Effects of Elicitation on Abeliophyllum distichum Leaf Callus and Changes in Verbascoside Content
Abstract
1. Introduction
2. Results
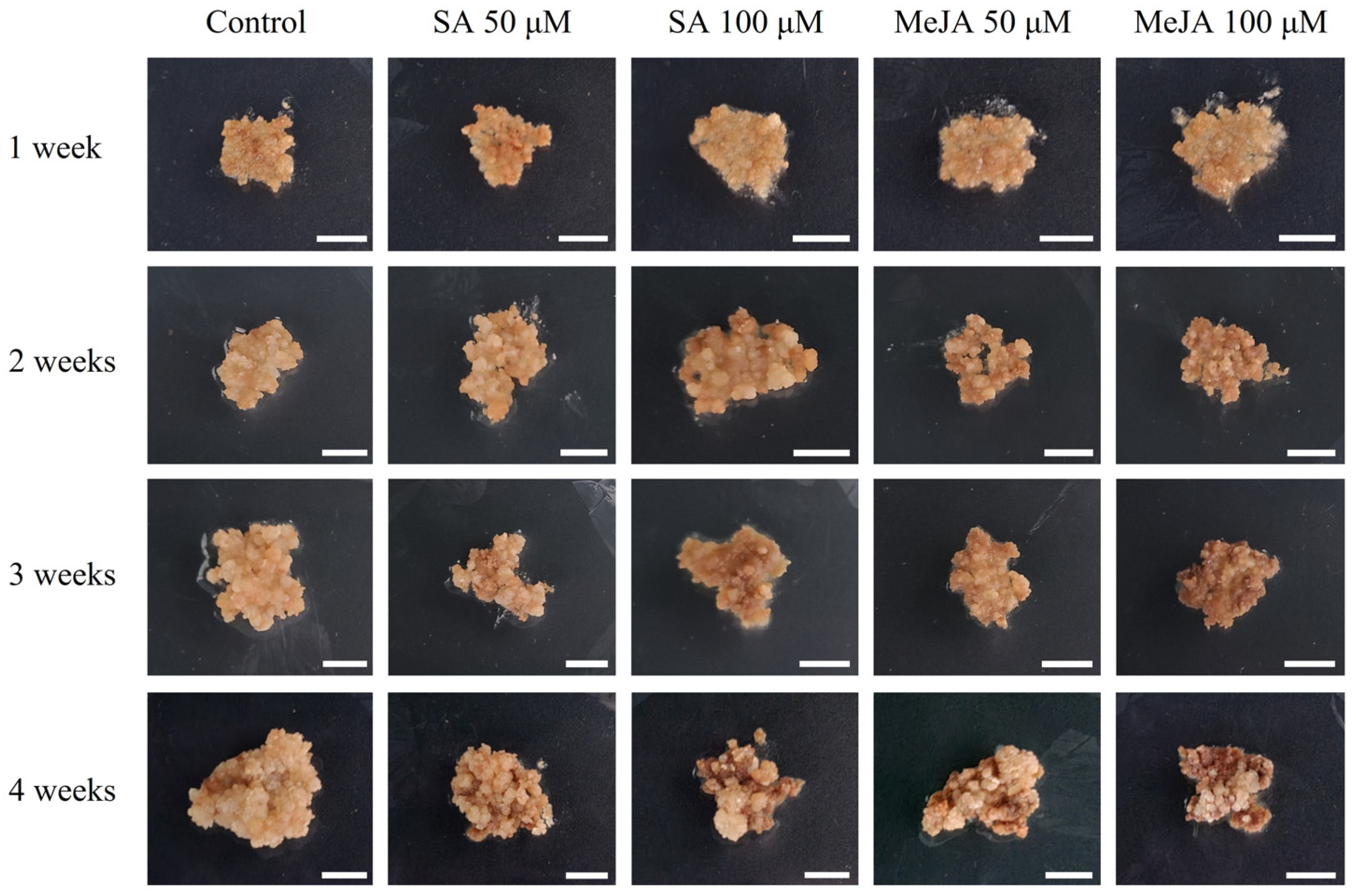
2.1. Salicylic Acid and Methyl Jasmonate Elicitation in Petri Dishes
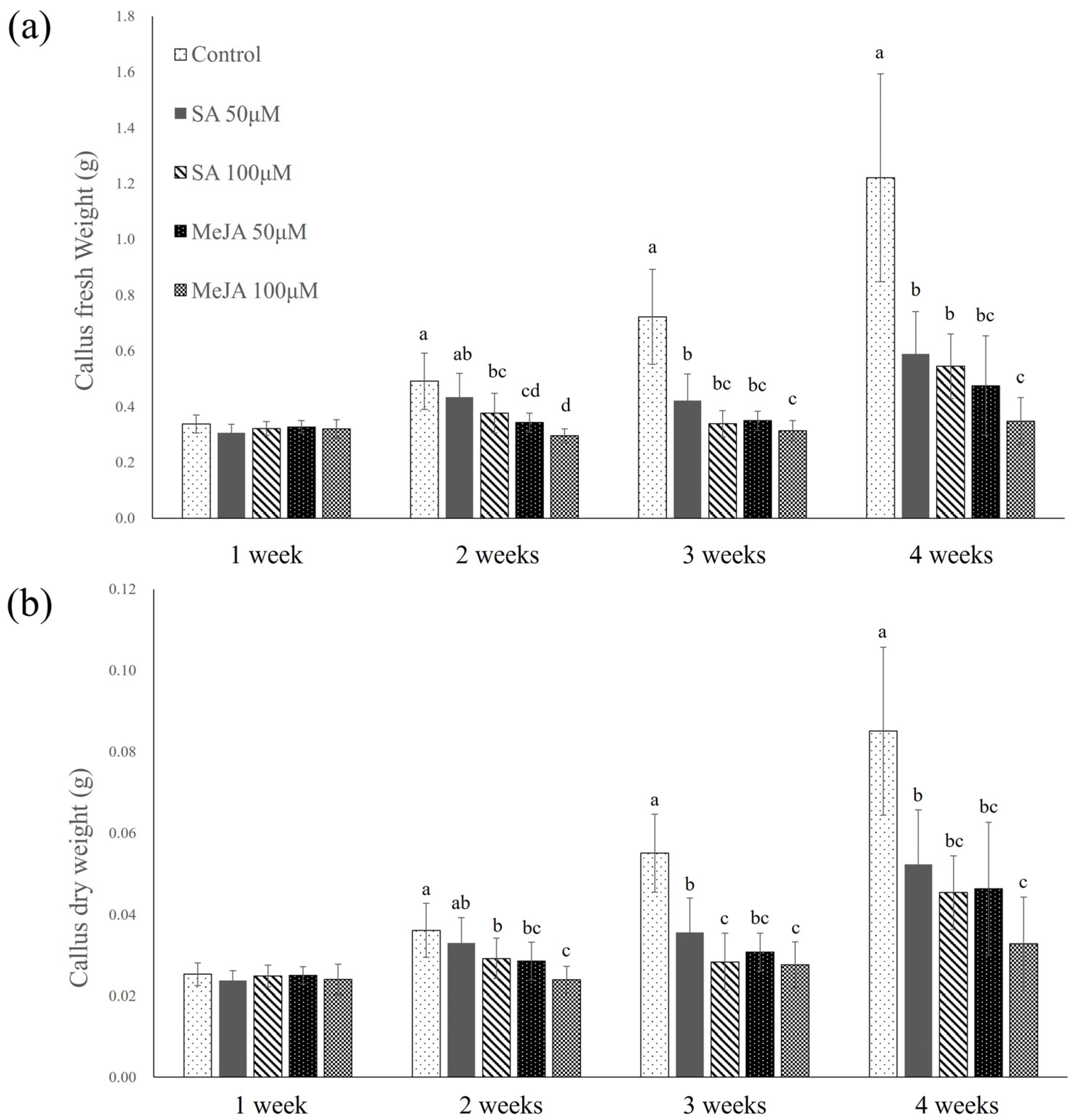
2.1.1. Fresh and Dry Weights of Callus
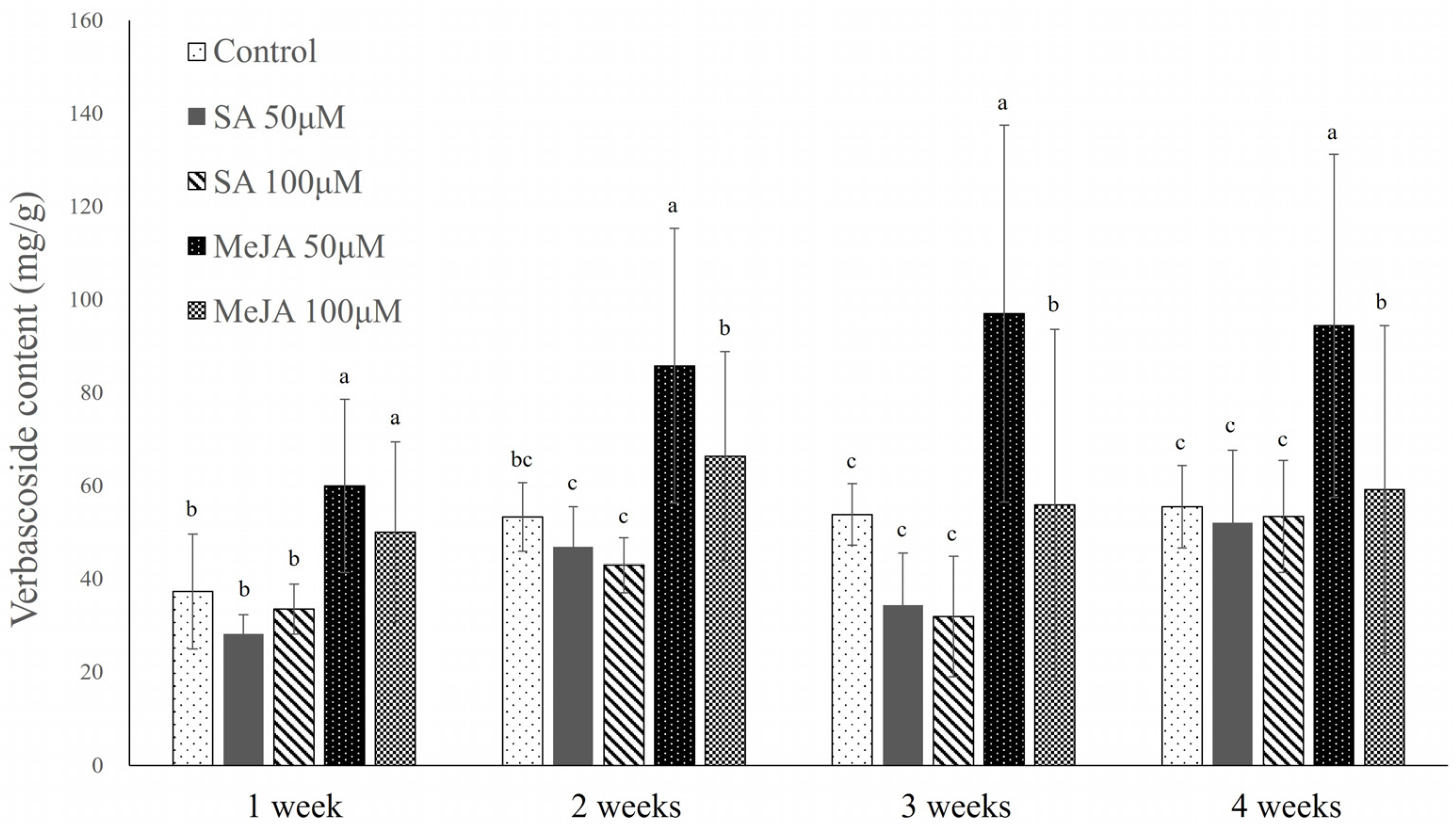
2.1.2. Verbascoside Content
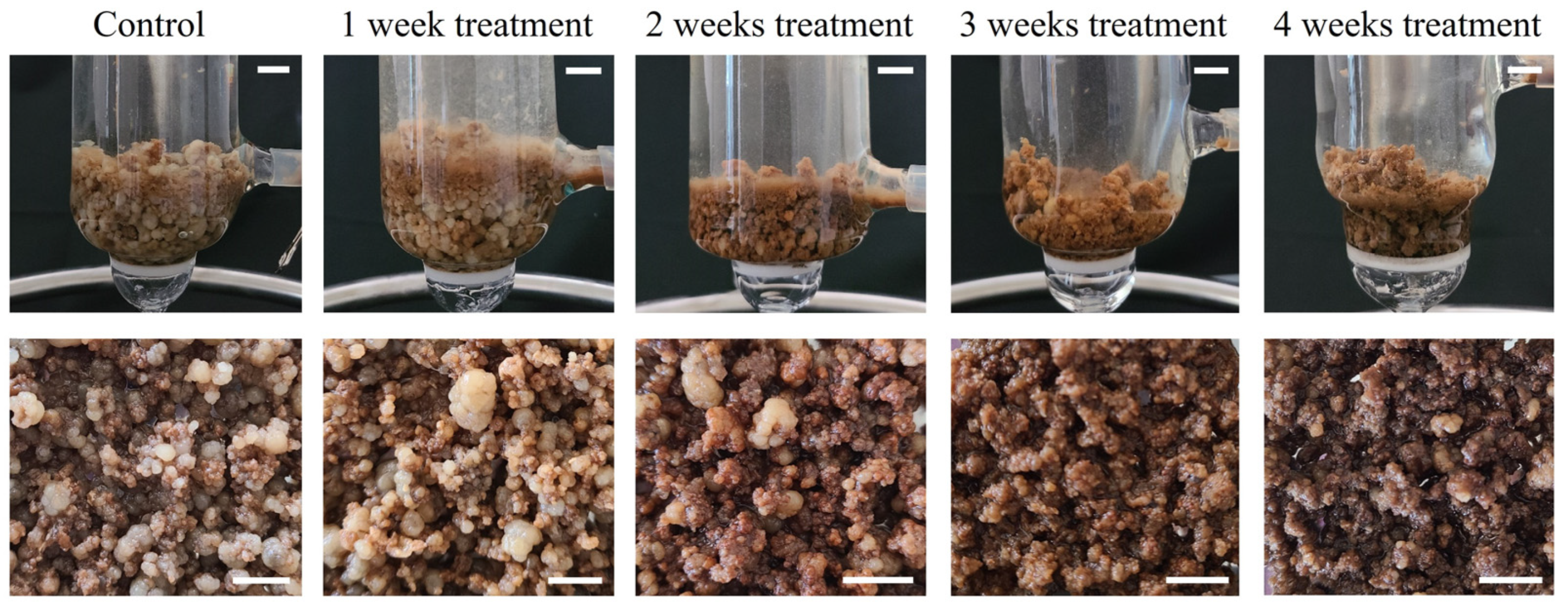
2.2. Methyl Jasmonate Elicitation in Bioreactors
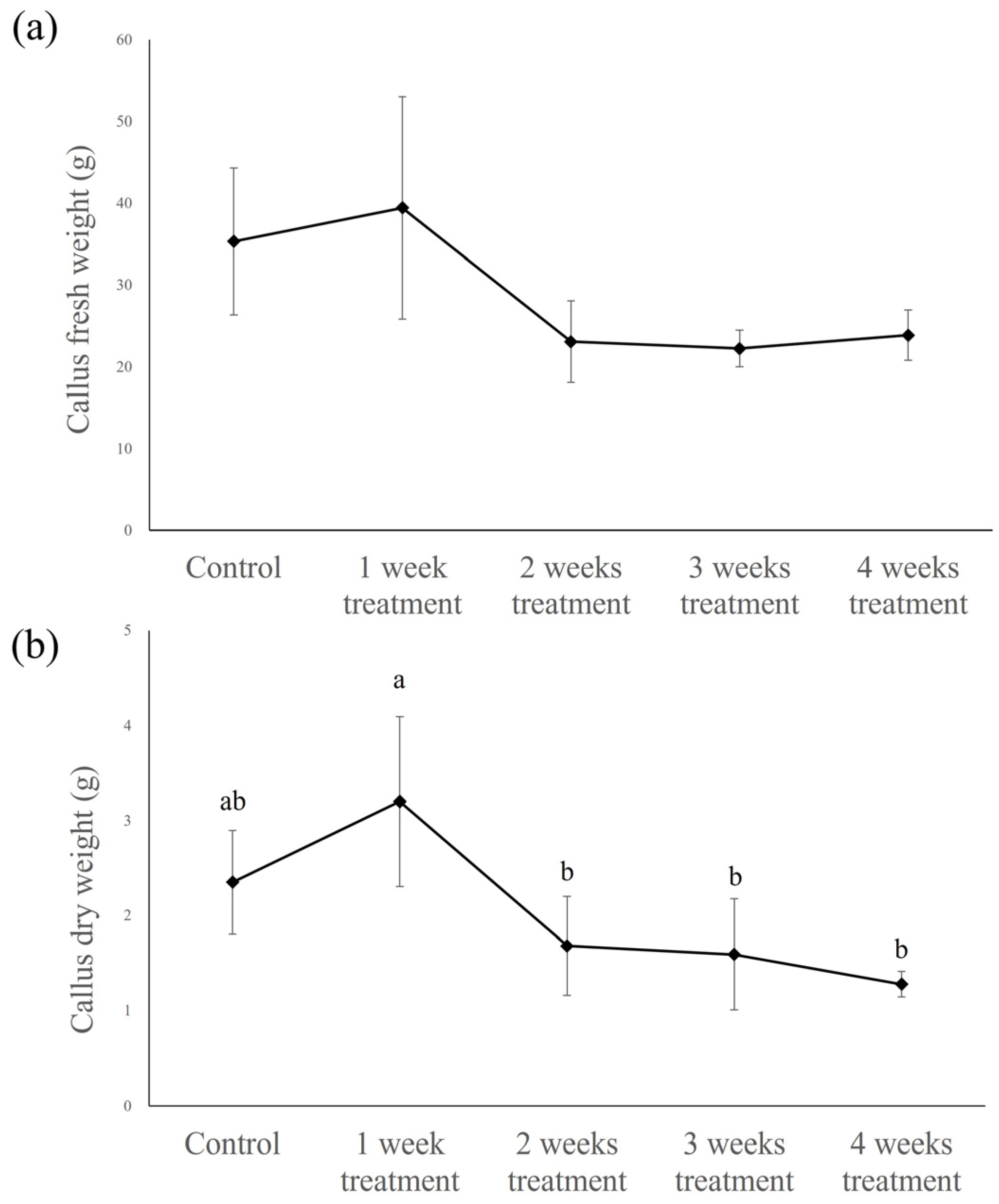
2.2.1. Fresh and Dry Weight of Callus
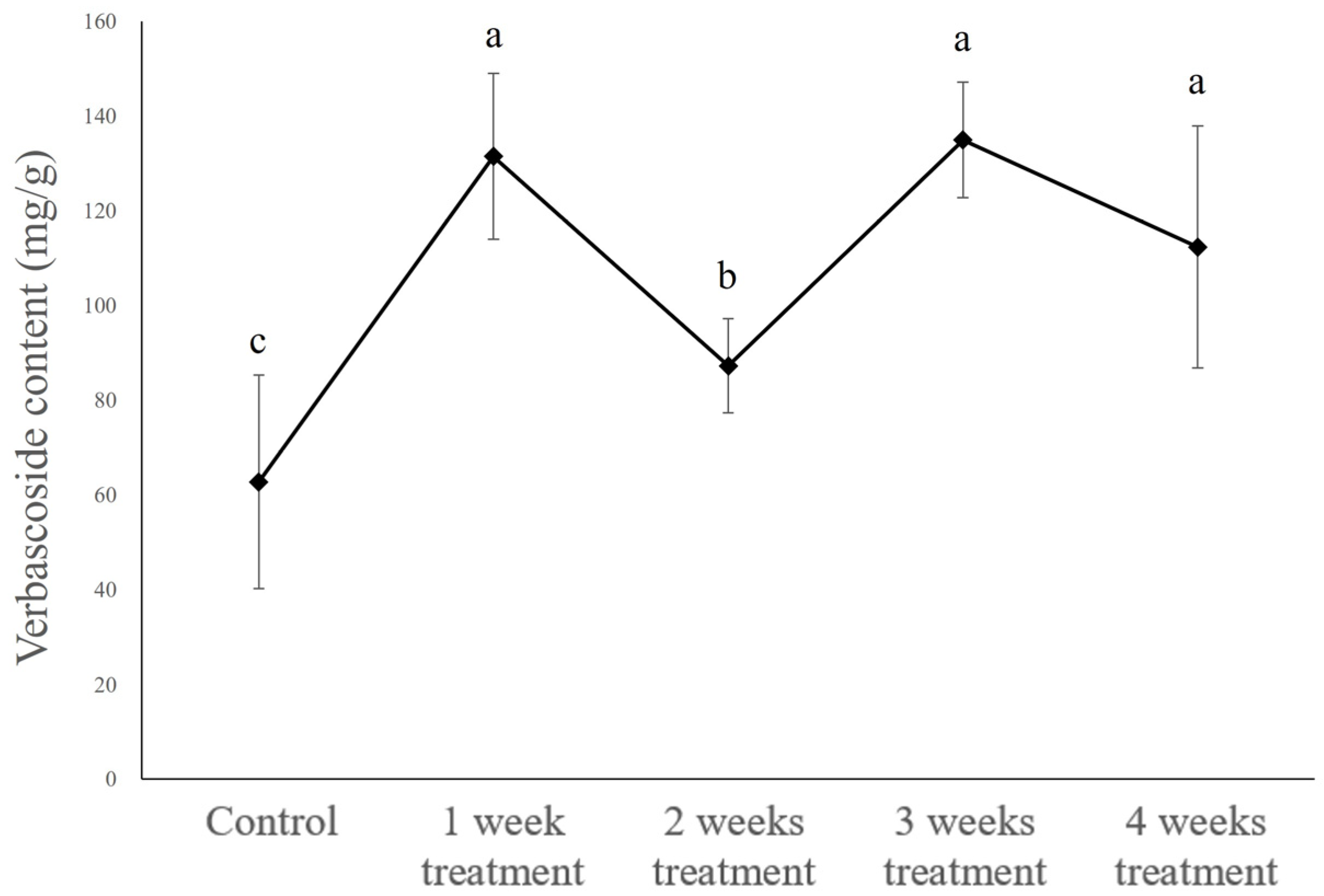
2.2.2. Verbascoside Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Elicitation with Salicylic Acid and Methyl Jasmonate
4.3. Methyl Jasmonate Elicitation Period in Bioreactor
4.4. Analysis of Verbascoside Content
4.4.1. Extraction of Verbascoside from Callus
4.4.2. Determination of Verbascoside Content Using Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- You, J.H.; Lee, C.H. Analysis on herbaceous communities and flora around Abeliophyllum distichum habitats. Korean J. Plant Res. 2005, 18, 315–324. [Google Scholar]
- Choi, J.H.; See, E.J.; Sung, J.; Choi, K.M.; Kim, H.; Kim, J.S.; Lee, J.; Efferth, T.; Hyun, T.K. Polyphenolic compounds, antioxidant and anti-inflammatory effects of Abeliophyllum distichum Nakai extract. J. Appl. Bot. Food Qual. 2017, 90, 266–273. [Google Scholar] [CrossRef]
