Antioxidant, Anti-Inflammatory, and Antiproliferative Activity of a Callus Culture of Prionosciadium dissectum (Apiaceae)
Abstract
1. Introduction
2. Results and Discussion
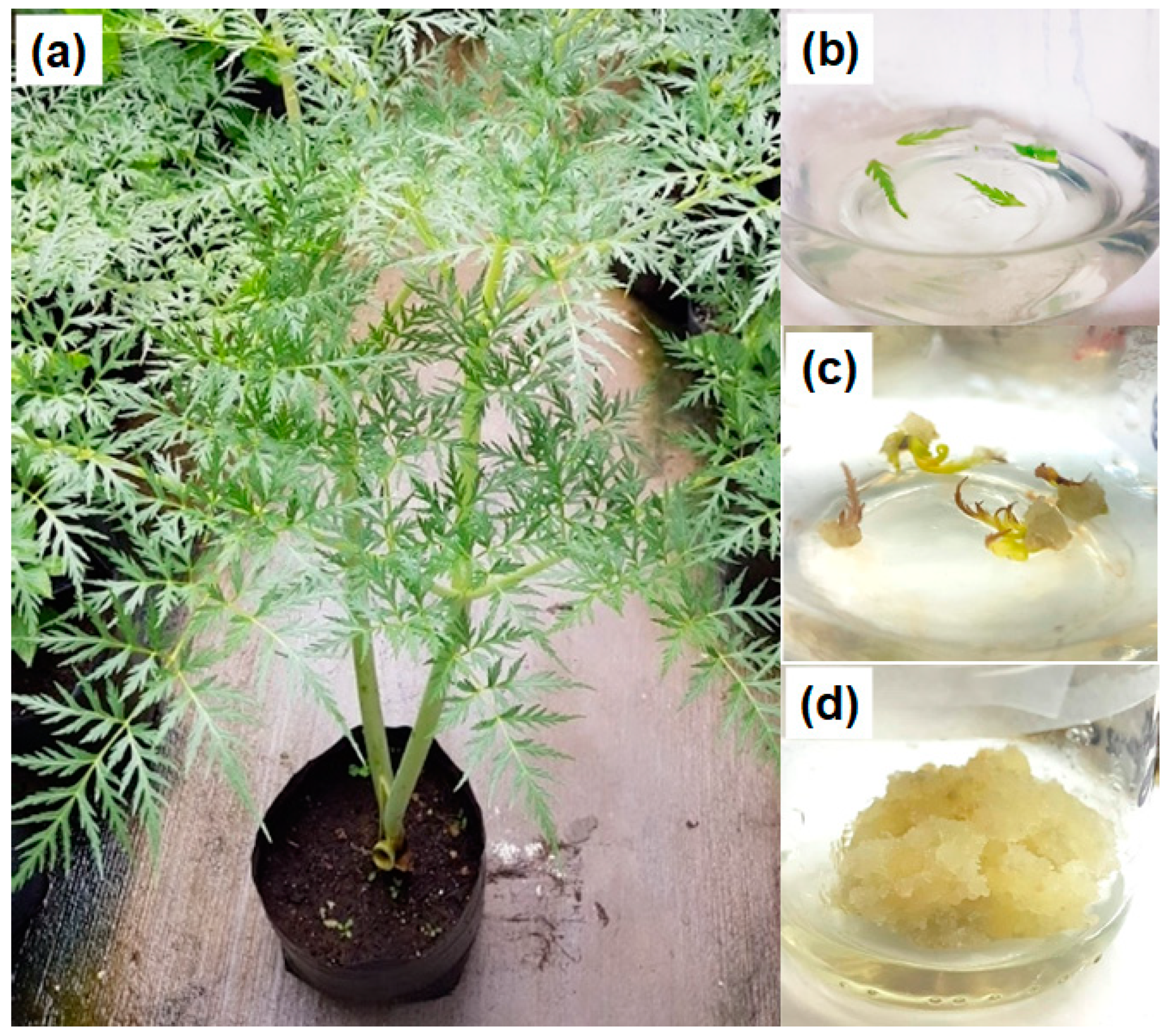
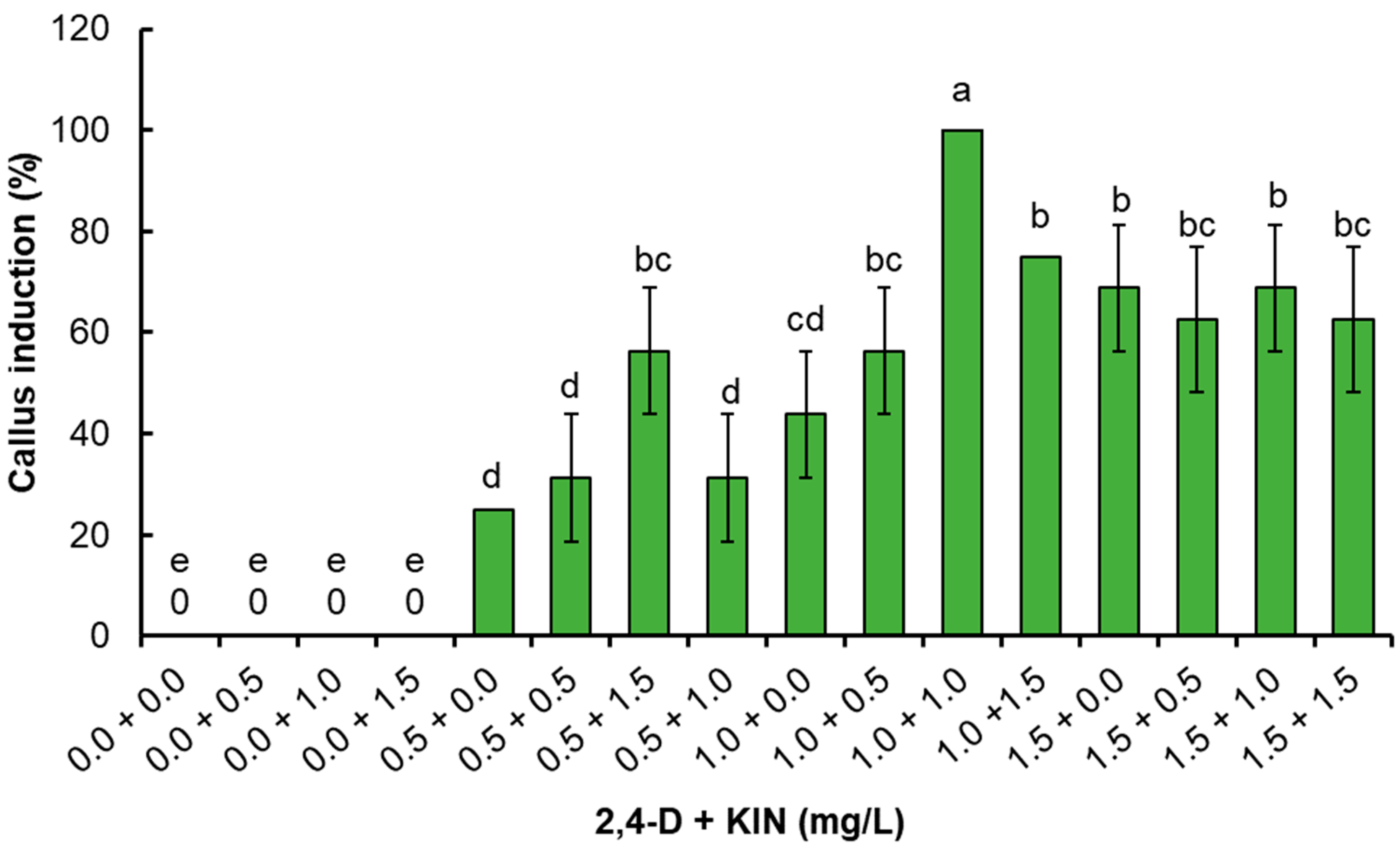
2.1. Callus Culture
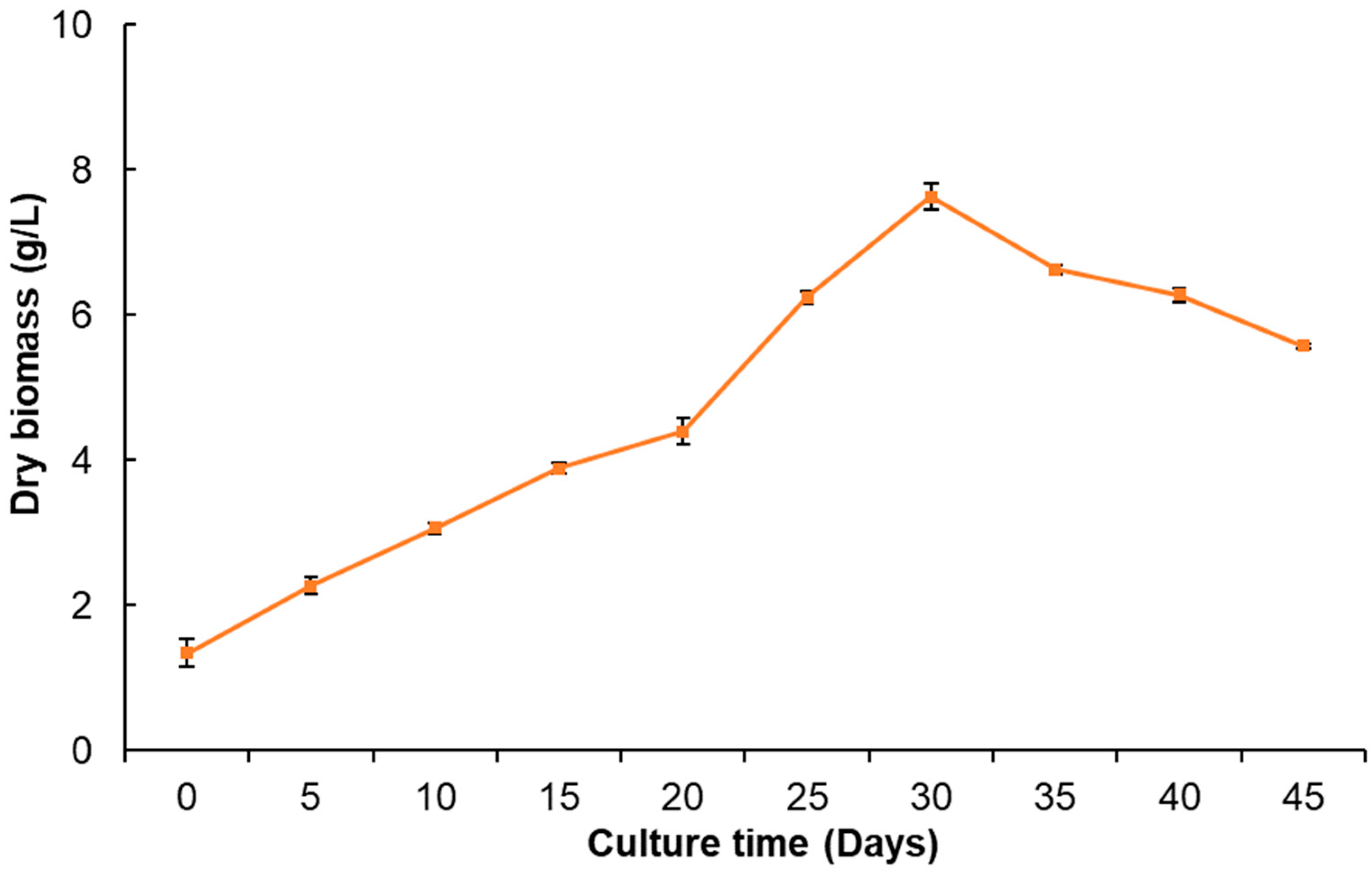
2.2. Callus Culture Growth
2.3. Yield of Callus Extracts
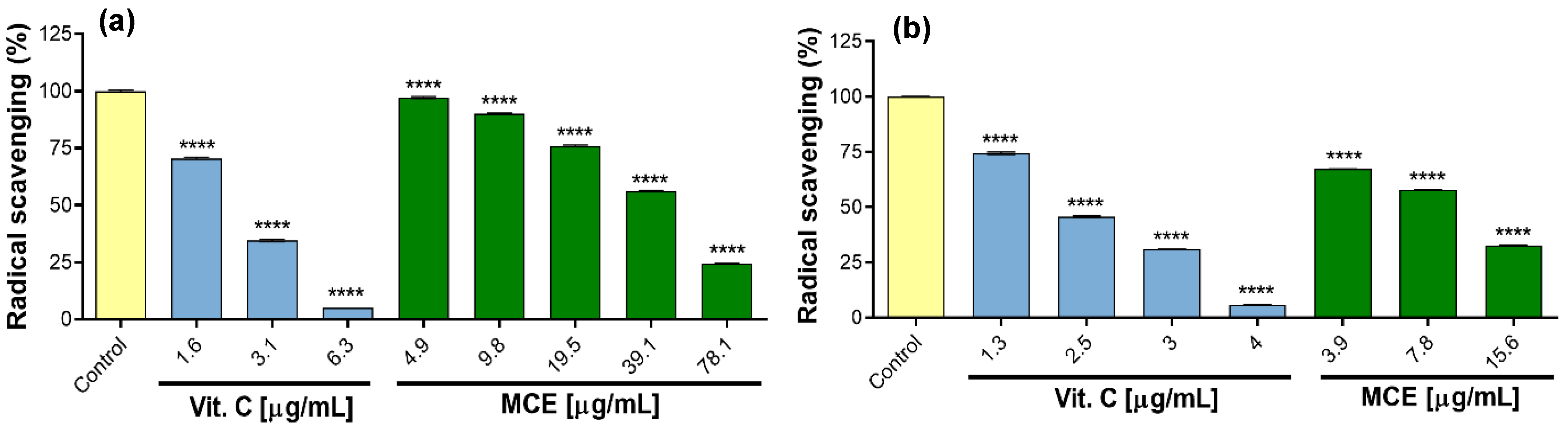
2.4. Total Phenolic Content and Antioxidant Activity
2.5. Anti-Inflammatory Activity in RAW 264.7 Cells
2.5.1. Effect of Extracts on Cell Viability
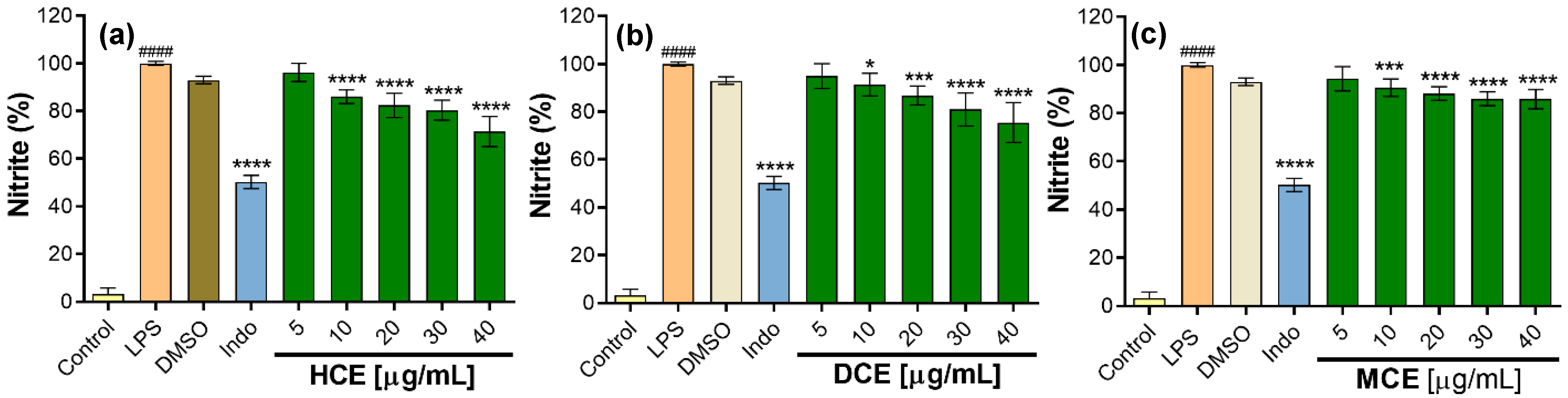
2.5.2. Effect of Extracts on the Inhibition of NO Production
2.6. Effect of Extracts on Antiproliferative Cancer Cells
3. Materials and Methods
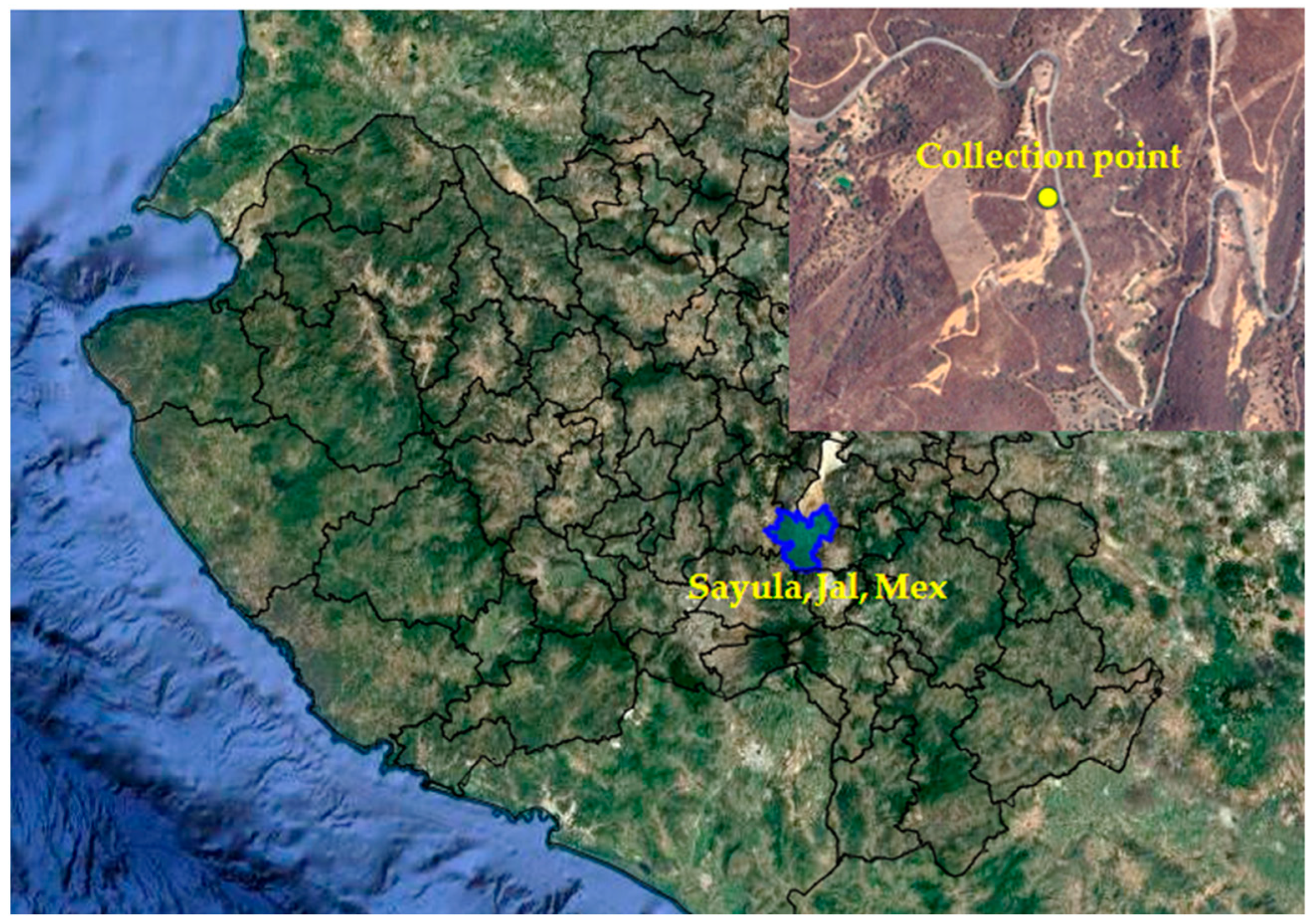
3.1. Plant Material
3.2. Callus Induction
3.3. Callus Culture Establishment and Growth Kinetics
3.4. Callus Biomass Preparation and Extraction Process
3.5. Determination of Total Phenolic Content
3.6. In Vitro Assessment of Antioxidant Activity
3.7. In Vitro Anti-Inflammatory Activity
3.8. Antiproliferative Activity on Cancer Cells
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, Y.; Mujib, A.; Mamgain, J.; Kumar, S.; Dewir, Y.H.; Magyar-Tábori, K. Synthesis and accumulation of phytocompounds in field-tissue-culture grown (stress) root tissues and simultaneous defense response activity in Glycyrrhiza glabra L. Sustainability 2024, 16, 1613. [Google Scholar] [CrossRef]
- Maqbool, M.; Ishtiaq, M.; Mazhar, M.W.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Enhancing bioactive metabolite production in Aerva sanguinolenta callus cultures through silver nanoparticle and salicylic acid elicitation. Sustainability 2023, 15, 10395. [Google Scholar] [CrossRef]
- Al-Qudah, T.S.; Shibli, R.A.; Zatimeh, A.; Tahtamouni, R.W.; Al-Zyoud, F. A Sustainable approach to in vitro propagation and conservation of Salvia dominica L.: A wild medicinal plant from Jordan. Sustainability 2023, 15, 14218. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Tauro, S.; Dhokchawle, B.; Mohite, P.; Nahar, D.; Nadar, S.; Coutinho, E. Natural anticancer agents: Their therapeutic potential, challenges and promising outcomes. Curr. Med. Chem. 2024, 31, 848–870. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.d.