Cell Wall Invertase 4 Governs Sucrose–Hexose Homeostasis in the Apoplast to Regulate Wood Development in Poplar
Abstract
1. Introduction
2. Results
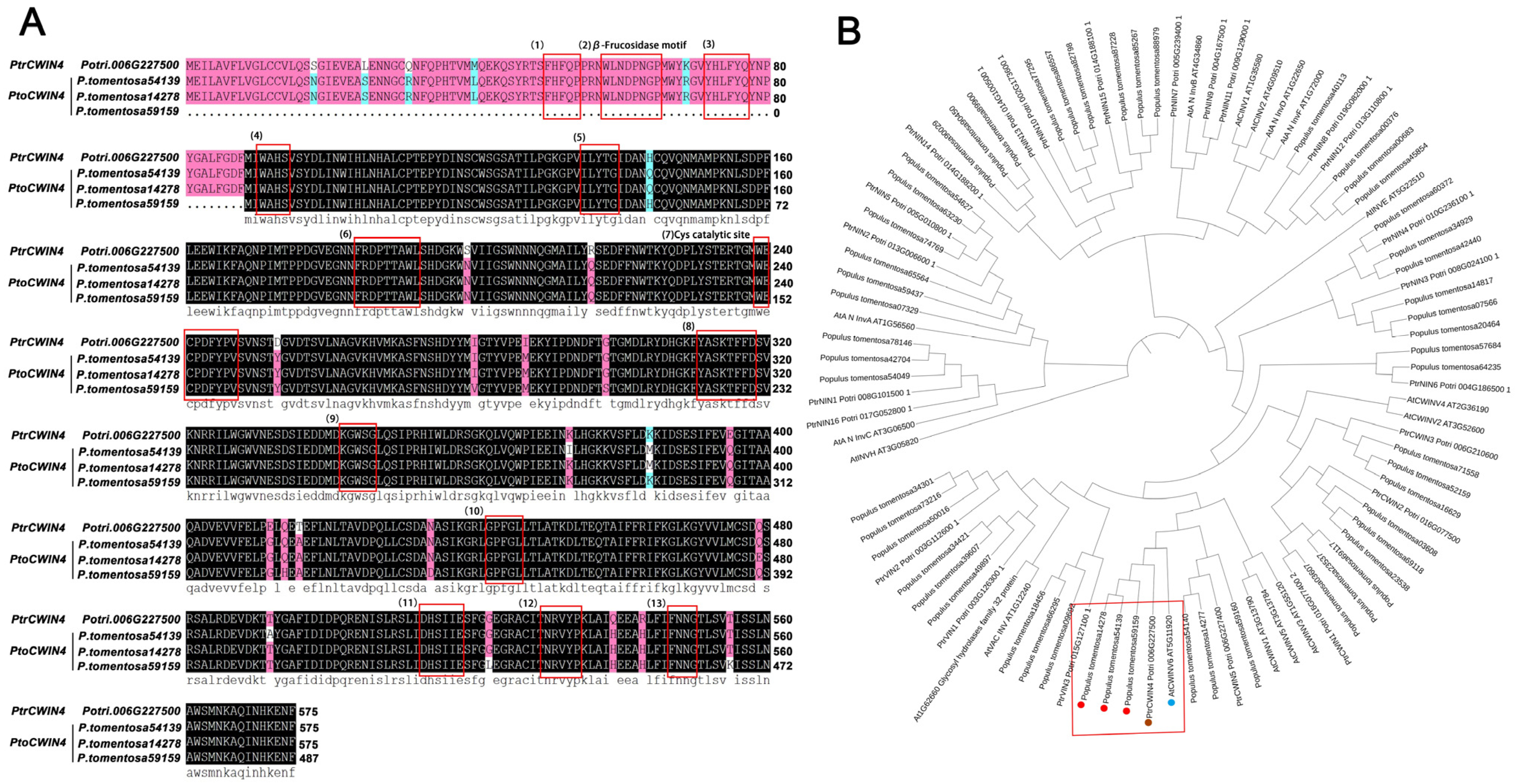
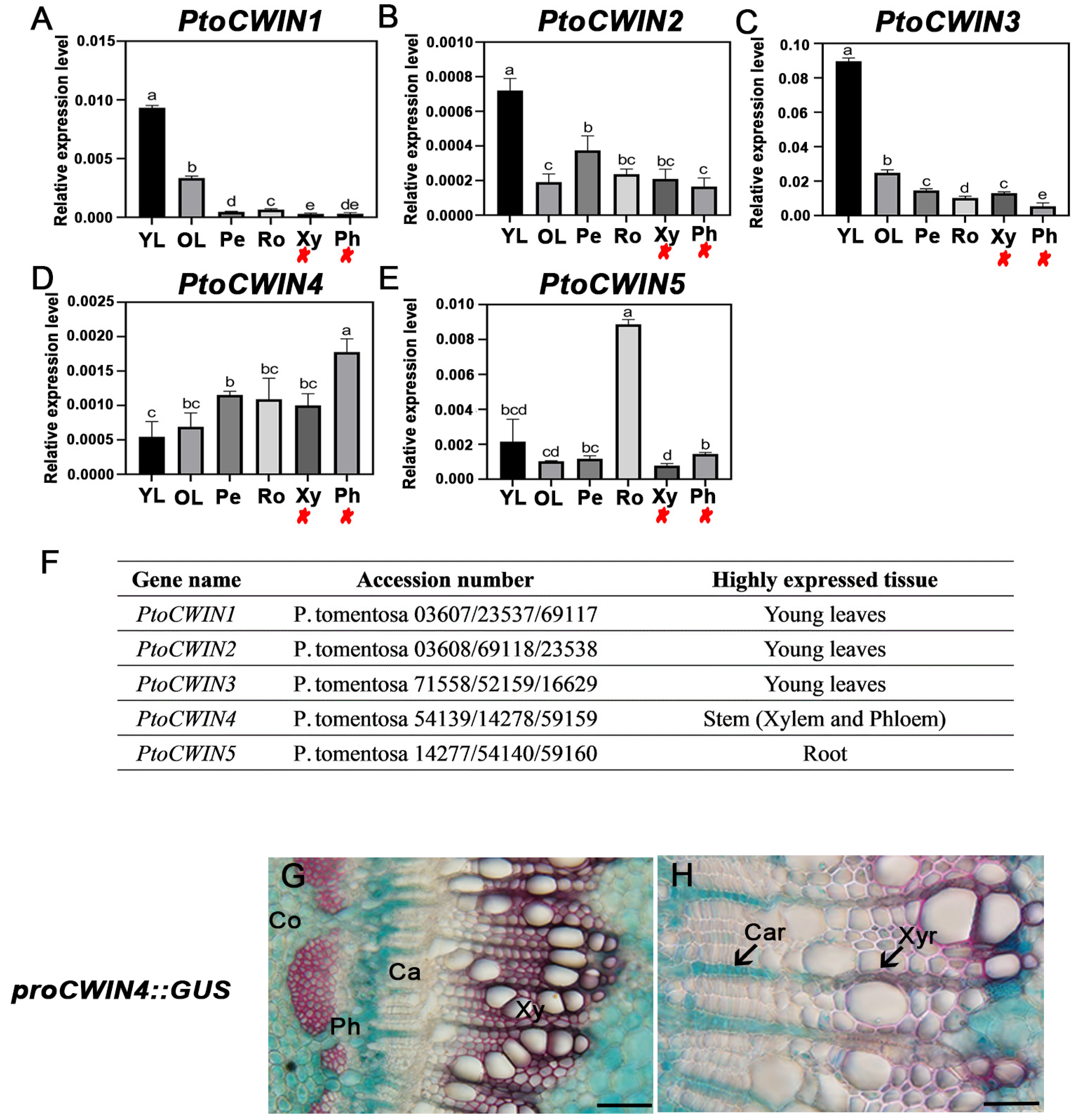
2.1. Characterization of PtoCWIN4 from P. tomentosa
2.2. Construction and Identification of Transgenic Poplars
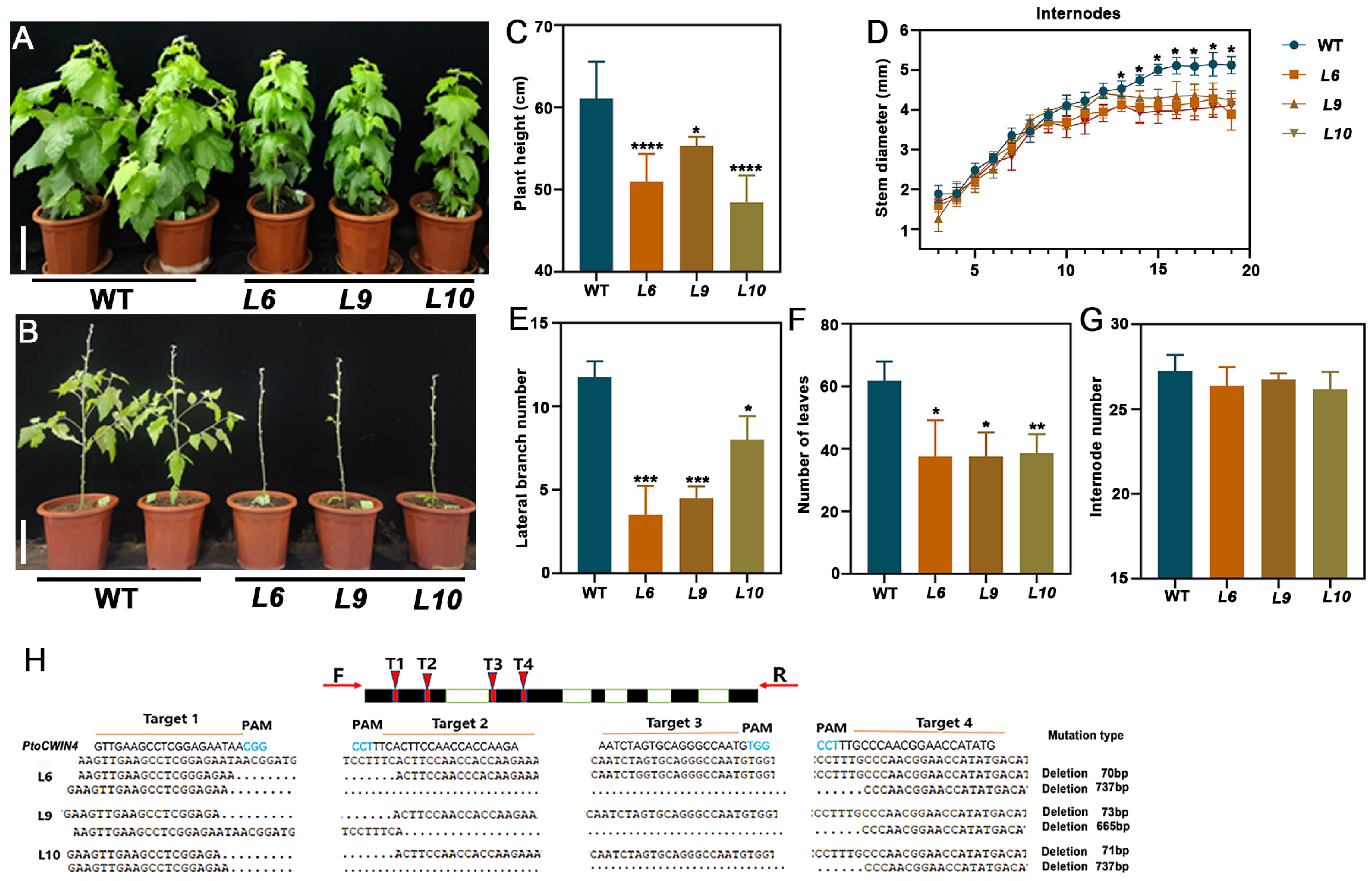
2.3. Knockout of PtoCWIN4 Alters the Phenotype of Poplar
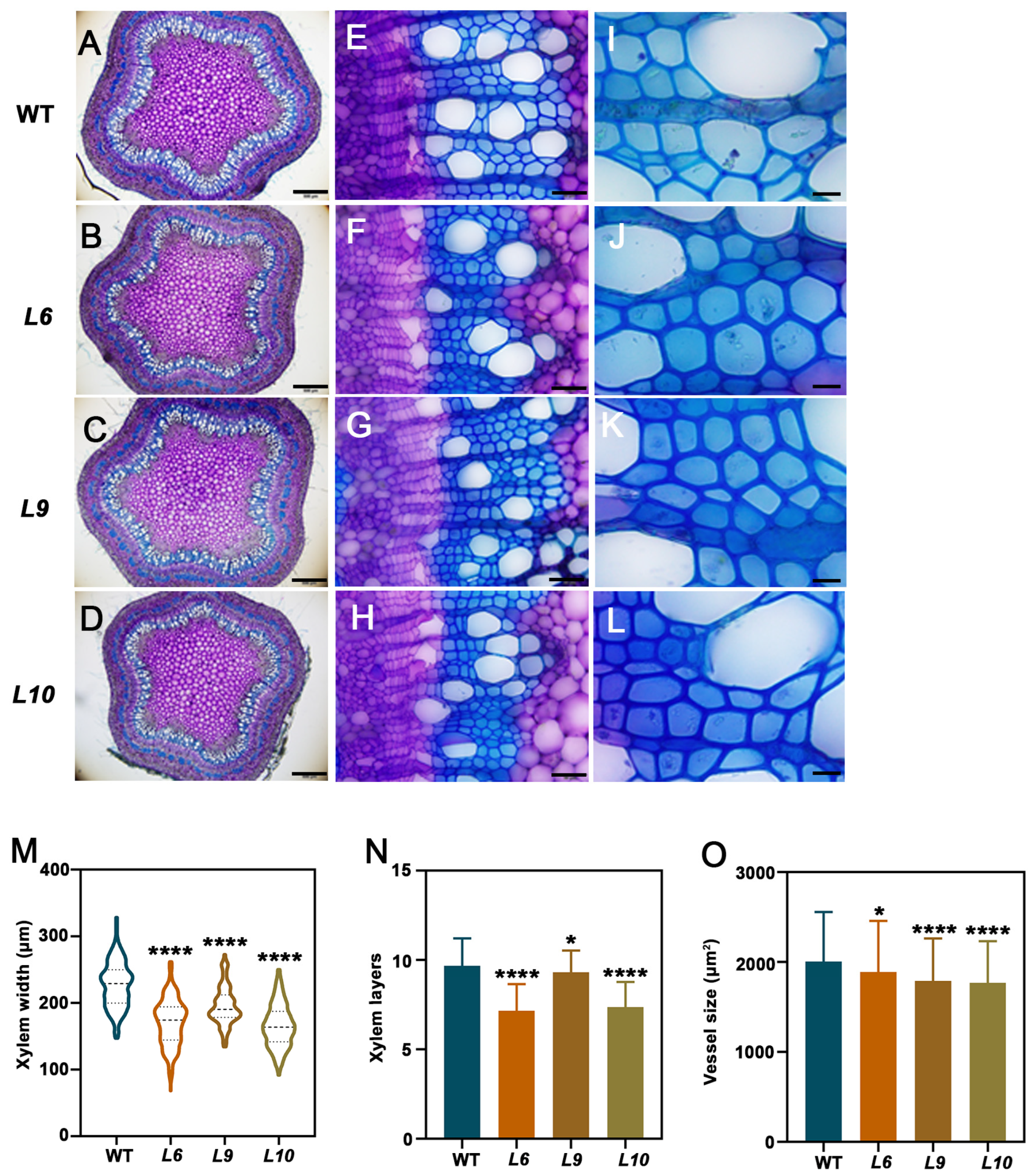
2.4. PtoCWIN4 Plays a Functional Role in Regulating Xylem Development in Poplar
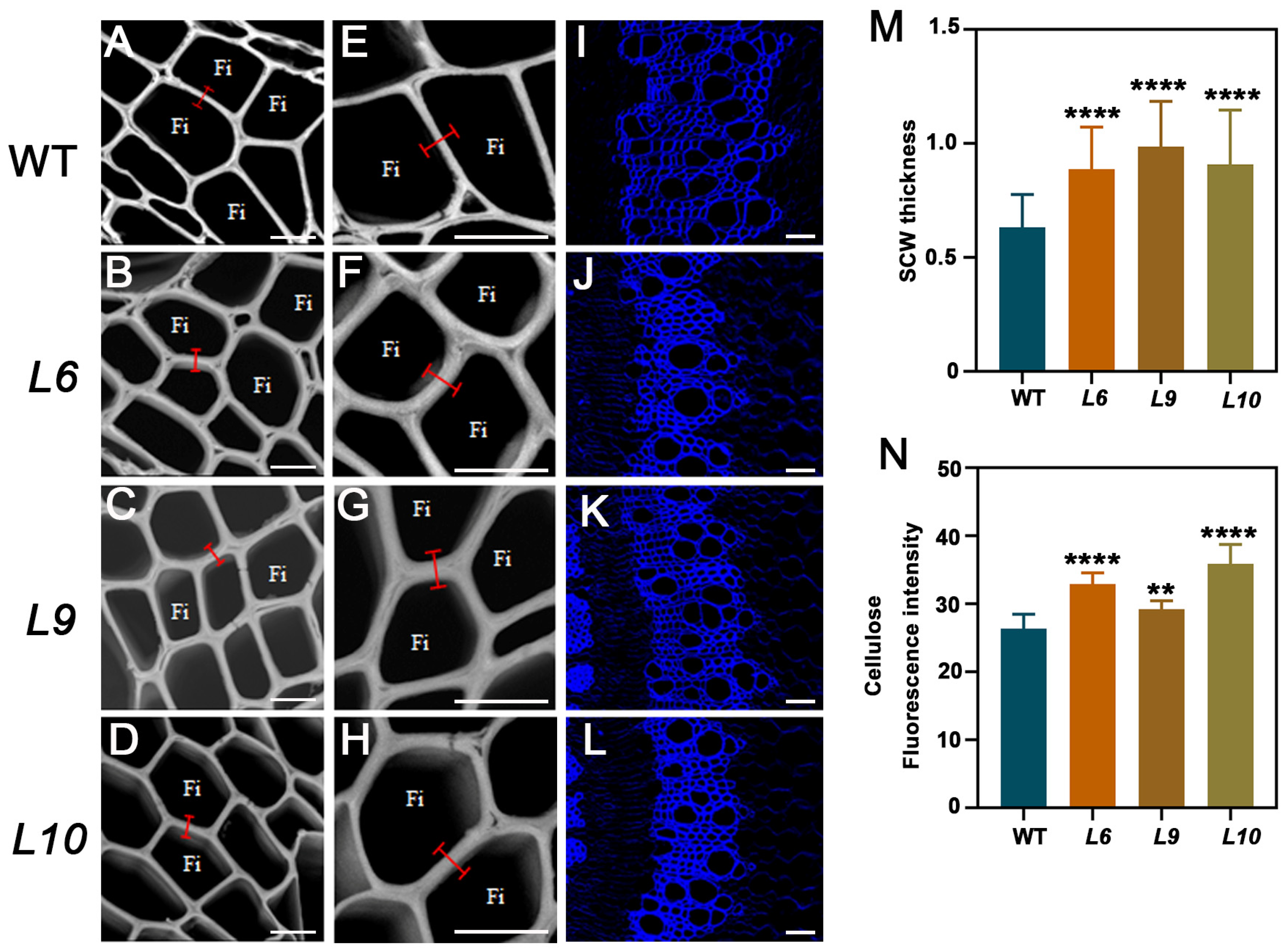
2.5. PtoCWIN4 Knockout Alters the Composition of Secondary Cell Walls in Xylem
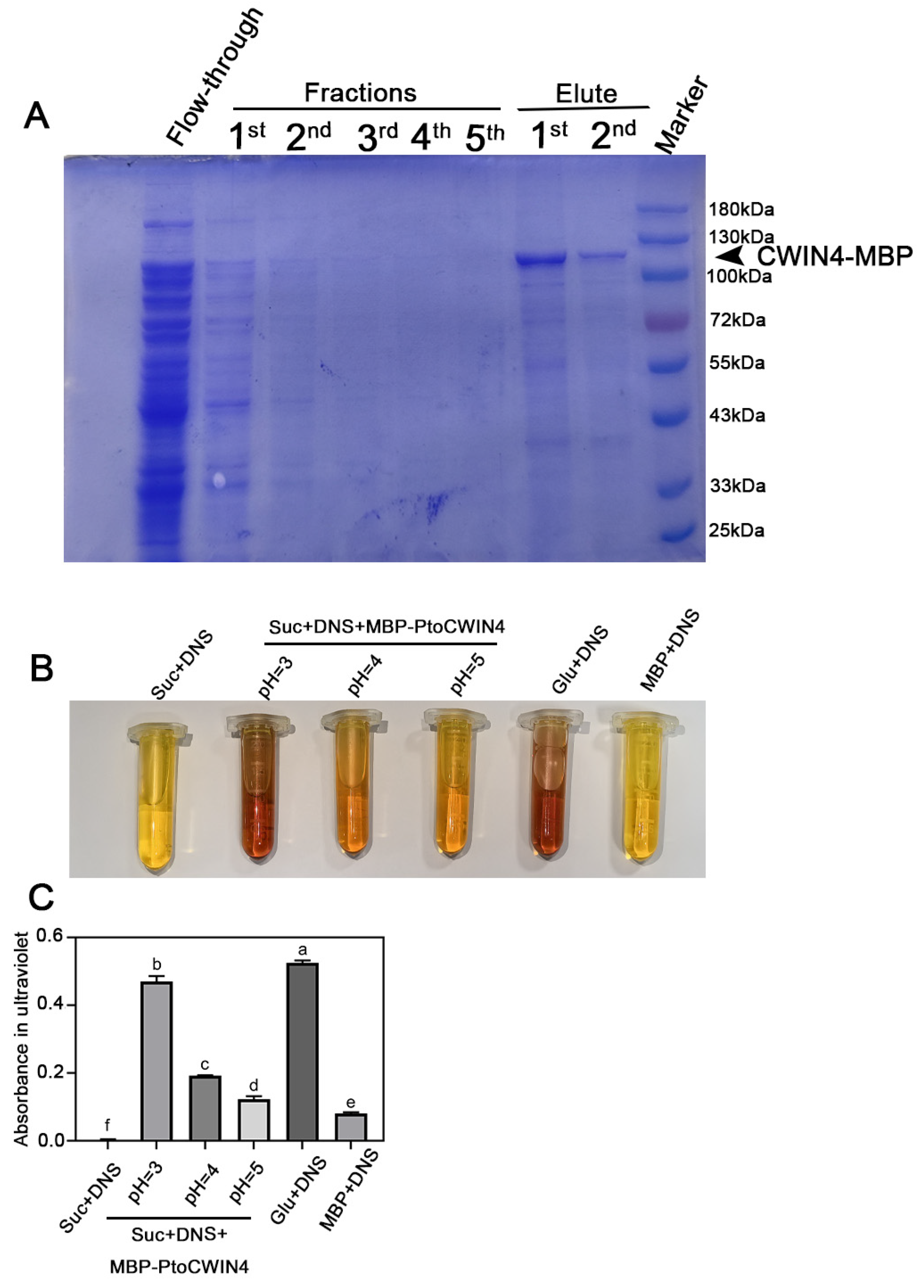
2.6. PtoCWIN4 Effectively Hydrolyzes Sucrose In Vitro
2.7. PtoCWIN4 Affects Soluble Sugar Accumulation in Poplar Stems
3. Discussion
3.1. Knockout of PtoCWIN4 Increases SCW Thickness
3.2. PtoCWIN4 Orchestrates Xylem Development and Branching Patterns in Poplar Through Sugar-Signaling-Mediated Metabolic Regulation
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Extraction and Real-Time Quantitative PCR
4.3. Vector Construction and Transformation of Poplar
4.4. Histochemical Staining and Wood Anatomy
4.5. GUS Analysis
4.6. Scanning Electron Microscopy
4.7. Calcofluor White Staining
4.8. Confocal Microscopy
4.9. SCW Composition Determination
4.10. Prokaryotic Expression and Purification of MBP-PtoCWIN4 Protein
