In Vitro Production of Smilax brasiliensis Seedlings, Callus Induction, Chemical Profile, and Assessment of Antioxidant Activity
Abstract
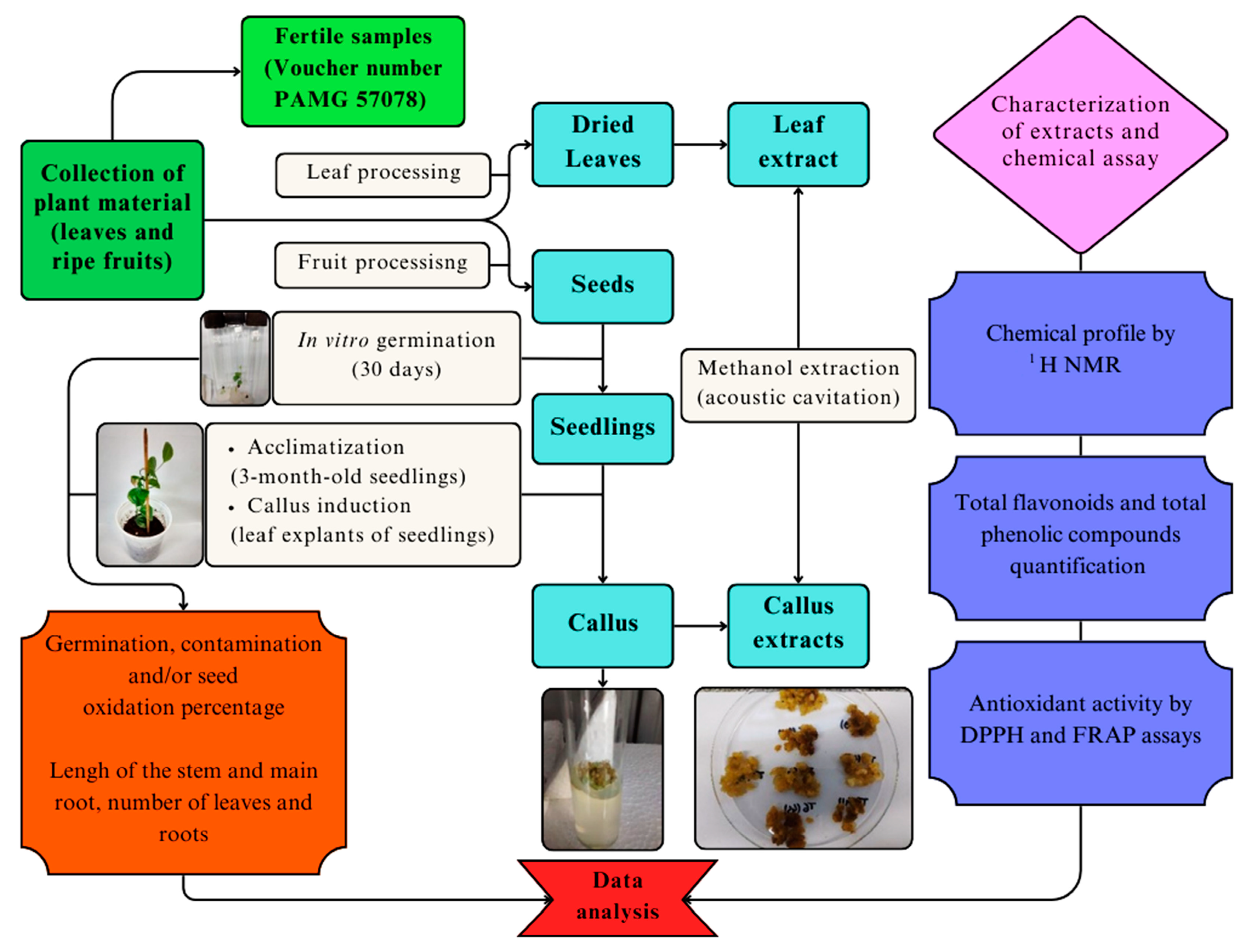
1. Introduction
2. Results
2.1. In Vitro Germination
2.2. Seedling Acclimatization
2.3. Callus Induction
2.4. Obtaining Extracts
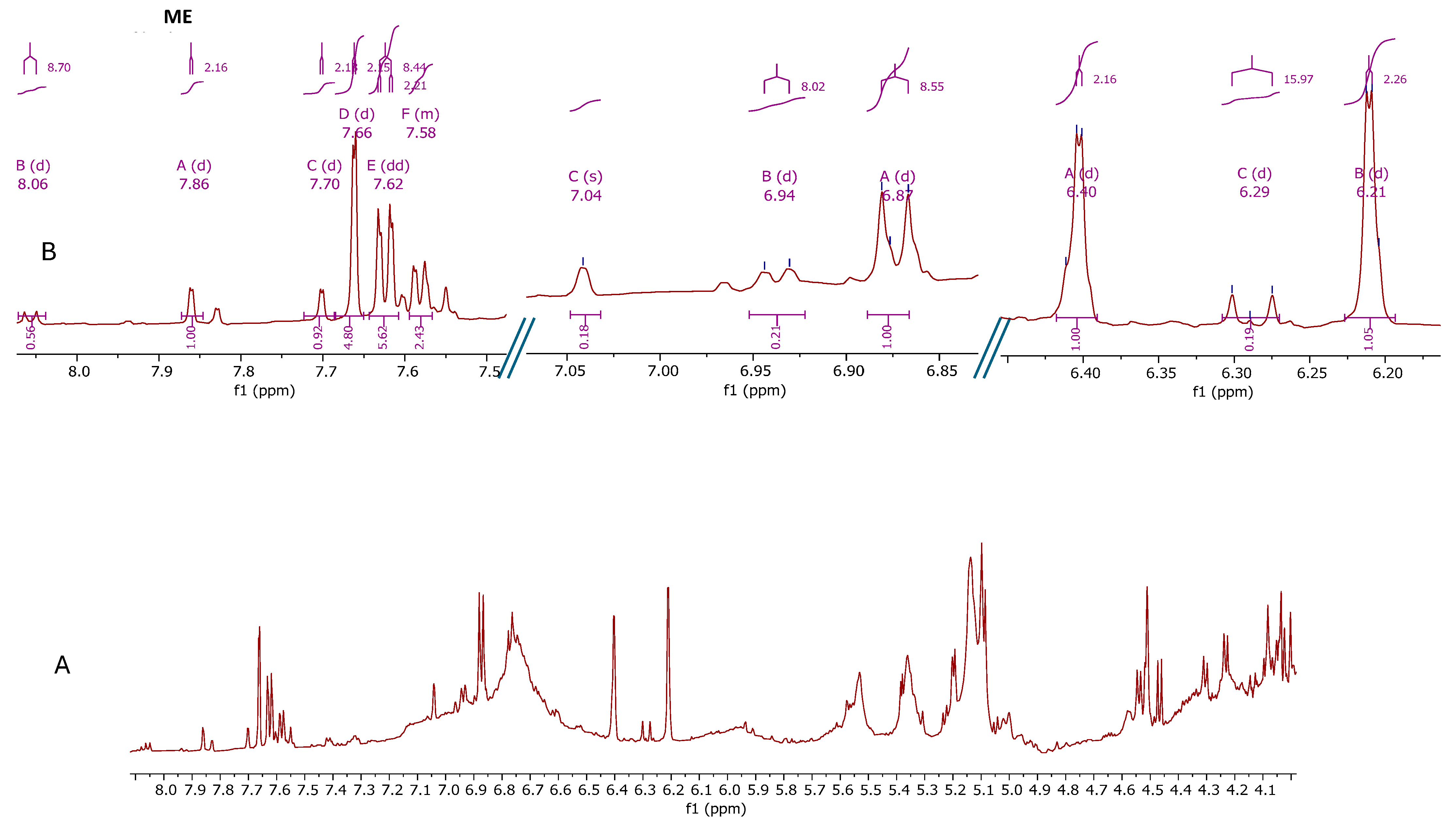