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Moazzami Farida, S.H.; Ajani, Y.; Sadr, M.; Mozaffarian, V. Ethnobotanical applications and their correspondence with phylogeny in Apiaceae-Apioideae. Res. J. Pharmacogn. 2018, 5, 79–97. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Redouan, F.Z.; Benítez, G.; Picone, R.M.; Crisafulli, A.; Yebouk, C.; Bouhbal, M.; Merzouki, A. Traditional medicinal knowledge of Apiaceae at Talassemtane National Park (Northern Morocco). S. Afr. J. Bot. 2020, 131, 118–130. [Google Scholar] [CrossRef]
- Fatemikia, S.; Abbasipour, H.; Saeedizadeh, A. Phytochemical and acaricidal study of the Galbanum, Ferula gumosa Boiss. (Apiaceae) essential oil against Tetranychus urticae Koch (Tetranychidae). J. Essent. Oil-Bear. Plants 2017, 20, 185–195. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Shin, K.H.; Kim, B.K.; Kang, S.S. Anti-tumor activities of decursinol angelate and decursin from Angelica gigas. Arch. Pharmacal Res. 2003, 26, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Sinan, K.I. Phytochemical characterization and bioactivities of five Apiaceae species: Natural sources for novel ingredients. Ind. Crop. Prod. 2019, 135, 107–121. [Google Scholar] [CrossRef]
- Shaikh, R.; Pund, M.; Dawane, A.; Iliyas, S. Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J. Tradit. Complement. Med. 2014, 4, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Özdemir, Ö.; Koca, M.; Demirci, B.; Aksakal, Ö.; Turkez, H.; Baser, K.H.C. Cytotoxic effect and molecular docking studies of essential oils of Cymbocarpum erythraeum (DC.) Boiss. (Apiaceae) as potential inhibitors of cholinesterase. J. Essent. Oil Res. 2020, 32, 436–448. [Google Scholar] [CrossRef]
- Gomaa, S.E.; Friedersdorf, M.; El Enshasy, H.A.; Abou-Donia, M.B. In vitro comparative study for anti-proliferative activity of some plant extracts, Fam. Apiaceae, on HeLa cell line. Indonesian J. Pharm. 2020, 31, 108. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, J.A.; Seo, C.S.; Ha, H.; Lee, H.; Son, J.K.; Shin, H.K. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264. 7 cells via upregulation of heme oxygenase-1. Food Chem. Toxicol. 2011, 49, 1047–1055. [Google Scholar] [CrossRef]