4.11. Soluble Sugar Analysis
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruan, Y.L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Heyer, A.G.; Raap, M.; Schroeer, B.; Marty, B.; Willmitzer, L. Cell wall invertase expression at the apical meristem alters floral, architectural, and reproductive traits in Arabidopsis thaliana. Plant J. 2004, 39, 161–169. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, R.H.; Rier, J.P. Experimental Induction of Vascular Tissues in Callus of Angiosperms. Am. J. Bot. 1963, 50, 418–430. [Google Scholar] [CrossRef]
- Jeffs, R.A.; Northcote, D.H. The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. J. Cell. Sci. 1967, 2, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Roberts, L.W.; Wilson, P.M.W.; Gresshoff, P.M. Stimulatory and Inhibitory Effects of Sucrose Concentration on Xylogenesis in Lettuce Pith Explants; Possible Mediation by Ethylene Biosynthesis. Ann. Bot. 1994, 73, 65–73. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ratke, C.; Gorzsás, A.; Kumar, M.; Mellerowicz, E.J.; Niittylä, T. Aspen SUCROSE TRANSPORTER3 allocates carbon into wood fibers. Plant Physiol. 2013, 163, 1729–1740. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Zhang, J.; Song, C.; Li, Y.; Li, J.; Lu, M. Expression and localization of SWEETs in Populus and the effect of SWEET7 overexpression in secondary growth. Tree Physiol. 2021, 41, 882–899. [Google Scholar] [CrossRef]
