The Impact of Seed Treatment with Cold Plasma on Antioxidants, Sugars, and Pigments in Needles of Norway Spruce Is Genotype-Dependent
Abstract
1. Introduction
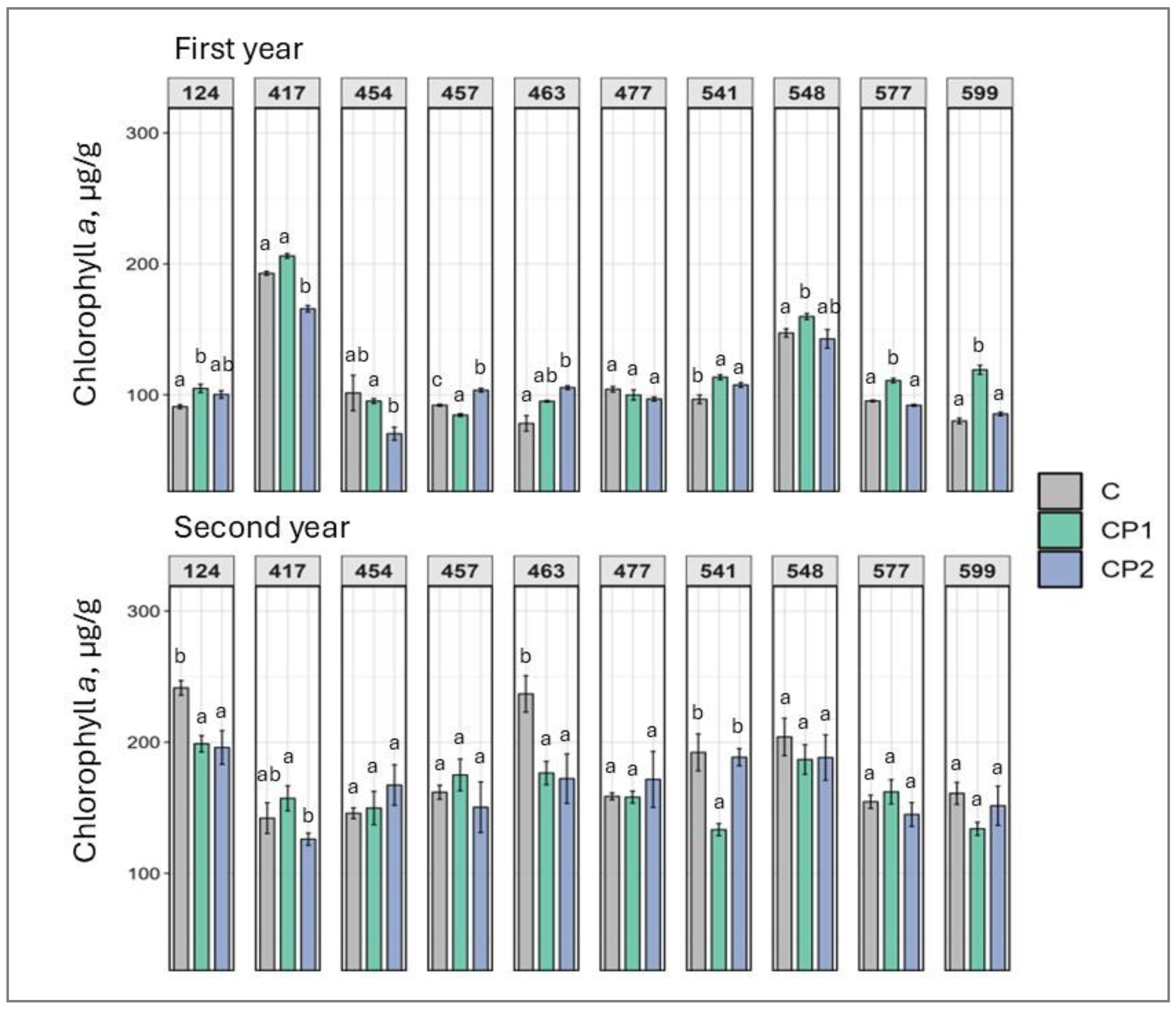
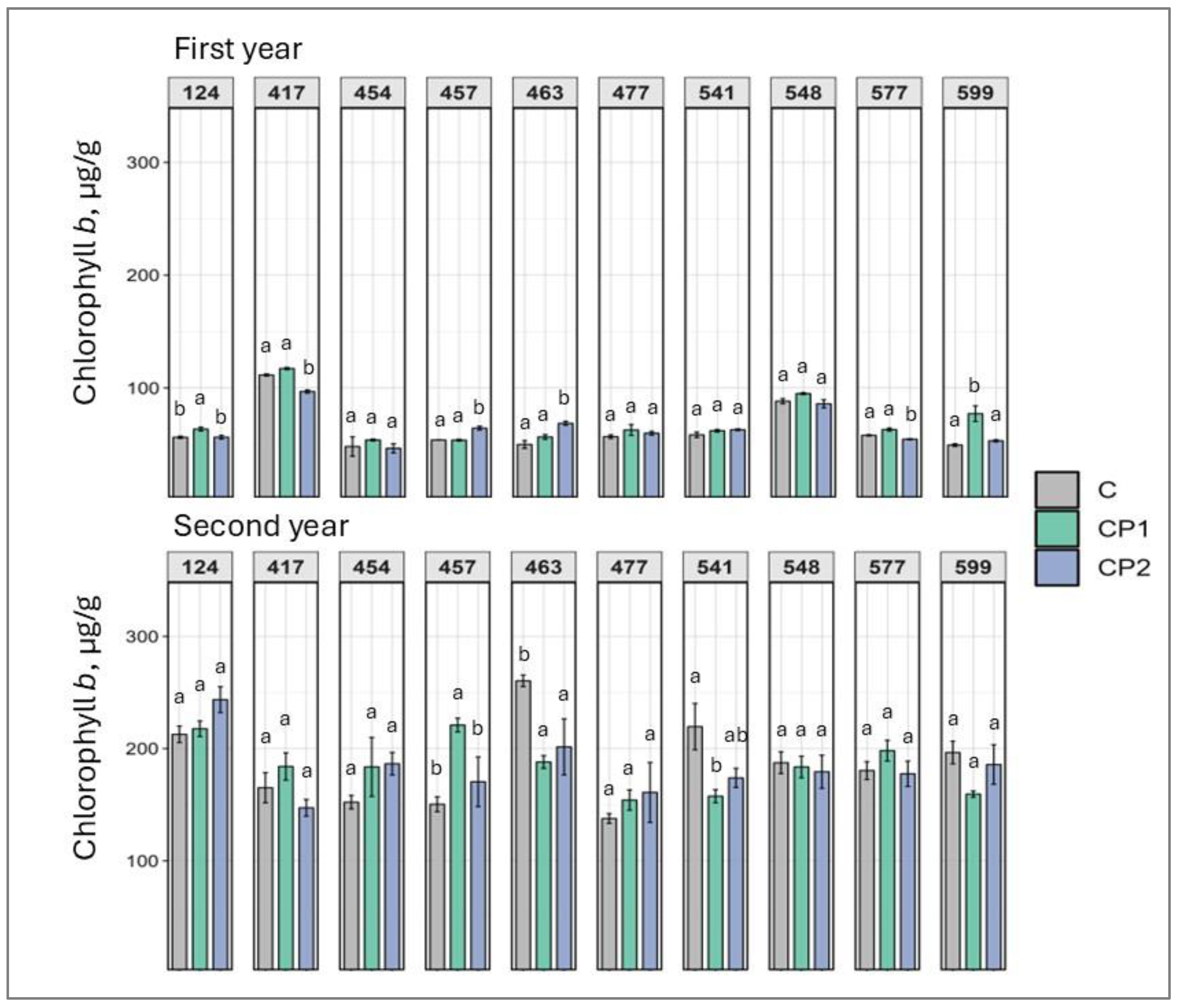
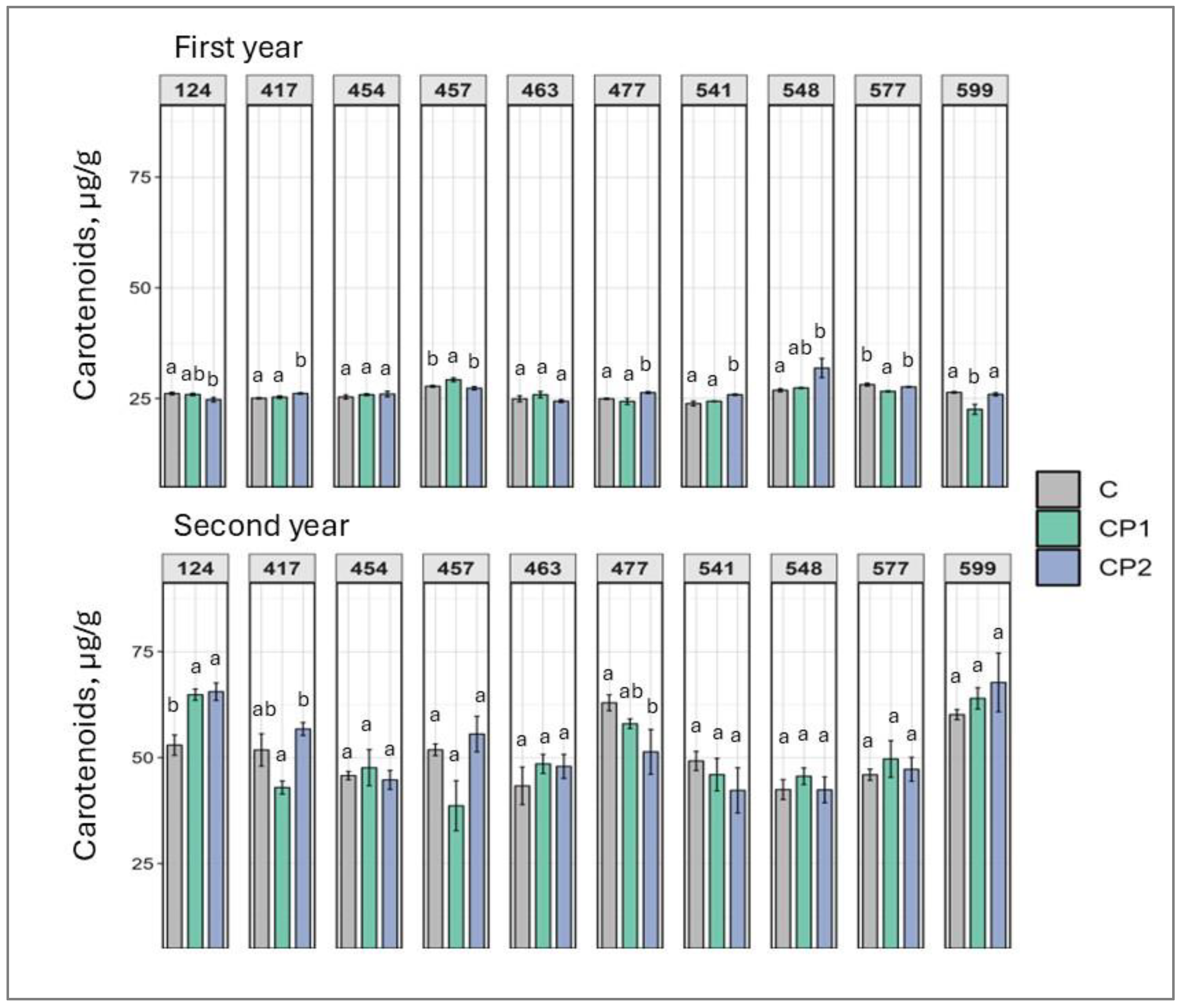
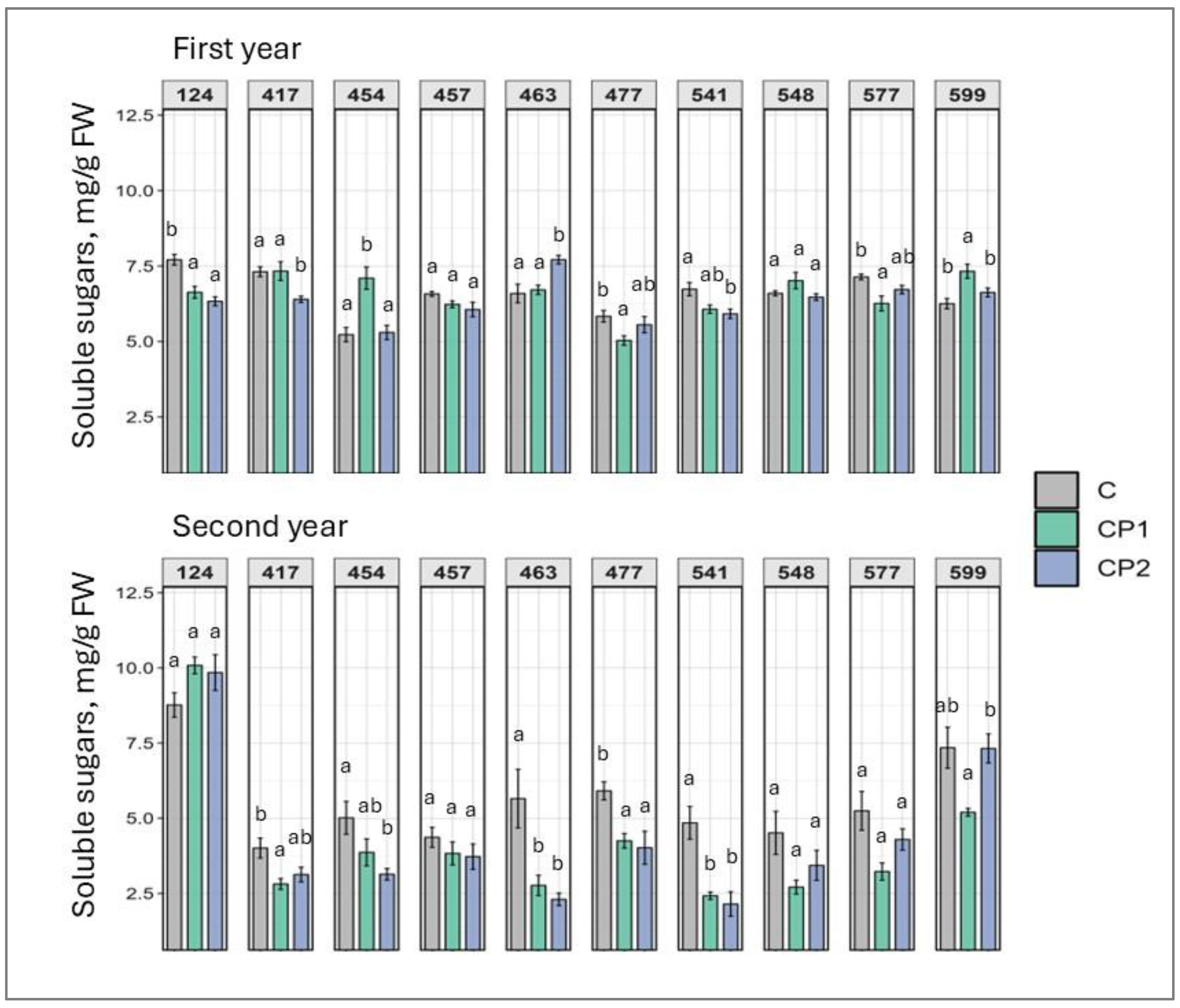
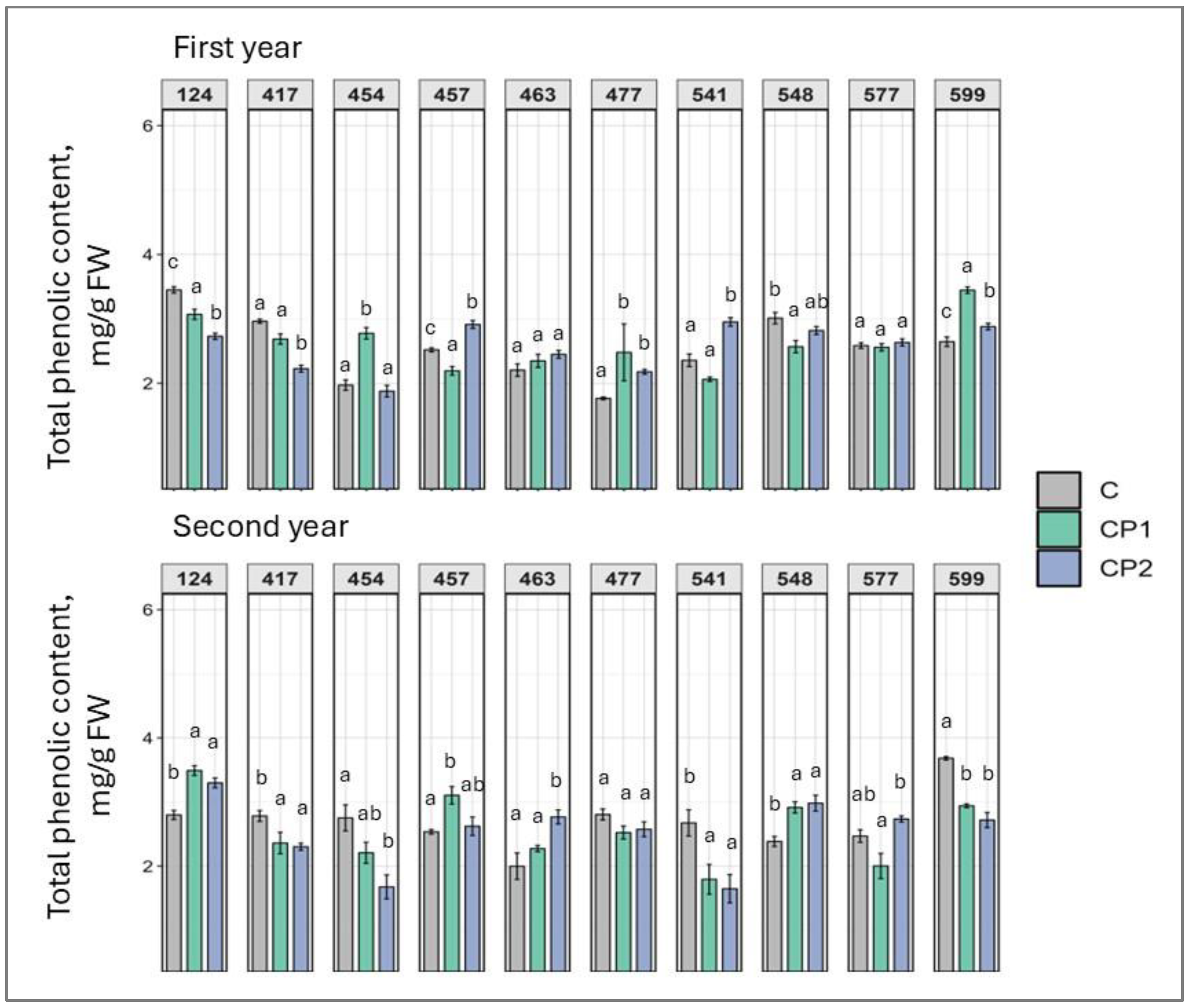
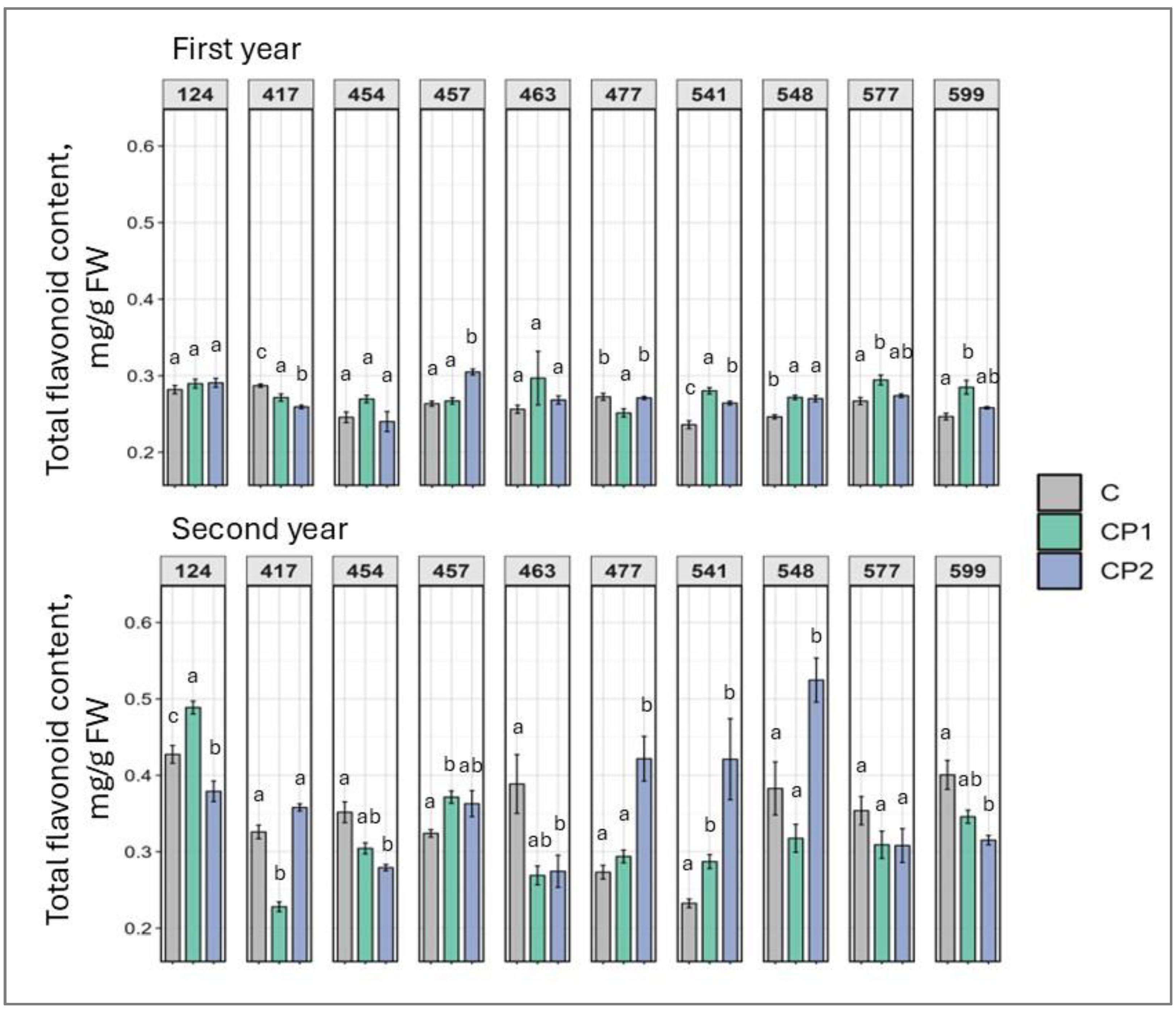
2. Results
3. Discussion
4. Materials and Methods
4.1. Sowing Material
4.2. Treatment with Cold Plasma
4.3. Growth Conditions
4.4. Sampling
4.5. Biochemical Analyses
4.5.1. Extract Preparation
4.5.2. TPC Analysis
4.5.3. TFC Analysis
4.5.4. Chlorophyll a, Chlorophyll b, and Carotenoid Content Analysis
4.5.5. Lipid Peroxidation Analysis
4.5.6. Soluble Sugar Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | cold plasma |
| CP1 | CP treatment for 1 min |
| CP2 | CP treatment for 2 min |
| DBD | dielectric barrier discharge |
| MDA | malondialdehyde |
| SM | secondary metabolites |
| TFC | total flavonoid content |
| TPC | total phenolic content |
References