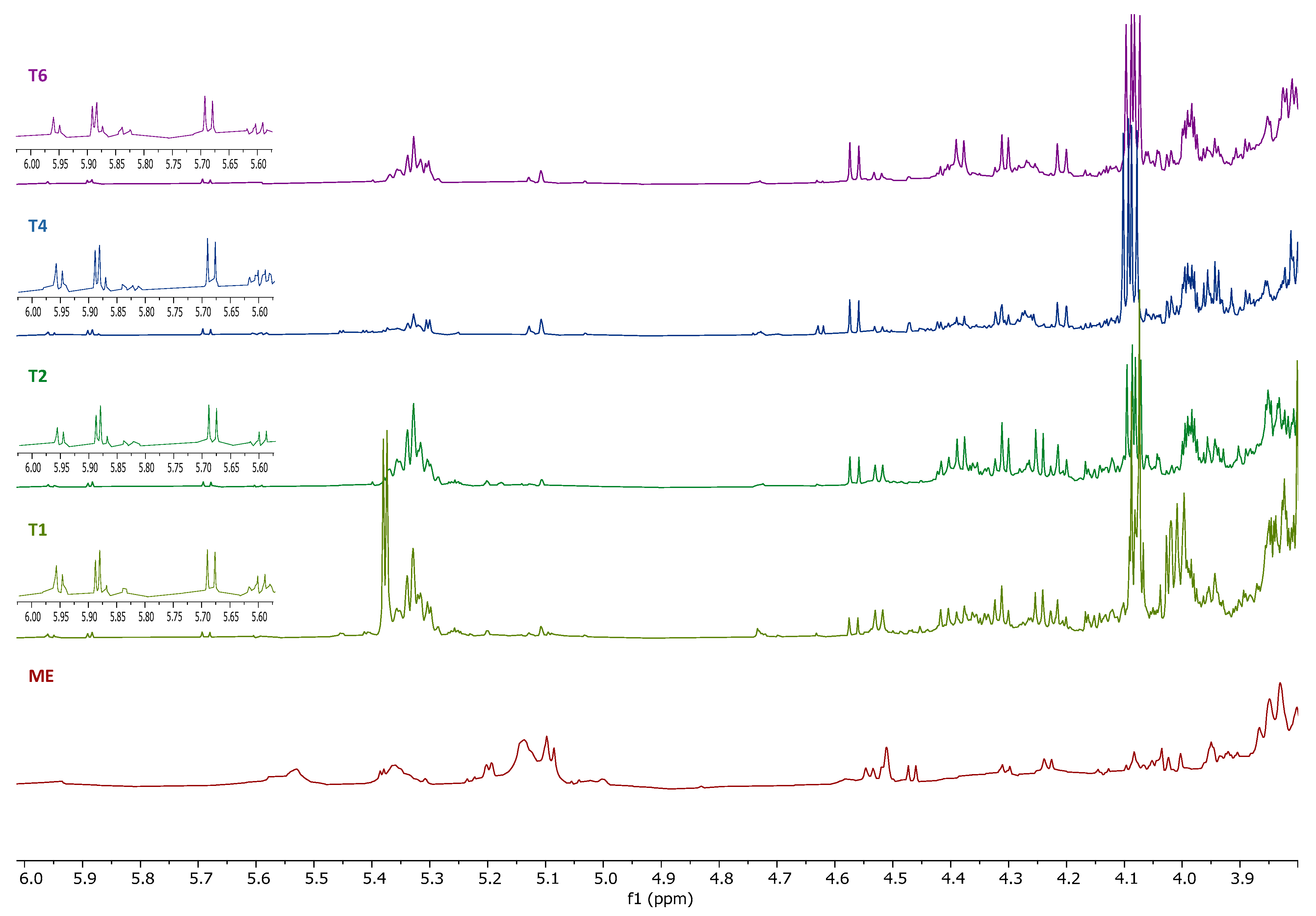
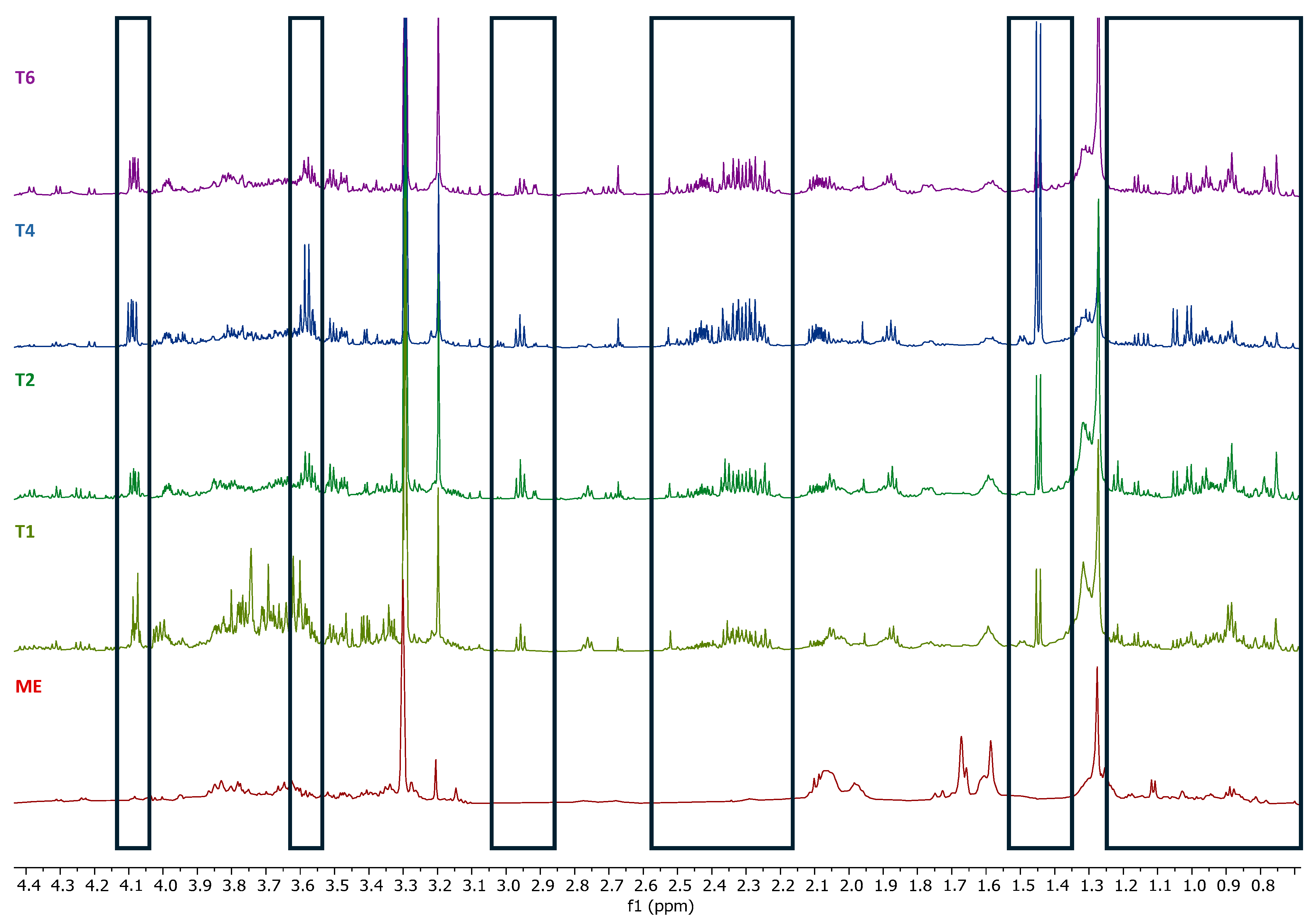
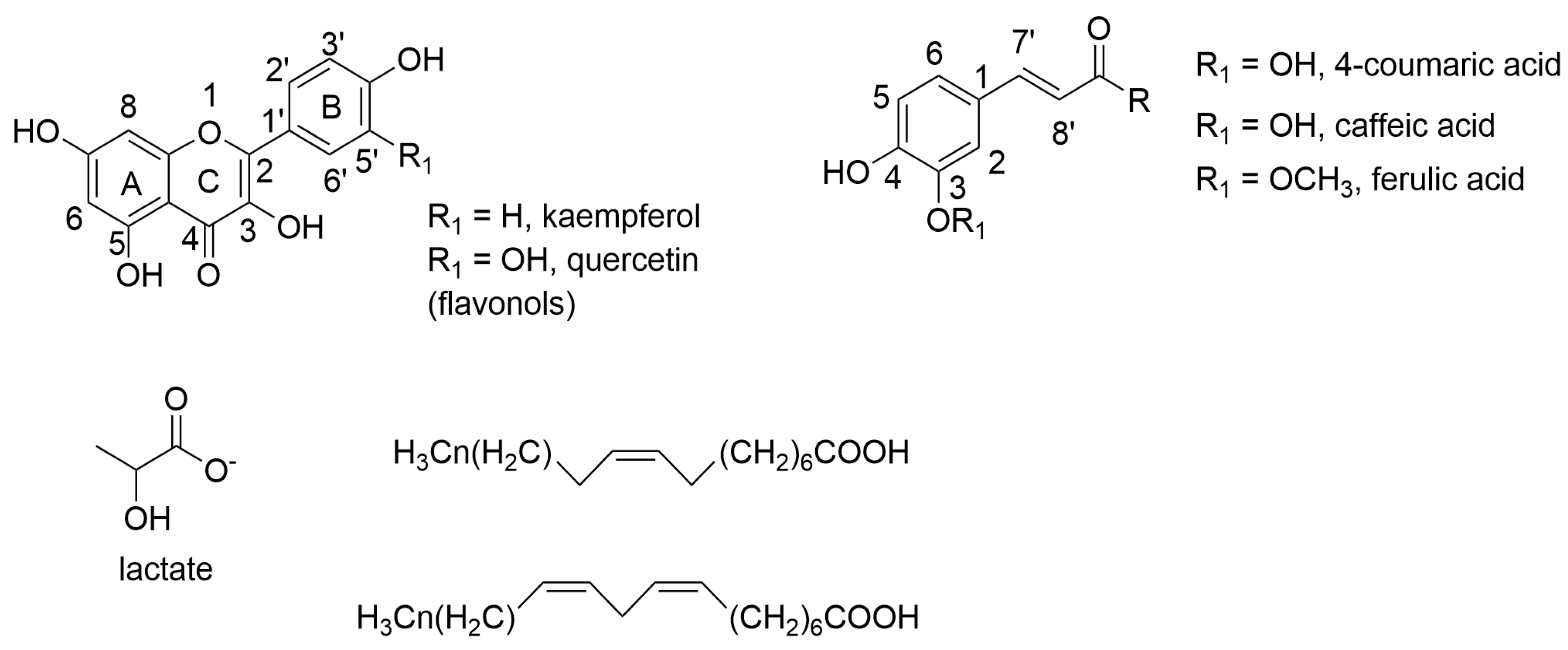
2.5. Determination of the Metabolic Profile of Methanol Extracts by Nuclear Magnetic Resonance (NMR)
2.6. Determination of the Total Phenolic Compound, Flavonoid Content, and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. In Vitro Germination
4.4. Seedlings Acclimatization
4.5. Callus Induction
4.6. Obtaining Extracts
4.7. Determination of the Metabolic Profile of Methanol Extracts by Nuclear Magnetic Resonance (NMR)
4.8. Determination of the Content of Total Phenolic Compounds and Flavonoids
4.9. Assessment of Antioxidant Activity
4.10. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breitbach, U.B.; Niehues, M.; Lopes, N.P.; Faria, J.E.Q.; Brandão, M.G.L. Amazonian Brazilian medicinal plants described by C.F.P. von Martius in the 19th century. J. Ethnopharmacol. 2013, 147, 180–189. [Google Scholar] [CrossRef]
- Guarim Neto, G.; Morais, G. Medicinal plants resources in the Cerrado of Mato Grosso State, Brazil: A review. Acta Bot. Bras. 2003, 17, 561–584. [Google Scholar] [CrossRef]
- Andreata, R.H.P. Smilacaceae, Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brasil, 2015. Available online: http://floradobrasil2015.jbrj.gov.br/FB224 (accessed on 10 January 2025).