- Payyavula, R.S.; Tay, K.H.; Tsai, C.J.; Harding, S.A. The sucrose transporter family in Populus: The importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J. 2011, 65, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Zhang, B.; Roach, M.; Rende, U.; Gorzs_as, A.; Kumar, M.; Burgert, I.; Niittylä, T.; Sundberg, B. Deficient sucrose synthase activity indeveloping wood does not specifically affect cellulose biosynthesis, but causesan overall decrease in cell wall polymers. New Phytol. 2014, 203, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Rende, U.; Wang, W.; Gandla, M.L.; Jönsson, L.J.; Niittylä, T. Cytosolic invertase contributes to the supply of substrate for cellulose biosynthesis in developing wood. New Phytol. 2017, 214, 796–807. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Marriott, P.E.; Gómez, L.D.; McQueen-Mason, S.J. Unlocking the potential of lignocellulosic biomass through plant science. New Phytol. 2016, 209, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon Supply and the Regulation of Cell Wall Synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar] [CrossRef]
- Roach, M.; Arrivault, S.; Mahboubi, A.; Krohn, N.; Sulpice, R.; Stitt, M.; Niittylä, T. Spatially resolved metabolic analysis reveals a central role for transcriptional control in carbon allocation to wood. J. Exp. Bot. 2017, 68, 3529–3539. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Llewellyn, D.J.; Furbank, R.T. Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 2003, 15, 952–964. [Google Scholar] [CrossRef]