- Stenlid, J.; Oliva, J. Phenotypic interactions between tree hosts and invasive forest pathogens in the light of globalization and climate change. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150455. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest Health in a Changing World. Microb. Ecol. 2015, 69, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Arnerup, J.; Nemesio-Gorriz, M.; Lundén, K.; Asiegbu, F.O.; Stenlid, J.; Elfstrand, M. The primary module in Norway spruce defence signalling against H. annosum s.l. seems to be jasmonate-mediated signalling without antagonism of salicylate-mediated signalling. Planta 2013, 237, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.M.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Mageroy; Krokene, P.; Viejo, M. Climatic and stress memory in trees-and how to study it. For. Mycriobiol. Acad. Press 2025, 43, 399–418. [Google Scholar] [CrossRef]
- Popa, A.; van der Maaten, E.; Popa, I.; van der Maaten-Theunissen, M. Early warning signals indicate climate change-induced stress in Norway spruce in the Eastern Carpathians. Sci. Total Environ. 2024, 912, 169167. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Šilinskas, B.; Varnagiryte-Kabašinskiene, I.; Aleinikovas, M.; Beniušiene, L.; Aleinikoviene, J.; Škema, M. Scots pine and Norway spruce wood properties at sites with different stand densities. Forests 2020, 11, 587. [Google Scholar] [CrossRef]