- Martins, A.R.; Abreu, A.G.; Bajay, M.M.; Villela, P.M.S.; Batista, C.E.A.; Monteiro, M.; Alves-Pereira, A.; Figueira, G.M.; Pinheiro, J.B.; Appezzato-da-Glória, B.; et al. Development and characterization of microsatellite markers for the medicinal plant Smilax brasiliensis (Smilacaceae) and related species. Appl. Plant Sci. 2013, 1, 1200507. [Google Scholar] [CrossRef]
- Andreata, R.H.P. Smilacaceae. In Flora fanerogâmica do estado de São Paulo; Instituto de Botânica: São Paulo, SP, Brasil, 2003; pp. 323–332. [Google Scholar]
- Silva, I.C.A.; Fonseca, J.C.; Medeiros, J.E.S.; Amado, P.A.; Castro, A.H.F.; Lima, L.A.R.S. Chapter 7: Phytochemical aspects and biological properties of Smilax brasiliensis Sprengel. In Phytochemicals: Plant Sources and Potential Health Benefits, 1st ed.; Ryan, I., Ed.; Nova Science Publishers: New York, NY, USA, 2019; pp. 175–189. [Google Scholar]
- Amado, P.A.; Ferraz, V.; Silva, D.B.; Carollo, C.A.; Castro, A.H.F.; Lima, L.A.R.S. Chemical composition, antioxidant and cytotoxic activities of extracts from the leaves of Smilax brasiliensis Sprengel (Smilacaceae). Nat. Prod. Res. 2018, 32, 610–615. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Fonseca, J.C.; Ferraz, V.; Castro, A.H.F.; Lima, L.A.R.S. Phytotoxic and antioxidant effects of dichloromethane fraction of Smilax brasiliensis Sprengel. Nat. Prod. Res. 2021, 35, 1676–1681. [Google Scholar] [CrossRef]
- Amado, P.A.; Castro, A.H.F.; Alves, S.N.; Silva, D.B.; Carollo, C.A.; Lima, L.A.R.S. Phenolic compounds: Antioxidant and larvicidal potential of Smilax brasiliensis Sprengel leaves. Nat. Prod. Res. 2020, 34, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.C.; Barbosa, M.A.; Silva, I.C.A.; Duarte-Almeida, J.M.; Castro, A.H.F.; dos Santos Lima, L.A.R. Antioxidant and allelopathic activities of Smilax brasiliensis Sprengel (Smilacaceae). South Afr. J. Bot. 2017, 111, 336–340. [Google Scholar] [CrossRef]
- Amado, P.A.; Castro, A.H.F.; Zanuncio, V.S.S.; Stein, V.C.; Silva, D.B.; Lima, L.A.R.d.S. Assessment of allelopathic, cytotoxic, genotoxic and antigenotoxic potential of Smilax brasiliensis Sprengel leaves. Ecotoxicol. Environ. Saf. 2020, 192, e110310. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.C.A.; Azevedo, L.S.; Castro, A.H.F.; Farias, K.S.; Zanuncio, V.S.S.; Silva, D.B.; Lima, L.A.R.S. Chemical profile, antioxidante potential and toxicity of Smilax brasiliensis Sprengel (Smilacaceae) stems. Food Res. Int. 2023, 168, 112781. [Google Scholar] [CrossRef]
- Santos, M.R.A.; Paiva, R.; Gomes, G.A.C.; Paiva, P.O.; Paiva, L. Studies of Smilax japecanga Grisebach seed dormancy breakage. Ciênc. Agrotec. 2003, 27, 319–324. [Google Scholar] [CrossRef]
- Mangena, P. Analysis of correlation between seed vigour, germination and multiple shoot induction in soybean (Glycine max L. Merr.). Helyon 2021, 7, e07913. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.F.; Mir, H.; Mehta, S.; Sohane, R.K. Estudo do estabelecimento in vitro e indução de calos em Banana cv. Grande Naine. Rev. Atual Ciência Apl. Tecnol. 2019, 33, 1–5. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- George, E.F.; Michael, A.; Klerk, H.G. Plant Propagation by Tissue Culture, 3 ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 205–226. [Google Scholar]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Dagla, H.R. Plant tissue culture: Historical developments and applied aspects. Resonance 2012, 17, 759–767. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Braga, K.Q.; Sousa, F.M.; Coimbra, M.C.; Chagas, R.C.R. Callus induction and bioactive phenolic compounds production from Byrsonima verbascifolia (L.) DC. (Malpighiaceae). Rev. Ciênc. Agron. 2016, 47, 143–151. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.T.; Medeiros, F.S.; de Souza Filho, J.D.; Sena, M.M.; Terra, W.C.; Pimenta, L.P.S. NMR-based metabolomic screening for metabolites associated with resistance to Meloidogyne javanica in Annona muricata roots. J. Braz. Chem. Soc. 2019, 30, 1276–1283. [Google Scholar] [CrossRef]
