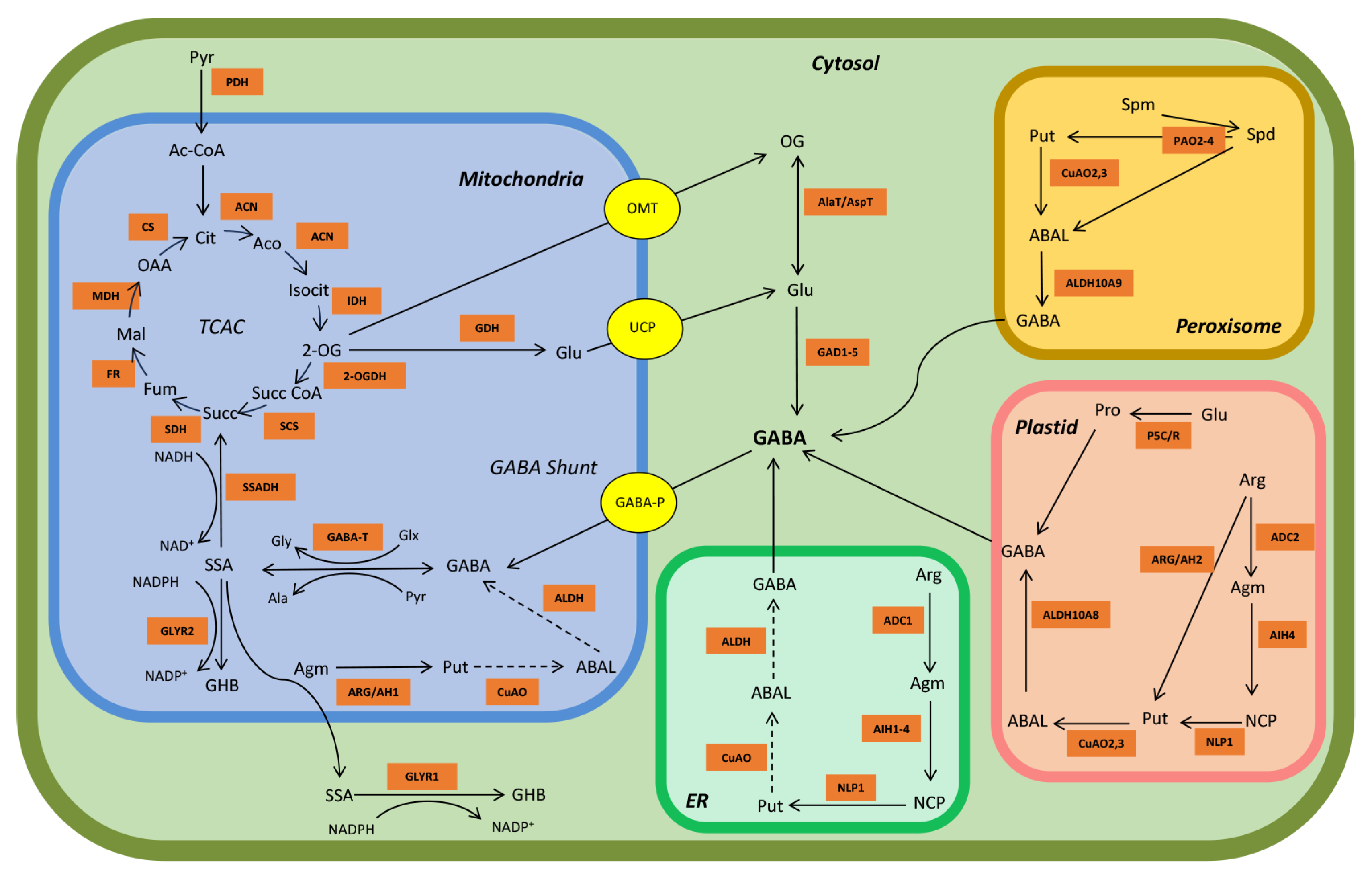
2. GABA Metabolism: The GABA Shunt
3. Alternative GABA Biosynthetic Pathways
4. Effect of Salinity Stress on the Tricarboxylic Acid Cycle
5. Stress-Induced Activation of GABA Metabolism Restores Energy Production and Respiration
| Plant Species | Growth Condition | Organ/ Tissue | NaCl Treatment | Control [GABA] | Salt [GABA] | Fold Stimulation | Reference |
|---|---|---|---|---|---|---|---|
| Tomato (Solanum lycopersicum L.) | Hydroponics | Leaf | 175 mM, 2 d | ~28 μmol g−1 FM | ~42 μmol g−1 FM | 0.5 | [79] |
| 175 mM, 4 d | ~23 μmol g−1 FM | ~30 μmol g−1 FM | 0.3 | ||||
| White clover (Trifolium repens L.) | Tissue culture | Seedling | 100 mM, 7 d | ~0.32 μmol g−1 DM | ~0.24 μmol g−1 DM | ~0.3 | [80] |
| Legume shrub (Caragana intermedia L.) | Sand | Root | 300 mM, 2 d | ~5 nmol g−1 FM | ~35 nmol g−1 FM | ~6 | [81] |
| Arabidopsis (Arabidopsis thaliana [L.] Heynh.) | Tissue culture | Shoot | 150 mM, 4 d | 0.7 μmol g−1 DM | 11 μmol g−1 DM | 15 | [75] |
| Root | 7.5 μmol g−1 DM | 9.9 μmol g−1 DM | 0.3 | ||||
| Hydroponics | Root | 150 mM, 1 d | 6.7 μmol g−1 DM | 6.9 μmol g−1 DM | 0.03 | [74] | |
| Soil | Shoot | 150 mM, 14 d | 0.02 μmol g−1 FM | 0.040 μmol g−1 FM | 1 | [38] | |
| Root | 0.4 μmol g−1 FM | 1.3 μmol g−1 FM | 2.3 | ||||
| Tissue culture | Root | 100 mM, 15 min | ~0.13 μmol g−1 FM | 0.6 μmol g−1 FM | ~3.6 | [42] | |
| Soil | Shoot | 150 mM, 2 d | ~0.02 μmol g−1 FM | ~0.07 μmol g−1 FM | ~2.5 | [34] | |
| Rice (Oryza sativa L.) | Tissue culture | Leaf | 150 mM, 7 d | 34 μmol g−1 DM | 46 μmol g−1 DM | 0.35 | [82] |
| Wheat (Triticum durum Desf.) | Hydroponics | Shoot | 100 mM, 10 d, 350 μmol m−2 s−1 PAR | ~1 μmol g−1 DM | ~1.5 μmol g−1 DM | 1.5 | [83] |
| 100 mM, 10 d, 900 μmol m−2 s−1 PAR | ~8 μmol g−1 DM | ~46 μmol g−1 DM | 6 | ||||
| Corn (Zea mays L.) | Hydroponics | Leaf | 150 mM; 12, 36 and 60 h | ~252, 290, 290 μmol g−1 FM | ~290, 533, 436 μmol g−1 FM | 0.13, 0.84, 0.5 | [71] |
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GABA | 4-Aminobutyrate |
| GAD | Glutamate decarboxylase |
| GDH | Glutamate dehydrogenase |
| TCAC | Tricarboxylic aid cycle |
| Km | Michaelis-Menten constant |
| AO | Amine oxidase |
| AMADH | Aminoaldehyde dehydrogenase |
| ALDH | Aldehyde dehydrogenase |
References
- Steward, F.C.; Thompson, J.F.; Dent, C.E. γ-Aminobutyric acid: A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar] [CrossRef]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. 2Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic acids: The pools of fixed carbon involved in redox Regulation and energy balance in higher plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef]
- De Oliveira, H.O.; Siqueira, J.A.; Medeiros, D.B.; Fernie, A.R.; Nunes-Nesi, A.; Araújo, W.L. Harnessing the dynamics of plant organic acids metabolism following abiotic stresses. Plant Physiol. Biochem. 2025, 220, 109465. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hoque, M.A. A review on plant responses to soil salinity and amelioration strategies. Open J. Soil Sci. 2019, 9, 219–231. [Google Scholar] [CrossRef]