- Uto, T.; Tung, N.H.; Taniyama, R.; Miyanowaki, T.; Morinaga, O.; Shoyama, Y. Anti-inflammatory activity of constituents isolated from aerial part of Angelica acutiloba Kitagawa. Phytother. Res. 2015, 29, 1956–1963. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of total polyphenols, flavonoids and anti-inflammatory potential of three Apiaceae species grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Arana-Argáez, V.; Alonso-Castro, A.J.; Yáñez-Barrientos, E.; Euan-Canto, A.; Torres-Romero, J.C.; Isiordia-Espinoza, M.A.; González-Ibarra, A.A. In vitro and in vivo anti-inflammatory effects of an ethanol extract from the aerial parts of Eryngium carlinae F. Delaroche (Apiaceae). J. Ethnopharmacol. 2020, 266, 113406. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Phytochemical profile, chemotaxonomic studies, and in vitro antioxidant activities of two endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chem. Biodiversity 2016, 13, 1290–1306. [Google Scholar] [CrossRef]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017, 109, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.G. Nutraceutical potential of Apiaceae. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 1311–1341. [Google Scholar] [CrossRef]
- Hamidian, M.; Salehi, A.; Naghiha, R.; Dehnavi, M.M.; Castangia, I.; Mirfathi, M.N. The comparative perspective of phytochemistry and biological properties of the Apiaceae family plants. Sci. Rep. 2023, 13, 12390. [Google Scholar] [CrossRef]
- Guzmán, R.C.; Nuñez-López, N.M.; Canales-Piña, S.; Morales-Arias, J.G. Prionosciadium tamayoi (Apiaceae), una especie nueva del occidente de México. Rev. Mex. Biodivers. 2020, 91, 913338. [Google Scholar] [CrossRef]
- Valencia-Islas, N.; Abbas, H.; Bye, R.; Toscano, R.; Mata, R. Phytotoxic compounds from Prionosciadium watsoni. J. Nat. Prod. 2002, 65, 828–834. [Google Scholar] [CrossRef]
- Torres-Valencia, J.M.; Chávez-Ríos, O.E.; Cerda-García-Rojas, C.M.; Burgueño-Tapia, E.; Joseph-Nathan, P. Dihydrofurochromones from Prionosciadium thapsoides. J. Nat. Prod. 2008, 71, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Siatka, T. Production of anthocyanins in callus cultures of Angelica archangelica. Nat. Prod. Commun. 2018, 13, 1645–1648. [Google Scholar] [CrossRef]