- Rodriguez, Y.P.; Gerendiain, A.Z.; Pappinen, A.; Peltola, H.; Pulkkinen, P. Differences in wood decay by Heterobasidion parviporum in cloned Norway spruce (Picea abies). Can. J. For. Res. 2009, 39, 26–35. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Dietrich, P.; Maharjan, R.S.; Thissen, A.; Didwal, P.N.; Shinde, M.; Laux, P.; Luch, A.; Mathe, V.; et al. Emerging cold plasma treatment and machine learning prospects for seed priming: A step towards sustainable food production. RSC Adv. 2022, 12, 10467–10488. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zhou, R.W.; De Groot, G.; Bazaka, K.; Murphy, A.B.; Ostrikov, K.K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 5601. [Google Scholar] [CrossRef]
- Li, K.; Zhong, C.; Shi, Q.; Bi, H.; Gong, B. Cold plasma seed treatment improves chilling resistance of tomato plants through hydrogen peroxide and abscisic acid signaling pathway. Free Radic. Biol. Med. 2021, 172, 286–297. [Google Scholar] [CrossRef]
- Veerana, M.; Mumtaz, S.; Rana, J.N.; Javed, R.; Panngom, K.; Ahmed, B.; Akter, K.; Choi, E.H. Recent Advances in Non-Thermal Plasma for Seed Germination, Plant Growth, and Secondary Metabolite Synthesis: A Promising Frontier for Sustainable Agriculture. Plasma Chem. Plasma Process. 2024, 44, 2263–2302. [Google Scholar] [CrossRef]