- Bandehagh, A.; Taylor, N.L. Can alternative metabolic pathways and shunts overcome salinity induced inhibition of central carbon metabolism in crops? Front. Plant Sci. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Studart-Guimaraes, C.; Fait, A.; Nunes-Nesi, A.; Carrari, F.; Usadel, B.; Fernie, A.R. Reduced expression of succinyl-coenzyme A ligase can be compensated for by up-regulation of the γ-aminobutyrate shunt in illuminated tomato leaves. Plant Physiol. 2007, 145, 626–639. [Google Scholar] [CrossRef]
- Araújo, W.L.; Nunes-Nesi, A.; Trenkamp, S.; Bunik, V.I.; Fernie, A.R. Inhibition of 2-oxoglutarate dehydrogenase in potato tuber suggests the enzyme is limiting for respiration and confirms its importance in nitrogen assimilation. Plant Physiol. 2008, 148, 1782–1796. [Google Scholar] [CrossRef]
- Joshi, J.; Folz, J.S.; Gregory, J.F., III; McCarty, D.R.; Fiehn, O.; Hanson, A.D. Rethinking the PDH bypass and GABA shunt as thiamin-deficiency workarounds. Plant Physiol. 2019, 181, 389–393. [Google Scholar] [CrossRef]
- Benidickson, K.H.; Raytek, L.M.; Hoover, G.H.; Flaherty, E.F.; Shelp, B.J.; Snedden, W.A.; Plaxton, W.C. Glutamate decarboxylase-1 is essential for efficient acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. New Phytol. 2023, 240, 2372–2385. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Does the GABA shunt regulate cytosolic GABA? Trends Plant Sci. 2020, 25, 422–424. [Google Scholar] [CrossRef]
- Satyanarayan, V.; Nair, P.M. Purification and characterization of glutamate decarboxylase from Solanum tuberosum. Eur. J. Biochem. 1985, 150, 53–60. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Busch, K.B.; Fromm, H. Plant succinic semialdehyde dehydrogenase. Cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiol. 1999, 121, 589–598. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Zarei, A.; Simpson, J.P.; Trobacher, C.P.; Allan, W.L. Strategies and tools for studying GABA metabolism and function: II. Integrated analysis. Botany 2012, 90, 781–793. [Google Scholar] [CrossRef]
- Breitkreuz, K.E.; Shelp, B.J. Subcellular compartmentation of the 4-aminobutyrate shunt in protoplasts from developing soybean cotyledons. Plant Physiol. 1995, 108, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.D.; Fox, G.G.; Laurie, S.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. Ammonium assimilation and the role of γ-aminobutyric acid in pH homeostasis in carrot cell suspensions. Plant Physiol. 1994, 106, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.A.; Bown, A.W.; Breitkreuz, K.E.; Guinel, F.C. The synthesis of [gamma]-aminobutyric acid in response to treatments reducing cytosolic pH. Plant Physiol. 1994, 104, 865–871. [Google Scholar] [CrossRef]