- Huang, B.; Han, L.; Li, S.; Yan, C. Optimization of induction, subculture conditions, and growth kinetics of Angelica sinensis (Oliv.) Diels callus. Pharmacogn. Mag. 2015, 11, 574–578. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Kang, D.I.; Thi, L.T.; Jeong, B.R. Effect of explant type and plant growth regulators on callus induction, growth and secondary metabolites production in Cnidium officinale Makino. Mol. Biol. Rep. 2018, 45, 1919–1927. [Google Scholar] [CrossRef]
- Suran, D.; Bolor, T.; Bayarmaa, G.A. In vitro seed germination and callus induction of Ferula ferulaeoides (Steud.) Korov. (Apiaceae). Mong. J. Biol. Sci. 2016, 14, 53–58. [Google Scholar] [CrossRef]
- Matos, E.; Marcano, M.; Azócar, C.J.; Mora, A. Establecimiento y multiplicación in vitro de cinco cultivares de apio (Arracacia xanthorrhiza Bancroft) colectados en Venezuela. Bioagro 2015, 27, 121–130. [Google Scholar]
- Golkar, P.; Akbari, R.; Bazarganipour, M.; Javed, R. Biochemical and phytochemical responses of Ammi visnaga L. (Apiaceae) callus culture elicited by SiO2 and graphene Oxide-SiO2 nanoparticles. Plant Physiol. Biochem. 2023, 200, 107741. [Google Scholar] [CrossRef] [PubMed]
- Batool, H.; Fatima, I.; Safdar, N.; Yasmin, A. Elicitors mediated bio-preservative potential targeting seedless fruits using Capsicum annuum L. (Solanaceae) and Foeniculum vulgare Mill. (Apiaceae) calli extracts. Acta Physiol. Plant. 2023, 45, 19. [Google Scholar] [CrossRef]
- Farvardin, A.; Ebrahimi, A.; Hosseinpour, B.; Khosrowshahli, M. Effects of growth regulators on callus induction and secondary metabolite production in Cuminum cyminum. Nat. Prod. Res. 2017, 31, 1963–1970. [Google Scholar] [CrossRef]
- Paque, S.; Weijers, D. Q&A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14, 67. [Google Scholar] [CrossRef]
- da Luz Costa, J.; da Silva, A.L.L.; Bier, M.C.J.; Brondani, G.E.; Gollo, A.L.; Letti, L.A.J.; Erasmo, E.A.L.; Soccol, C.R. Callus growth kinetics of physic nut (Jatropha curcas L.) and content of fatty acids from crude oil obtained in vitro. Appl. Biochem. Biotechnol. 2015, 176, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Hundare, A.; Dhaytadak, B.; Kudale, S.; Joshi, N. Growth kinetics and diosgenin estimation from callus cultures of Costus speciosus (Koen. ex. Retz.). Nat. Prod. Res. 2018, 32, 1809–1816. [Google Scholar] [CrossRef]