- Eom, J.; Thomas, S.S.; Sung, N.Y.; Kim, D.S.; Cha, Y.S.; Kim, K.A. Abeliophyllum distichum ameliorates high-fat diet-induced obesity in C57BL/6J mice by upregulating the AMPK pathway. Nutrients 2020, 12, 3320. [Google Scholar] [CrossRef]
- Yoo, T.K.; Kim, J.S.; Hyun, T.K. Polyphenolic composition and anti-melanoma activity of white forsythia (Abeliophyllum distichum nakai) organ extracts. Plants 2020, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.Y. Enhancement of anti-wrinkle activities of Abeliophyllum distichum Nakai through low temperature extraction process. Korean J. Med. Crop Sci. 2015, 23, 231–236. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, J.H.; Pang, Q.Q.; Cho, E.J.; Lee, S. Antioxidant activity and acteoside analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yoshida, K.; Kondo, Y.; Inoue, K. Production of cornoside in Abeliophyllum distichum cell suspension cultures. Phytochemistry 1998, 48, 273–277. [Google Scholar] [CrossRef]
- Sarkhail, P.; Nikan, M.; Sarkheil, P.; Gohari, A.R.; Ajani, Y.; Hosseini, R.; Hadjiakhoondi, A.; Saeidnia, S. Quantification of verbascoside in medicinal species of Phlomis and their genetic relationships. DARU J. Pharm. Sci. 2014, 22, 32. [Google Scholar] [CrossRef]