- Baum, G.; Chen, Y.; Arazi, T.; Takatsuji, H.; Fromm, H. A plant glutamate decarboxylase containing a calmodulin binding domain. J. Biol. Chem. 1993, 268, 19610–19617. [Google Scholar] [CrossRef]
- Snedden, W.A.; Arazi, T.; Fromm, H.; Shelp, B.J. Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol. 1995, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Chiu, G.; Bajwa, V.S. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. I. Pathway structure. Botany 2012, 90, 651–668. [Google Scholar] [CrossRef]
- Snedden, W.A.; Koutsia, N.; Baum, G.; Fromm, H. Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin-binding domain. J. Biol. Chem. 1996, 271, 4148–4153. [Google Scholar] [CrossRef]
- Trobacher, C.P.; Zarei, A.; Liu, J.; Clark, S.M.; Bozzo, G.G.; Shelp, B.J. Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef]
- McLean, M.D.; Yevtushenko, D.P.; Deschene, A.; Van Cauwenberghe, O.R.; Makhmoudova, A.; Potter, J.W.; Bown, A.W.; Shelp, B.J. Overexpression of glutamate decarboxylase in transgenic tobacco plants confers resistance to the northern root-knot nematode. Mol. Breed. 2003, 11, 277–285. [Google Scholar] [CrossRef]
- Deng, X.; Xu, X.; Liu, Y.; Zhang, Y.; Yang, L.; Zhang, S.; Xu, J. Induction of γ-aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae. J. Integr. Plant Biol. 2020, 62, 1797–1812. [Google Scholar] [CrossRef]
- Turano, F.J.; Fang, T.K. Characterization of two glutamate decarboxylase cDNA clones from Arabidopsis. Plant Physiol. 1998, 117, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Gut, H.; Dominici, P.; Pilati, S.; Astegno, A.; Petoukhov, M.V.; Svergun, D.I.; Grütter, M.G.; Capitani, G. A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 2009, 392, 334–351. [Google Scholar] [CrossRef]
- Zarei, A.; Chiu, G.Z.; Yu, G.; Trobacher, C.P.; Shelp, B.J. Salinity-regulated expression of genes involved in GABA metabolism and signaling. Botany 2017, 95, 621–627. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Zik, M.; Arazi, T.; Snedden, W.A.; Fromm, H. Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol. Biol. 1998, 37, 967–975. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, N.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Mekonnen, D.W. Oversensitivity of Arabidopsis gad1/2 mutant to NaCl treatment reveals the importance of GABA in salt stress responses. Afr. J. Plant Sci. 2017, 11, 252–263. [Google Scholar] [CrossRef]
- Yu, G.H.; Zou, J.; Feng, J.; Peng, X.B.; Wu, J.Y.; Wu, Y.L.; Palanivelu, R.; Sun, M.X. Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J. Exp. Bot. 2014, 65, 3235–3248. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.Y.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrian, M.; Alcazar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Janowitz, T.; Kneifel, H.; Piotrowski, M. Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett. 2003, 544, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Ahmed, S.; Ge, L.; Avestakh, A.; Oloyede, B.; Phuntumart, V.; Kalinoski, A.; Morris, P.F. Spatial organization of putrescine synthesis in plants. Plant Sci. 2014, 349, 112232. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Ariyaratne, M.; Ahmed, S.; Ge, L.; Phuntumart, V.; Kalinoski, A.; Morris, P.F. Dual functioning of plant arginases provides a third route for putrescine synthesis. Plant Sci. 2017, 262, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Fraudentali, I.; Rodrigues-Pousada, R.A.; Angelini, R.; Ghuge, S.A.; Cona, A. Plant copper amine oxidases: Key players in hormone signaling leading to stress-induced phenotypic plasticity. Int. J. Mol. Sci. 2021, 22, 5136. [Google Scholar] [CrossRef]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef]
- Liao, J.; Wu, X.; Xing, Z.; Li, Q.; Duan, Y.; Fang, W.; Zhu, X. γ-Aminobutyric acid (GABA) accumulation in tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 2017, 65, 3013−3018. [Google Scholar] [CrossRef]
- Zarei, A.; Trobacher, C.P.; Shelp, B.J. NAD+-aminoaldehyde dehydrogenase candidates for 4-aminobutyrate (GABA) and β-alanine production during terminal oxidation of polyamines in apple fruit. FEBS Lett. 2015, 589, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Trobacher, C.P.; Shelp, B.J. Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci. Rep. 2016, 6, 35115. [Google Scholar] [CrossRef]
- Missihoun, T.D.; Schmitz, J.; Klug, R.; Kirch, H.H.; Bartels, D. Betaine aldehyde dehydrogenase genes from Arabidopsis with different sub-cellular localization affect stress responses. Planta 2011, 233, 369–382. [Google Scholar] [CrossRef]
- Hou, Q.; Bartels, D. Comparative study of the aldehyde dehydrogenase (ALDH) gene superfamily in the glycophyte Arabidopsis thaliana and Eutrema halophytes. Ann. Bot. 2015, 115, 465–479. [Google Scholar] [CrossRef]
- Jacques, F.; Zhao, Y.; Kopečná, M.; Končitíková, R.; Kopečný, D.; Rippa, S.; Perrin, Y. Roles for ALDH10 enzymes in γ-butyrobetaine synthesis, seed development, germination, and salt tolerance in Arabidopsis. J. Exp. Bot. 2020, 71, 7088–7102. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, Y.; Cao, Y.; Chen, Y.; Zou, Z.; Li, F.; Shen, Q.; Yang, X.; Ma, Y.; Fang, W.; et al. CsCuAOs and CsAMADH1 are required for putrescine-derived Y-aminobutyric acid accumulation in tea. Foods 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, Y.; Cheng, N.; Zhang, K.; Duan, Y.; Fang, S.; Shen, Q.; Yang, X.; Fang, W.; Zhu, X. CsCuAO1 associated with CsAMADH1 confers drought tolerance by modulating GABA levels in tea plants. Int. J. Mol. Sci. 2024, 25, 992. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef]