- Bernabé-Antonio, A.; Álvarez, L.; Salcedo-Pérez, E.; Toral, F.L.D.; Anzaldo-Hernández, J.; Cruz-Sosa, F. Fatty acid profile of intact plants of two different sites and callus cultures derived from seed and leaf explants of Calophyllum brasiliense Cambess: A new resource of non-edible oil. Ind. Crop. Prod. 2015, 77, 1014–1019. [Google Scholar] [CrossRef]
- Álvarez de León, R.A.; García Higueros, A.L.; Olivia Rentana, N.V.; Rojas Sazo, A.M. Determinación de Actividad Inhibitoria In Vitro de Extractos Diclorometánicos y Metanólicos y Aceites Esenciales de Doce Especies Condimentarías y Medicinales Guatemaltecas Contra Listeria Monocytogenes. Bachelor’s Thesis, Universidad de San Carlos de Guatemala, Facultad de Ciencias Químicas y Farmacéuticas, Guatemala, Guatemala, 2010. [Google Scholar]
- Palomo, I.; Gutiérrez, C.M.; Astudillo, L.; Rivera, C.; Torres, C.; Guzmán, L.; Moore-Carrasco, R.; Carrasco, G.; Alarcón, L.M. Efecto antioxidante de frutas y hortalizas de la zona central de chile. Rev. Chil. Nutr. 2009, 36, 152–158. [Google Scholar] [CrossRef]
- Wu, X.W.; Wei, W.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Yang, Y.F.; Zhong, G.Y.; Liu, H.N.; Yang, S.L. Anti-Inflammatory phenolic acid esters from the roots and rhizomes of Notopterygium incisium and their permeability in the human caco-2 monolayer cell model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-EL-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of drugs and chemicals toxicity: Biomedical value of antioxidants. Oxid. Med. Cell Long. 2018, 2018, 6276438. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungǎu, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, metabolic, and degenerative disorders: Biomedical value of antioxidants. Oxid. Med. Cell Long. 2018, 2018, 2098123. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Vasić, S.; Stanković, M.; Čomić, L.; Topuzović, M. In vitro biological activity of secondary metabolites from Seseli rigidum Waldst. et Kit. Apiaceae. Acta Biol. Hung. 2015, 66, 395–405. [Google Scholar] [CrossRef]