- Barratt, D.H.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef]
- Amor, Y.; Haigler, C.H.; Johnson, S.; Wainscott, M.; Delmer, D.P. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. USA 1995, 92, 9353–9357. [Google Scholar] [CrossRef]
- Haigler, C.H.; Ivanova-Datcheva, M.; Hogan, P.S.; Salnikov, V.V.; Hwang, S.; Martin, K.; Delmer, D.P. Carbon partitioning to cellulose synthesis. Plant Mol. Biol. 2001, 47, 29–51. [Google Scholar] [CrossRef]
- Fujii, S.; Hayashi, T.; Mizuno, K. Sucrose synthase is an integral component of the cellulose synthesis machinery. Plant Cell Physiol. 2010, 51, 294–301. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Aubry, E.; Hoffmann, B.; Vilaine, F.; Gilard, F.; Klemens, P.A.W.; Guerard, F.; Gakiere, B.; Neuhaus, H.E.; Bellini, C.; Dinant, S.; et al. A vacuolar hexose transport is required for xylem development in the inflorescence stem. Plant Physiol. 2022, 188, 1229–1247. [Google Scholar] [CrossRef]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Bocock, P.N.; Morse, A.M.; Dervinis, C.; Davis, J.M. Evolution and diversity of invertase genes in Populus trichocarpa. Planta 2008, 227, 565–576. [Google Scholar] [CrossRef]