- Poolman, M.G.; Miguet, L.; Sweetlove, L.J.; Fell, D.A. A genome-scale metabolic model of Arabidopsis and some of its properties. Plant Physiol. 2009, 15, 1570–1581. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gomez, N.M.; Mattson, N.; Wajid, N.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2010, 10, 938. [Google Scholar] [CrossRef]
- Hanning, I.; Heldt, H.W. On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves (partitioning between respiration and export of redox equivalents and precursors for nitrate assimilation products). Plant Physiol. 1993, 103, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Araújo, W.L.; Fernie, A.R. Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol. 2011, 155, 101–107. [Google Scholar] [CrossRef]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 2014, 15, 552. [Google Scholar] [CrossRef] [PubMed]
- Fougere, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Zhong, M.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S.; Yuan, R.; Tang, Y. Effects of exogenous putrescine on glycolysis and Krebs cycle metabolism in cucumber leaves subjected to salt stress. Plant Growth Regul. 2016, 79, 319–330. [Google Scholar] [CrossRef]
- Li, P.C.; Yang, X.Y.; Wang, H.M.; Ting, P.A.N.; Yang, J.Y.; Wang, Y.Y.; Yang, X.U.; Yang, Z.F.; Xu, C.W. Metabolic responses to combined water deficit and salt stress in maize primary roots. J. Integr. Agric. 2021, 20, 109–119. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Wang, S.; Guo, J.; Dou, H.; Qiao, J.; Yang, Q.; Shao, R.; Wang, H. Exogenous γ-aminobutyric acid (GABA) improves salt-inhibited nitrogen metabolism and the anaplerotic reaction of the tricarboxylic acid cycle by regulating GABA-shunt metabolism in maize seedlings. Ecotoxicol. Environ. Saf. 2013, 254, 114756. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS ONE 2013, 8, E55431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Du, Y.; Chen, S.; Tang, H. Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; El Amrani, A.; Berger, A.; Mouille, G.; Soubigou-Taconnat, L.; Bouchereau, A.; Deleu, C. γ-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ. 2013, 36, 1009–1018. [Google Scholar] [CrossRef]
- Renault, H.; Roussel, V.; El Amrani, A.; Arzel, M.; Renault, D.; Bouchereau, A.; Deleu, C. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Kumari, S.; Naaz, M.; Siddique, K.H.M.; Khan, M.I.R. GABA-dependent ethylene response mitigates salt-induced growth and yield inhibition through stabilizing carbon energy, nutrients accumulation and metabolomic fingerprinting in wheat. Plant Physiol. Biochem. 2025, 226, 110020. [Google Scholar] [CrossRef] [PubMed]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The role of the γ-aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 1–21. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Shi, S.Q.; Shi, A.; Jiang, Z.P.; Qi, L.W.; Sun, X.M.; Li, C.X.; Liu, J.F.; Xiao, W.F.; Zhang, S.G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010, 33, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Brikis, C.J.; Zarei, A.; Chiu, G.Z.; Deyman, K.L.; Liu, J.; Trobacher, C.P.; Hoover, G.J.; Subedi, S.; DeEll, J.R.; Bozzo, G.G.; et al. Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic. Res. 2018, 5, 61. [Google Scholar] [CrossRef]