- Pimenta, L.P.; Kim, H.K.; Verpoorte, R.; Choi, Y.H. NMR-based metabolomics: A probe to utilize biodiversity. Methods Mol. Biol. 2013, 1055, 117–127. [Google Scholar] [CrossRef]
- Fan, T.W.M. Metabolite profiling by one-and two-dimensional NMR analysis of complex mixtures. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 161–219. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, S.; Liang, F.; Liang, J.; Huang, G.; Liu, M.; Huang, H. Structural characterization of steroidal saponins from Smilax trinervula using ultra high-performance liquid chromatography coupled with LtQ-orbitrap mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2015, 974, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.G.; Vega, S.; Ganosa, D.; Pérez de Paz, P.; Díaz Diaz, D. Novel triterpenes and bioactive compounds isolated from Smilax canariensis Brouss. ex Willd. Separations 2025, 12, 74. [Google Scholar] [CrossRef]
- Hamid, A.A.; Aiyelaagbe, O.O.; Negi, A.S.; Luqman, S.; Kaneez, F.S. Bioguided isolation and antiproliferative activity of constituents from Smilax korthalsii A.D.C. leaves. J. Chin. Chem. Soc. 2016, 63, 562–571. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, X.; Zhang, D.; Li, Y.; Zhang, L.; Song, B.; Yue, Z.; Song, X.; Tang, H. Steroidal constituents from roots and rhizomes of Smilacina japonica. Molecules 2018, 23, 798. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2010, 47, 5–16. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Brinckmann, J.A.; Yang, X.; He, J. Introduction to the special issue: Saving plants, saving lives: Trade, sustainable harvest and conservation of traditional medicinals in Asia. J. Ethnopharmacol. 2019, 229, 288–292. [Google Scholar] [CrossRef]
- Asigbaase, M.; Adusu, D.; Anaba, L.; Abugre, S.; Kang-Milung, S.; Acheamfour, S.A.; Adamu, I.; Ackah, D.K. Conservation and economic benefits of medicinal plants: Insights from forest-fringe communities of Southwestern Ghana. Trees For. People 2023, 14, 100462. [Google Scholar] [CrossRef]
- Engelmann, F.; Rao, R. Major Research challenges and directions for future research. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2013; pp. 511–526. [Google Scholar]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Palhares, D.; Tiné, M.A.; Vinha, D.; Silveira, C.E.S.; Zaidan, L.B.P. Studies on the seeds of Smilax goyazana A.DC. (Smilacaceae). Phyton 2009, 49, 117–130. [Google Scholar]
- D’Antuono, L.F.; Lovato, A. Germination trials and domestication potential of three native species with edible sprouts: Ruscus aculeatus L., Tamus communis L. and Smilax aspera L. Acta Hortic. 2003, 598, 211–218. [Google Scholar] [CrossRef]
- Rosa, S.G.T.; Ferreira, A.G. Germination of medicinal plant Smilax campestris Griseb. (Salsaparrilha). Acta Hortic. 1999, 502, 105–112. [Google Scholar] [CrossRef]
- Martins, A.R.; Soares, A.N.; Bombo, A.B.; Fidelis, A.; Novembre, A.D.L.C.; Glória, B.A. Germination and seedling morphology of four South American Smilax (Smilacaceae). Rev. Biol. Trop. 2012, 60, 495–504. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; Nardi, F.S.; Filippi, D.; Trentin, T.S.; Anzolin, A.P.; Bertol, C.D.; Nienow, A.A.; Calvete, E.O. Mycorrhization of strawberry plantlets potentiates the synthesis of phytochemicals during ex vitro acclimatization. Acta Sci. Agron. 2022, 44, e55682. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Korbozova, N.K.; Kudrina, N.O.; Kobylina, T.N.; Kurmanbayeva, M.S.; Meduntseva, N.D.; Tolstikova, T.G. The influence of abiotic stress factors on the morphophysiological and phytochemical aspects of the acclimation of the plant Rhodiola semenowii Boriss. Plants 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Palei, S.; Das, A.K.; Rout, G.R. In vitro studies of strawberry–an important fruit crop: A review. J. Plant Sci. Res. 2015, 31, 115–131. [Google Scholar]