- Li, J.; Wu, L.; Foster, R.; Ruan, Y.L. Molecular regulation of sucrose catabolism and sugar transport for development, defence and phloem function. J. Integr. Plant Biol. 2017, 59, 322–335. [Google Scholar] [CrossRef]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Palmer, W.M.; Ru, L.; Jin, Y.; Patrick, J.W.; Ruan, Y.L. Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements. Mol. Plant 2015, 8, 315–328. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, Y.; Ma, L.; Yan, X.; Yuan, M.; Chen, J.; Cheng, Y.; Yang, X.; Qiao, Q.; Zhang, L.; et al. A cell wall invertase modulates resistance to fusarium crown rot and sharp eyespot in common wheat. J. Integr. Plant Biol. 2023, 65, 1814–1825. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Cell Wall Invertase Promotes Fruit Set under Heat Stress by Suppressing ROS-Independent Cell Death. Plant Physiol. 2016, 172, 163–180. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Chourey, P.S. Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theor. Appl. Genet. 1999, 98, 485–495. [Google Scholar] [CrossRef]
- Sundell, D.; Street, N.R.; Kumar, M.; Mellerowicz, E.J.; Kucukoglu, M.; Johnsson, C.; Kumar, V.; Mannapperuma, C.; Delhomme, N.; Nilsson, O.; et al. AspWood: High-Spatial-Resolution Transcriptome Profiles Reveal Uncharacterized Modularity of Wood Formation in Populus tremula. Plant Cell 2017, 29, 1585–1604. [Google Scholar] [CrossRef]
- Kacuráková, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef]