- Lefahal, M.; Zaabat, N.; Ayad, R.; Makhloufi, E.H.; Djarri, L.; Benahmed, M.; Laouer, H.; Nieto, G.; Akkal, S. In vitro assessment of total phenolic and flavonoid contents, antioxidant and photoprotective activities of crude methanolic extract of aerial parts of Capnophyllum peregrinum (L.) Lange (Apiaceae) growing in Algeria. Medicines 2018, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Mangoale, R.M.; Afolayan, A.J. Comparative phytochemical constituents and antioxidant activity of wild and cultivated Alepidea amatymbica Eckl & Zeyh. BioMed Res. Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Puente Vigo, S.L. Compuestos Bioactivos y Capacidad Antioxidante de Extractos de Hoja de Sacha Culantro (Erynguim foetidum L.) y de Aceite de Copaiba (Copaifera paupera) Procedentes de la Provincia de Coronel Portillo, Ucayali. Bachelor’s Thesis, Universidad Científica del Sur, Lima, Perú, 2019. [Google Scholar]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive compounds and antioxidant, antiperoxidative, and antihemolytic properties investigation of three Apiaceae species grown in the southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Boveiri Dehsheikh, A.; Mahmoodi Sourestani, M.; Kiss, T.; Hohmann, J.; Csupor, D. Ducrosia spp., rare plants with promising phytochemical and pharmacological characteristics: An updated review. Pharmaceuticals 2020, 13, 175. [Google Scholar] [CrossRef]
- Ashour, M.L.; Wink, M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef]
- Kang, H.; Bang, T.S.; Lee, J.W.; Lew, J.H.; Eom, S.H.; Lee, K.; Choi, H.Y. Protective effect of the methanol extract from Cryptotaenia japonica Hassk. against lipopolysaccharide-induced inflammation in vitro and in vivo. BMC Complement. Altern. Med. 2012, 12, 199. [Google Scholar] [CrossRef]
- Park, G.; Kim, E.; Son, Y.J.; Yoon, D.H.; Sung, G.H.; Aravinthan, A.; Park, Y.C.; Kim, J.H.; Cho, J.Y. Anti-inflammatory effect of torilidis fructus ethanol extract through inhibition of Src. Pharm. Biol. 2017, 55, 2074–2082. [Google Scholar] [CrossRef]
- Mekhora, C.; Muangnoi, C.; Chingsuwanrote, P.; Dawilai, S.; Svasti, S.; Chasri, K.; Tuntipopipat, S. Eryngium foetidum suppresses inflammatory mediators produced by macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Shin, Y.; Lee, K.H.; Kim, T.J. Anethum graveloens flower extracts inhibited a lipopolysaccharide-induced inflammatory response by blocking iNOS expression and NF-κB activity in macrophages. Biosci. Biotechnol. Biochem. 