- Melo-Oliveira, R.; Oliveira, I.C.; Coruzzi, G.M. Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc. Natl. Acad. Sci. USA 1996, 93, 4718–4723. [Google Scholar] [CrossRef]
- Kumar, R.G.; Shah, K.V.; Dubey, R.S. Salinity induced behavioural changes in malate dehydrogenase and glutamate dehydrogenase activities in rice seedlings of differing salt tolerance. Plant Sci. 2000, 156, 23–34. [Google Scholar] [CrossRef]
- Lasa, B.; Frechilla, S.; Aparicio-Tejo, P.M.; Lamsfus, C. Role of glutamate dehydrogenase and phosphoenolpyruvate carboxylase activity in ammonium nutrition tolerance in roots. Plant Physiol. Biochem. 2020, 40, 969–976. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.S.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef]
- Grzechowiak, M.; Sliwiak, J.; Jaskolski, M.; Ruszkowski, M. Structural and functional studies of Arabidopsis thaliana glutamate dehydrogenase isoform 2 demonstrate enzyme dynamics and identify its calcium binding site. Plant Physiol. Biochem. 2023, 201, 107895. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Anokhina, G.B.; Oya, P.S.; Dedov, Y.I. Catalytic and molecular aspects of the functioning of glutamate-dehydrogenase isoforms in corn Zea mays L. Appl. Biochem. Microbiol. 2021, 57, 236–242. [Google Scholar] [CrossRef]
- Yamaya, T.; Oaks, A.; Matsumoto, H. Characteristics of glutamate dehydrogenase in mitochondria prepared from corn shoots. Plant Physiol. 1984, 76, 1009–1013. [Google Scholar] [CrossRef]
- Dubois, F.; Tercé-Laforgue, T.; Gonzalez-Moro, M.-B.; Estavillo, J.M.; Sangwan, R.; Gallais, A.; Hirel, B. Glutamate dehydrogenase in plants: Is there a new story for an old enzyme? Plant Physiol. Biochem. 2003, 41, 565–576. [Google Scholar] [CrossRef]
- Purnell, M.P.; Botella, J.R. Tobacco isoenzyme 1 of NAD(H)-dependent glutamate dehydrogenase catabolizes glutamate in vivo. Plant Physiol. 2007, 143, 530–539. [Google Scholar] [CrossRef]
- Purnell, M.P.; Skopelitis, D.S.; Roubelakis-Angelakis, K.A.; Botella, J.R. Modulation of higher-plant NAD(H)-dependent glutamate dehydrogenase activity in transgenic tobacco via alteration of beta subunit levels. Planta 2005, 222, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Tercé-Laforgue, T.; Bedu, M.; Dargel-Grafin, C.; Dubois, F.; Gibon, Y.; Restivo, F.M.; Hirel, B. Resolving the role of plant glutamate dehydrogenase: II. Physiological characterization of plants overexpressing the two enzyme subunits individually or simultaneously. Plant Cell Physiol. 2013, 54, 1635–1647. [Google Scholar] [CrossRef]