- Schlauer, J.; Budzianowski, J.; Kukulczanka, K.; Ratajczak, L. Acteoside and related phenylethanoid glycosides in Byblis liniflora Salisb. plants propagated in vitro and its systematic significance. Acta Soc. Bot. Pol. 2004, 73, 1–15. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Orhan, I.E.; Cankaya, I.I.T.; Kostadinova, E.P.; Georgiev, M.I. Treasure from garden: Chemical profiling, pharmacology and biotechnology of mulleins. Phytochem. Rev. 2014, 13, 417–444. [Google Scholar] [CrossRef]
- Peng, X.M.; Gao, L.; Huo, S.X.; Liu, X.M.; Yan, M. The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of d-gal and AlCl3. Phytother. Res. 2015, 29, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. Evid.-Based Complement. Altern. Med. 2017, 2017, 3574012. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.Y.; Kang, H.S.; Jeong, C.H.; Moon, H.; Whang, W.K.; Kim, C.J.; Sim, S.S. The effect of acteoside on histamine release and arachidonic acid release in RBL-2H3 mast cells. Arch. Pharm. Res. 2006, 29, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, S.; Jung, M.Y.; Ham, I.H.; Whang, W.K. Anti-inflammatory phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch. Pharm. Res. 2009, 32, 7–13. [Google Scholar] [CrossRef]
- Kang, K.H.; Jang, S.K.; Kim, B.K.; Park, M.K. Antibacterial phenylpropanoid glycosides from Paulownia tomentosa Steud. Arch. Pharm. Res. 1994, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic profile, antibacterial, antimutagenic and antitumour evaluation of Veronica urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Chathuranga, K.; Kim, M.S.; Lee, H.C.; Kim, T.