2012, 76, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Jeong, S.H.; Guo, H.; Park, J.B. Anti-inflammatory and cytotoxic effects of methanol, ethanol, and water extracts of Angelicae dahuricae Radix. J. Oral Sci. 2016, 58, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, J.D.; Yeo, J.H.; Son, H.J.; Park, S.B.; Park, G.H.; Eo, H.J.; Jeong, J.B. Heracleum moellendorffii roots inhibit the production of pro-inflammatory mediators through the inhibition of NF-κB and MAPK signaling, and activation of ROS/Nrf2/HO-1 signaling in LPS-stimulated RAW264.7 cells. BMC Complement. Altern. Med. 2019, 19, 310. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chang, W.L.; Lee, S.C.; Liu, Y.G.; Lin, H.C.; Chen, C.J.; Yen, C.Y.; Yu, D.S.; Lin, S.Z.; Harn, H.J. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 2003, 73, 2383–2394. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, S.Z.; Chang, W.L.; Cheng, Y.L.; Harn, H.J. Requirement for ERK activation in acetone extract identified from Bupleurum scorzonerifolium induced A549 tumor cell apoptosis and keratin 8 phosphorylation. Life Sci. 2005, 76, 2409–2420. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. The proliferative inhibition and apoptotic mechanism of saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 1231–1242. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, H.Z.; Li, Y.C.; Wei, G.Q.; Geng, Y.; Ma, C.Y. Antitumor constituents from Anthriscus sylvestris (L.) Hoffm. Asian Pac. J. Cancer Prev. 2014, 15, 2803–2807. [Google Scholar] [CrossRef]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) C.A.Sm—Medicinal plants used in South Africa. BMC Complement. Altern. Med. 2018, 18, 315. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, T.; Yasuzawa, T.; Ueshima, S. Effects of Coriandrum sativum on migration and invasion abilities of cancer cells. J. Nut. Sci. Vitaminol. 2020, 66, 468–477. [Google Scholar] [CrossRef]