- Dilip, D.; Modupalli, N.; Rahman, M.M.; Kariyat, R. Atmospheric cold plasma alters plant traits and negatively affects the growth and development of fall armyworm in rice. Sci. Rep. 2025, 15, 3680. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]
- Sarinont, T.; Amano, T.; Attri, P.; Koga, K.; Hayashi, N.; Shiratani, M. Effects of plasma irradiation using various feeding gases on growth of Raphanus sativus L. Arch. Biochem. Biophys. 2016, 605, 129–140. [Google Scholar] [CrossRef] [PubMed]
- de Groot, G.J.J.B.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, srep05859. [Google Scholar] [CrossRef]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Malakauskienė, A.; Norkevičienė, E.; Šlepetienė, A.; Stukonis, V.; Olšauskaitė, V.; Padarauskas, A.; et al. Effect of seed treatment with cold plasma and electromagnetic field on red clover germination, growth and content of major isoflavones. J. Phys. D. Appl. Phys. 2020, 53, 264001. [Google Scholar] [CrossRef]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1700068. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Jasim, S.F.; Abdulbaqi, N.J.; Al-Zubaidi, L.A.; Jasim, A.D. Evaluation of Atmospheric Cold Plasma Technique Activity on Phenylpropanoids Gene Expression and Essential Oil Contents and Different Traits of Ocimum basilicum L. Baghdad Sci. J. 2022, 19, 966–975. [Google Scholar] [CrossRef]
- Lyu, X.; Chen, Y.; Gao, S.; Cao, W.; Fan, D.; Duan, Z.; Xia, Z. Metabolomic and transcriptomic analysis of cold plasma promoting biosynthesis of active substances in broccoli sprouts. Phytochem. Anal. 2023, 34, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhang, N.; Ji, H.; Zhang, X.; Dong, C.; Yu, J.; Yan, S.; Chen, C.; Liang, L. Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato. Foods 2022, 11, 4088. [Google Scholar] [CrossRef]
- Ebrahimibasabi, E.; Ebrahimi, A.; Momeni, M.; Amerian, M.R. Elevated expression of diosgenin-related genes and stimulation of the defense system in Trigonella foenum-graecum (Fenugreek) by cold plasma treatment. Sci. Hortic. 2020, 271, 109494. [Google Scholar] [CrossRef]
- Čėsnienė, I.; Čėsna, V.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė-Šėžienė, V. Seed Treatment with Cold Plasma and Electromagnetic Field: Changes in Antioxidant Capacity of Seedlings in Different Picea abies (L.) H. Karst Half-Sib Families. Plants 2024, 13, 2021. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Sirgedaitė-Šėžienė, V.; Mildažienė, V.; Žemaitis, P.; Ivankov, A.; Koga, K.; Shiratani, M.; Baliuckas, V. Long-term response of Norway spruce to seed treatment with cold plasma: Dependence of the effects on the genotype. Plasma Process. Polym. 2021, 18, 2000159. [Google Scholar] [CrossRef]
- Bozhanova, V.; Marinova, P.; Videva, M.; Nedjalkova, S.; Benova, E. Effect of Cold Plasma on the Germination and Seedling Growth of Durum Wheat Genotypes. Processes 2024, 12, 544. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A. Phenolic metabolites in the resistance of northern forest trees to pathogens—Past experiences and future prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138697. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Yang, C.M.; Shih, T.H.; Lin, S.H.; Pham, G.T.; Nguyen, H.C. Chlorophyll biosynthesis and transcriptome profiles of chlorophyll b-deficient type 2b rice (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12380. [Google Scholar] [CrossRef]
- Beniušytė, E.; Čėsnienė, I.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators. Plants 2023, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Čėsnienė, I.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė, V. Seed Treatment with Electromagnetic Field Induces Different Effects on Emergence, Growth and Profiles of Biochemical Compounds in Seven Half-Sib Families of Silver Birch. Plants 2023, 12, 3048. [Google Scholar] [CrossRef] [PubMed]
- Hanusz, Z.; Tarasinska, J.; Zielinski, W. Shapiro–Wilk Test with Known Mean. REVSTAT-Stat. J. 2016, 14, 89–100. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s honestly significant difference (HSD) test. Encycl. Res. Des. 2010, 3, 1–5. [Google Scholar]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. In The Corsini Encyclopedia of Psychology; Weiner, I.B., Craighead, W.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups using Dunn’s Test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Sedgwick, P. Philip, Multiple significance tests: The Bonferroni correction. BMJ 2012, 344, e509. [Google Scholar] [CrossRef]
- Graves, S.; Piepho, H.P.; Selzer, M.L. Package ‘multcompView’. Vis. Paired Comp. 2015, 451, 452. [Google Scholar]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2016, 2, 1–189. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čėsnienė, I.; Čėsna, V.; Mildažienė, V.; Miškelytė, D.; Vaitiekūnaitė, D.; Sirgedaitė-Šėžienė, V. The Impact of Seed Treatment with Cold Plasma on Antioxidants, Sugars, and Pigments in Needles of Norway Spruce Is Genotype-Dependent. Plants 2025, 14, 1404. https://doi.org/10.3390/plants14091404
Čėsnienė I, Čėsna V, Mildažienė V, Miškelytė D, Vaitiekūnaitė D, Sirgedaitė-Šėžienė V. The Impact of Seed Treatment with Cold Plasma on Antioxidants, Sugars, and Pigments in Needles of Norway Spruce Is Genotype-Dependent. Plants. 2025; 14(9):1404. https://doi.org/10.3390/plants14091404
Chicago/Turabian StyleČėsnienė, Ieva, Vytautas Čėsna, Vida Mildažienė, Diana Miškelytė, Dorotėja Vaitiekūnaitė, and Vaida Sirgedaitė-Šėžienė. 2025. "The Impact of Seed Treatment with Cold Plasma on Antioxidants, Sugars, and Pigments in Needles of Norway Spruce Is Genotype-Dependent" Plants 14, no. 9: 1404. https://doi.org/10.3390/plants14091404
APA StyleČėsnienė, I., Čėsna, V., Mildažienė, V., Miškelytė, D., Vaitiekūnaitė, D., & Sirgedaitė-Šėžienė, V. (2025). The Impact of Seed Treatment with Cold Plasma on Antioxidants, Sugars, and Pigments in Needles of Norway Spruce Is Genotype-Dependent. Plants, 14(9), 1404. https://doi.org/10.3390/plants14091404