- Åkerholm, M.; Salmén, L. Interactions between wood polymers studied by dynamic FT-IR spectroscopy. Polymer 2001, 42, 963–969. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Mohebby, B. Application of ATR Infrared Spectroscopy in Wood Acetylation. J. Agric. Sci. Technol. 2008, 10, 253–259. [Google Scholar]
- Zhou, X.; Ren, S.; Lu, M.; Zhao, S.; Chen, Z.; Zhao, R.; Lv, J. Preliminary study of Cell Wall Structure and its Mechanical Properties of C3H and HCT RNAi Transgenic Poplar Sapling. Sci. Rep. 2018, 8, 10508. [Google Scholar] [CrossRef]
- Van Bel, A.J.E. Xylem-Phloem Exchange Via the Rays: The Undervalued Route of Transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Chaffey, N.; Barlow, P. The cytoskeleton facilitates a three-dimensional symplasmic continuum in the long-lived ray and axial parenchyma cells of angiosperm trees. Planta 2001, 213, 811–823. [Google Scholar] [CrossRef]
- Mahboubi, A.; Niittyla, T. Sucrose transport and carbon fluxes during wood formation. Physiol. Plant 2018, 164, 67–81. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Cheng, W.H.; Taliercio, E.W.; Chourey, P.S. Sugars modulate an unusual mode of control of the cell-wall invertase gene (Incw1) through its 3′ untranslated region in a cell suspension culture of maize. Proc. Natl. Acad. Sci. USA 1999, 96, 10512–10517. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; Osorio, S.; Wang, L.; Fernie, A.R.; Patrick, J.W.; Ruan, Y.L. Transcriptomic and metabolomics responses to elevated cell wall invertase activity during tomato fruit set. J. Exp. Bot. 2017, 68, 4263–4279. [Google Scholar] [CrossRef]