- Tercé-Laforgue, T.; Clément, G.; Marchi, L.; Restivo, F.M.; Lea, P.J.; Hirel, B. Resolving the role of plant NAD-glutamate dehydrogenase: III. Overexpressing individually or simultaneously the two enzyme subunits under salt stress induces changes in the leaf metabolic profile and increases plant biomass production. Plant Cell Physiol. 2015, 56, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Anokhina, G.B.; Selivanova, P.S.; Moskvina, P.P.; Igamberdiev, A.U. Biochemical and epigenetic regulation of glutamate metabolism in maize (Zea mays L.) leaves under salt stress. Plants 2024, 13, 2651. [Google Scholar] [CrossRef]
- Tcherkez, G.; Mahé, A.; Gauthier, P.; Mauve, C.; Gout, E.; Bligny, R.; Cornic, G.; Hodges, M. In folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid “cycle” in illuminated leaves. Plant Physiol. 2009, 151, 620–630. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Anokhina, G.B.; Shakhov, Z.N.; Moskvina, P.P.; Igamberdiev, A.U. The role of glutamate metabolism and the GABA shunt in bypassing the tricarboxylic acid cycle in the light. Int. J. Mol. Sci. 2024, 25, 12711. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.X.; Tercé-Laforgue, T.; Armengaud, P.; Clément, G.; Renou, J.P.; Pelletier, S.; Catterou, M.; Azzopardi, M.; Gibbon, Y.; Lea, P.J.; et al. Characterization of a NADH-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism. Plant Cell 2012, 24, 4044−4065. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zanegina, D.; Akinchits, E.; Brilkina, A.; Vodeneev, V. Salt-induced changes in cytosolic pH and photosynthesis in tobacco and potato leaves. Int. J. Mol. Sci. 2013, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Hao, D.-L.; Yang, G.Z. Regulation of cytosolic pH: The contributions of plant plasma membrane H+-ATPases and multiple transporters. Int. J. Mol. Sci. 2021, 222, 2998. [Google Scholar] [CrossRef]
- Laohavisit, A.; Richards, S.L.; Shabala, L.; Chen, C.; Colaço, R.D.D.R.; Swarbreck, S.M.; Shaw, E.; Dark, A.; Shabala, S.; Shang, Z.; et al. Salinity-induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein annexin1. Plant Physiol. 2013, 163, 253–262. [Google Scholar] [CrossRef]
- Singh, A.; Choudhary, K.K. Sodium-induced calcium signaling in plants under salinity stress. In Metals and Metalloids in Plant Signaling; Aftab, T., Ed.; Signaling and Communication in Plants; Springer Nature: Cham, Switzerland, 2024; pp. 201–214. [Google Scholar] [CrossRef]
- Cholewa, E.; Cholewinski, A.J.; Shelp, B.J.; Snedden, W.A.; Bown, A.W. Cold-shock-stimulated γ-aminobutyric acid synthesis is mediated by an increase in cytosolic Ca2+, not by an increase in cytosolic H+. Can. J. Bot. 1997, 75, 375–382. [Google Scholar] [CrossRef]
- Aurisano, N.; Bertani, A.; Reggiani, R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995, 36, 1525–1529. [Google Scholar] [CrossRef]
- Scott-Taggart, C.P.; Van Cauwenberghe, O.R.; McLean, M.D.; Shelp, B.J. Regulation of γ-aminobutyric acid synthesis in situ by glutamate availability. Physiol. Plant. 1999, 106, 363−369. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 23, 1237–1249. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 1, 152–159. [Google Scholar] [CrossRef]