-H.; Kim, J.H.; Chathuranga, W.A.G.; Ekanayaka, P.; Wijerathne, H.M.S.M.; Cho, W.K.; Kim, H.I.; et al. Anti-respiratory syncytial virus activity of Plantago asiatica and Clerodendrum trichotomum extracts in vitro and in vivo. Viruses 2019, 11, 604. [Google Scholar] [CrossRef]
- He, J.; Hu, X.P.; Zeng, Y.; Li, Y.; Wu, H.Q.; Qiu, R.Z.; Ma, W.J.; Li, T.; Li, C.Y.; He, Z.D. Advanced research on acteoside for chemistry and bioactivities. J. Asian Nat. Prod. Res. 2011, 13, 449–464. [Google Scholar] [CrossRef]
- Bastante, C.C.; Cardoso, L.C.; Ponce, M.T.F.; Serrano, C.M.; de la Ossa-Fernández, E.J.M. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluid. 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Xie, G.; Xu, Q.; Li, R.; Shi, L.; Han, Y.; Zhu, Y.; Wu, G.; Qin, M. Chemical profiles and quality evaluation of Buddleja officinalis flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 164, 283–295. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Salgueiro, L.; Canhoto, J. Plant tissue culture: Industrial relevance and future directions. Plants as Factor. Bioprod. Recent Dev. Appl. 2024, 188, 1–15. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Beigmohamadi, M.; Movafeghi, A.; Sharafi, A.; Jafari, S.; Danafar, H. Cell suspension culture of Plumbago europaea L. to-wards production of plumbagin. Iran. J. Biotech. 