- Le, C.S.; Schmelz, E.A.; Chourey, P.S. Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol. 2010, 153, 306–318. [Google Scholar]
- Fan, D.; Liu, T.; Li, C.; Jiao, B.; Li, S.; Hou, Y.; Luo, K. Efficient CRISPR/Cas9-mediated Targeted Mutagenesis in Populus in the First Generation. Sci. Rep. 2015, 5, 12217. [Google Scholar] [CrossRef]
- Chen, S.; Songkumarn, P.; Liu, J.; Wang, G.L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009, 150, 1111–1121. [Google Scholar] [CrossRef]
- Jia, Z.; Sun, Y.; Yuan, L.; Tian, Q.; Luo, K. The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol. Lett. 2010, 32, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, S.; Kim, J.-S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
- Wang, X.; Yao, S.; Htet, W.; Yue, Y.; Zhang, Z.; Sun, K.; Chen, S.; Luo, K.; Fan, D. MicroRNA828 negatively regulates lignin biosynthesis in stem of Populus tomentosa through MYB targets. Tree Physiol. 2022, 42, 1646–1661. [Google Scholar] [CrossRef]
- Fan, C.; Yu, H.; Qin, S.; Li, Y.; Alam, A.; Xu, C.; Fan, D.; Zhang, Q.; Wang, Y.; Zhu, W.; et al. Brassinosteroid overproduction improves lignocellulose quantity and quality to maximize bioethanol yield under green-like biomass process in transgenic poplar. Biotechnol. Biofuels 2020, 13, 9. [Google Scholar] [CrossRef]