- INEGI. Instituto Nacional de Estadística y Geografía (INEGI). Sistema de Consulta de Mapas. 2025. Available online: https://www.inegi.org.mx/app/mapas/ (accessed on 24 April 2025).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Debnath, T.; Park, P.J.; Nath, N.C.D.; Samad, N.B.; Park, H.W.; Lim, B.O. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011, 128, 697–703. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using Griess reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Basu, A.; Saito, K.; Meyer, K.; Ray, R.B.; Friedman, S.L.; Chang, Y.H.; Ray, R. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis 2006, 11, 1391–1400. [Google Scholar] [CrossRef]

| Extract | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| IHH | Hep3B | HepG2 | CasKi | HeLa | A549 | |
| HCE | 15.3 ± 1.5 | 21.7 ± 2.9 | 16.0 ± 1.0 | 17.3 ± 2.5 | 17.3 ± 3.5 | 18.3 ± 1.5 |
| DCE | 15.3 ± 1.5 | 24.3 ± 4.0 | 14.0 ± 3.6 | 19.0 ± 3.6 | 18.7 ± 2.9 | 17.7 ± 3.1 |
| MCE | 16.7 ± 2.9 | 19.0 ± 1.7 | 15.0 ± 3.5 | 18.0 ± 1.0 | 17.0 ± 3.0 | 19.0 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabé-Antonio, A.; Sánchez-Carranza, J.N.; Silva-Guzmán, J.A.; Romero-Estrada, A.; Pérez-Rodríguez, S.G.; Cruz-Sosa, F.; Sánchez-Ramos, M.; Nieto-Trujillo, A. Antioxidant, Anti-Inflammatory, and Antiproliferative Activity of a Callus Culture of Prionosciadium dissectum (Apiaceae). Plants 2025, 14, 1394. https://doi.org/10.3390/plants14091394
Bernabé-Antonio A, Sánchez-Carranza JN, Silva-Guzmán JA, Romero-Estrada A, Pérez-Rodríguez SG, Cruz-Sosa F, Sánchez-Ramos M, Nieto-Trujillo A. Antioxidant, Anti-Inflammatory, and Antiproliferative Activity of a Callus Culture of Prionosciadium dissectum (Apiaceae). Plants. 2025; 14(9):1394. https://doi.org/10.3390/plants14091394
Chicago/Turabian StyleBernabé-Antonio, Antonio, Jessica Nayelli Sánchez-Carranza, José Antonio Silva-Guzmán, Antonio Romero-Estrada, Samantha Guadalupe Pérez-Rodríguez, Francisco Cruz-Sosa, Mariana Sánchez-Ramos, and Aurelio Nieto-Trujillo. 2025. "Antioxidant, Anti-Inflammatory, and Antiproliferative Activity of a Callus Culture of Prionosciadium dissectum (Apiaceae)" Plants 14, no. 9: 1394. https://doi.org/10.3390/plants14091394
APA StyleBernabé-Antonio, A., Sánchez-Carranza, J. N., Silva-Guzmán, J. A., Romero-Estrada, A., Pérez-Rodríguez, S. G., Cruz-Sosa, F., Sánchez-Ramos, M., & Nieto-Trujillo, A. (2025). Antioxidant, Anti-Inflammatory, and Antiproliferative Activity of a Callus Culture of Prionosciadium dissectum (Apiaceae). Plants, 14(9), 1394. https://doi.org/10.3390/plants14091394