2019, 17, e2169. [Google Scholar] [CrossRef]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Sutini; Widiwurjani; Augustien, N.; Purwanto, D.A.; Muslihatin, W. The production of cinnamic acid secondary metabolites through in vitro culture of callus Camellia sinensis L with the elicitor of cobalt metal ions. In Proceedings of the International Conference on Biology and Applied Science, Malang, Indonesia, 20 March 2019; American Institute of Physics Inc.: Malang, Indonesia, 2019; Volume 2120, pp. 030028-1–030028-5. [Google Scholar] [CrossRef]
- Benjamin, E.D.; Ishaku, G.A.; Peingurta, F.A.; Afolabi, A.S. Callus culture for the production of therapeutic compounds. Am. J. Plant Biol. 2019, 4, 76–84. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of sec-ondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Bio-Technol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Song, L.; Zhao, T.; Luo, C.L.; Li, J.L.; Chen, S.S.; Li, D.D.; Liang, J.; Liu, H.C.; Luo, F.L.; Huang, M.J.; et al. Establishment of a callus induction system of Saxifraga stolonifera Meerb. and its response to different elicitors. S. Afr. J. Bot. 2024, 171, 447–453. [Google Scholar] [CrossRef]
- Cuaspud, O.; Mendoza, D.; Arias, J. Exogenous addition of methyl jasmonate and salicylic acid in immobilized cell cultures of Thevetia peruviana: Effect on the biomass, phenolic compounds and cardiac glycosides production. Plant Cell Tissue Organ Cult. PCTOC 2025, 160, 69. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.F.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.O.; et al. Production of Verbascoside, Isoverbascoside and Phenolic Acids in Callus, Suspension, and Bioreactor Cultures of Verbena officinalis and Biological Properties of Biomass Extracts. Molecules 2020, 25, 5609. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Frezza, C.; Beccaccioli, M.; Gelardi, L.; Bianco, A.; Bonina, F.P.; Nielsen, E. Production of verbascoside and its analogues in in vitro cultures of Verbascum thapsus L. Plant Cell Tissue Organ Cult. 2020, 140, 83–93. [Google Scholar] [CrossRef]
- Cambaz, E.; Çördük, N. Secondary metabolite production in callus culture of Verbascum scamandri Murb. Acta Soc. Bot. Pol. 2023, 92, 165894. [Google Scholar] [CrossRef]
- Gurav, S.S.; Gurav, N.S.; Patil, A.T.; Duragkar, N.J. Effect of explant source, culture media, and growth regulators on callo-genesis and expression of secondary metabolites of Curcuma longa. J. Herbs Spices Med. Plants 2020, 26, 172–190. [Google Scholar] [CrossRef]
- da Luz Costa, J.; Silva, A.L.L.d.; Bier, M.C.J.; Brondani, G.E.; Gollo, A.L.; Letti, L.A.J.; Erasmo, E.A.L.; Soccol, C.R. Callus growth kinetics of physic nut (Jatropha curcas L.) and content of fatty acids from crude oil obtained in vitro. Appl. Biochem. Biotechnol. 2015, 176, 892–902. [Google Scholar] [CrossRef]