- Willige, B.C.; Kutzer, M.; Tebartz, F.; Bartels, D. Subcellular localization and enzymatic properties of differentially expressed transketolase genes isolated from the desiccation tolerant resurrection plant Craterostigma plantagineum. Planta 2009, 229, 659–666. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Ren, Q.; Wang, Q.; Wen, Y.; Wang, Y.; Liang, R.; Ran, D.; Jia, Y.; Zhuo, X.; Luo, J.; et al. Cell Wall Invertase 4 Governs Sucrose–Hexose Homeostasis in the Apoplast to Regulate Wood Development in Poplar. Plants 2025, 14, 1388. https://doi.org/10.3390/plants14091388
Lu J, Ren Q, Wang Q, Wen Y, Wang Y, Liang R, Ran D, Jia Y, Zhuo X, Luo J, et al. Cell Wall Invertase 4 Governs Sucrose–Hexose Homeostasis in the Apoplast to Regulate Wood Development in Poplar. Plants. 2025; 14(9):1388. https://doi.org/10.3390/plants14091388
Chicago/Turabian StyleLu, Jing, Qiao Ren, Qilin Wang, Yaqi Wen, Yanhong Wang, Ruiqi Liang, Dingxin Ran, Yifeng Jia, Xinyu Zhuo, Jiangtao Luo, and et al. 2025. "Cell Wall Invertase 4 Governs Sucrose–Hexose Homeostasis in the Apoplast to Regulate Wood Development in Poplar" Plants 14, no. 9: 1388. https://doi.org/10.3390/plants14091388
APA StyleLu, J., Ren, Q., Wang, Q., Wen, Y., Wang, Y., Liang, R., Ran, D., Jia, Y., Zhuo, X., Luo, J., Wang, X., & Luo, K. (2025). Cell Wall Invertase 4 Governs Sucrose–Hexose Homeostasis in the Apoplast to Regulate Wood Development in Poplar. Plants, 14(9), 1388. https://doi.org/10.3390/plants14091388