- Gao, Q.; Deng, D.; Zeng, R.; Liu, Y.; Jiang, J.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X. GABA is a key player regulating the TCA cycle and polyamine metabolism under combined heat-drought stress in tea plants. Plant Stress 2025, 18, 101006. [Google Scholar] [CrossRef]
- Mekonnen, D.W.; Flügge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Growth Condition | Organ | NaCl Treatment | Control [GABA] 2 | Salt [GABA] 2 | Fold Stimulation | Reference |
|---|---|---|---|---|---|---|---|
| Wt 1 | Soil | Shoot | 150 mM, 2 d | ~0.018 | ~0.063 | ~2.5 | [34] |
| gad4 | ~0.020 | ~0.038–0.050 | ~0.9–1.5 | ||||
| gad1/2 | ~0.005 | ~0.007–0.018 | ~0.4–2.6 | ||||
| Wt | Soil | Shoot | 150 mM, 14 d | ~0.02 | ~0.04 | ~1 | [38] |
| Wt | Root | ~0.4 | ~1.3 | ~2.3 | |||
| gad1/2 | Shoot | n.d. 3 | n.d. | - | |||
| gad1/2 | Root | ~0.0125 | ~0.0125 | 0 | |||
| Wt | Tissue culture | Root | 100 mM, 15 min | ~0.39 | ~0.69 | ~0.77 | [42] |
| gad1/2 | 0.33 | ~0.45 | ~0.36 |
| Plant Species | Growth Condition | Organ/ Tissue | NaCl Treatment | Metabolite Increase | Metabolite Decrease | Reference |
|---|---|---|---|---|---|---|
| Wheat (Triticum aestivum L.) | Hydroponics | Leaf | 150 mM, 3 d | OG, Succ | Fum, Mal, Cit, Aco | [40] |
| Cucumber (Cucumis sativus L.) | Soil | Leaf | 75 mM, 7 d | - | Pyr, Cit, Succ, Mal | [69] |
| Corn (Zea mays L.) | Hydroponics | Root | 100 mM, 4 d | - | Mal, Fum, Cit, OG | [70] |
| Leaf | 150 mM; 12, 36, 60 h | - | Mal, Cit, Succ, OG | [71] | ||
| Barley (Hordeum vulgare L.) | Hydroponics | Root | 300 mM, 21 d | Cit, Isocit | Fum, OG | [72] |
| Shoot | - | Cit, Fum, OG, Mal, Succ | ||||
| Tobacco (Nicotiana tabacum L.) | Tissue culture | Shoot | 500 mM, 3 d | Succ | Mal, Fum | [73] |
| Arabidopsis (Arabidopsis thaliana [L.] Heynh.) | Soil | Shoot | 150 mM, 14 d | - | Fum, OAA, Mal, Cit | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Flaherty, E.J.; Shelp, B.J. Interaction of 4-Aminobutyrate (GABA) with the Tricarboxylic Acid Cycle in Plants Under Salinity Stress. Plants 2026, 15, 123. https://doi.org/10.3390/plants15010123
Flaherty EJ, Shelp BJ. Interaction of 4-Aminobutyrate (GABA) with the Tricarboxylic Acid Cycle in Plants Under Salinity Stress. Plants. 2026; 15(1):123. https://doi.org/10.3390/plants15010123
Chicago/Turabian StyleFlaherty, Edward J., and Barry J. Shelp. 2026. "Interaction of 4-Aminobutyrate (GABA) with the Tricarboxylic Acid Cycle in Plants Under Salinity Stress" Plants 15, no. 1: 123. https://doi.org/10.3390/plants15010123
APA StyleFlaherty, E. J., & Shelp, B. J. (2026). Interaction of 4-Aminobutyrate (GABA) with the Tricarboxylic Acid Cycle in Plants Under Salinity Stress. Plants, 15(1), 123. https://doi.org/10.3390/plants15010123