- Danaee, M.; Farzinebrahimi, R.; Abdul Kadir, M.; Sinniah, U.R.; Mohamad, R.; Mat Taha, R. Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Braz. J. Bot. 2015, 38, 265–272. [Google Scholar] [CrossRef]
- Arano-Varela, H.; Cruz-Sosa, F.; Estrada-Zúñiga, M.E.; Fernández, F.J. Effects of phenylalanine and methyl jasmonate on verbascoside production in Buddleja cordata Kunth cell suspension cultures. S. Afr. J. Bot. 2020, 135, 41–49. [Google Scholar] [CrossRef]
- Szymczyk, P.; Szymańska, G.; Kuźma, Ł.; Jeleń, A.; Balcerczak, E. Methyl jasmonate activates the 2C methyl-D-erithrytol 2, 4-cyclodiphosphate synthase gene and stimulates tanshinone accumulation in Salvia miltiorrhiza solid callus cultures. Molecules 2022, 27, 1772. [Google Scholar] [CrossRef]
- Chavan, J.J.; Kshirsagar, P.R.; Jadhav, S.G.; Nalavade, V.M.; Gurme, S.T.; Pai, S.R. Elicitor-mediated enhancement of biomass, polyphenols, mangiferin production and antioxidant activities in callus cultures of Salacia chinensis L. 3 Biotech. 2021, 11, 285. [Google Scholar] [CrossRef]
- Rijhwani, S.K.; Shanks, J.V. Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol. Prog. 1998, 14, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Piatczak, E.; Kuzma, L.; Wysokinska, H. The influence of methyl jasmonate and salicylic acid on secondary metabolite production in Rehmannia glutinosa Libosch. hairy root culture. Acta Biol. Cracoviensia Ser. Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Rahmat, E.; Chung, Y.; Nam, H.H.; Lee, A.Y.; Park, J.H.; Kang, Y. Evaluation of marker compounds and biological activity of in vitro regenerated and commercial Rehmannia glutinosa (Gaertn.) DC. Roots subjected to steam processing. Evid.-Based Complemet. Altern. Med. 2022, 2022, 1506703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.; Park, Y.-W.; Kang, J.; Jung, E.-S.; Lee, H. Effects of Elicitation on Abeliophyllum distichum Leaf Callus and Changes in Verbascoside Content. Plants 2025, 14, 1386. https://doi.org/10.3390/plants14091386
Choi D, Park Y-W, Kang J, Jung E-S, Lee H. Effects of Elicitation on Abeliophyllum distichum Leaf Callus and Changes in Verbascoside Content. Plants. 2025; 14(9):1386. https://doi.org/10.3390/plants14091386
Chicago/Turabian StyleChoi, Daeho, Yong-Woo Park, Jungmok Kang, Eun-Suk Jung, and Hwayong Lee. 2025. "Effects of Elicitation on Abeliophyllum distichum Leaf Callus and Changes in Verbascoside Content" Plants 14, no. 9: 1386. https://doi.org/10.3390/plants14091386
APA StyleChoi, D., Park, Y.-W., Kang, J., Jung, E.-S., & Lee, H. (2025). Effects of Elicitation on Abeliophyllum distichum Leaf Callus and Changes in Verbascoside Content. Plants, 14(9), 1386. https://doi.org/10.3390/plants14091386