- Sofiah, S.; Hakim, L.; Indriyani, S.; Robiansyah, I. Acclimation study of Smilax nageliana A.DC., a climber species endemic to East Java, Indonesia. Biodiversitas 2022, 23, 4082–4089. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Itharat, A. Effects of medium salt strength and plant growth regulators on shoot multiplication and root induction of Smilax corbularia. Pharmacologyonline 2013, 3, 1–7. [Google Scholar]
- Kumar, R.N.; Kaffoor, H.A.; Arumugasamy, K.; Shalimol, A.; Devi, V.A. In vitro callus induction of Smilax wightii A. DC an endemic medicinal plant. Kongunadu Res. J. 2015, 2, 97–101. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for a rapid growth and bioassays with tobacco tissues cultures. Plant Physiol. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Loredo-Carrillo, S.E.; Santos-Díaz, M.L.; Leyva, E.; Santos-Díaz, M.S. Establishment of callus from Pyrostegia venusta (Ker Gawl.) Miers and effect of abiotic stress on flavonoids and sterols accumulation. J. Plant Biochem. Biotechnol. 2013, 22, 312–318. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z.; He, X. Triterpenoids of sour jujube show pronounced inhibitory effect on human tumor cells and antioxidant activity. Fitoterapia 2014, 98, 137–142. [Google Scholar] [CrossRef]
- Buț, M.-G.; Tero-Vescan, A.; Pușcaș, A.; Jîtcă, G.; Marc, G. Exploring the inhibitory potential of phytosterols β-sitosterol, stigmasterol, and campesterol on 5-alpha reductase activity in the human prostate: An in vitro and in silico approach. Plants 2024, 13, 3146. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty acid composition, antioxidant, and in vitro anti-inflammatory activity of five cold-pressed Prunus seed oils, and their anti-biofilm effect against pathogenic bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, T.; Rana, S.S.; Ghosh, P. Microwave-assisted extraction of dragon fruit seed oil: Fatty acid profile and functional properties. J. Saudi Soc. Agric. Sci. 2023, 22, 149–157. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Haminiuk, C.W.I.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Granato, D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 5th ed; ANVISA: Brasília, Brasil, 2010. [Google Scholar]
- Morais, M.G.; Saldanha, A.A.; Azevedo, L.S.; Mendes, I.C.; Rodrigues, J.P.C.; Amado, P.A.; Farias, K.S.; Zanuncio, V.S.S.; Cassemiro, N.S.; Silva, D.B.; et al. Antioxidant and anti-inflammatory effects of fractions from ripe fruits of Solanum lycocarpum St. Hil. (Solanaceae) and putative identification of bioactive compounds by GC-MS and LC-DAD-MS. Food Res. Int. 2022, 156, e111145. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Pires, J.; Torres, P.B.; Santos, D.Y.A.C.; Chow, F. Ensaio Antioxidante Em Microplaca Do Poder De Redução Do Ferro (FRAP) Para Extratos De Algas; Instituto de Biociências, Universidade de São Paulo: Sao Paulo, SP, Brazil, 2016; Volume 12, pp. 1–6. [Google Scholar] [CrossRef]


| Treatments | Plant Growth Regulators (PGR) | Concentration (µg/mL) | Light |
|---|---|---|---|
| T1 | 2.4-D (dichlorophenoxyacetic acid) | 0.5 | Absence |
| T2 | 2.4-D (dichlorophenoxyacetic acid) | 1.0 | Absence |
| T4 | Picloram | 1.0 | Presence |
| T6 | Picloram + BAP (6-benzylaminopurine) | 0.5 + 0.5 | Absence |
| Treatments | CI (%) | Consistency | FM (g) | DM (g) | DMY (%) |
|---|---|---|---|---|---|
| T1 | 50% | friable | 1.3239 ± 0.5113 ab | 0.1797 ± 0.1298 a | 13.57 b |
| T2 | 25% | friable | 0.7901 ± 0.3020 b | 0.2411 ± 0.0773 a | 30.05 a |
| T4 | 75% | friable | 2.3656 ± 0.8435 a | 0.1361 ± 0.0469 a | 5.75 c |
| T6 | 100% | friable | 2.5730 ± 1.3246 a | 0.1232 ± 0.0720 a | 4.79 c |
| Samples | T1 | T2 | T4 | T6 | ME |
|---|---|---|---|---|---|
| Mass 1 (g) | 0.1210 | 0.0760 | 0.0950 | 0.0650 | 0.0950 |
| Yield 1 (%) | 12.10 | 7.60 | 9.50 | 6.50 | 9.50 |
| Mass 2 (g) | 0.0994 | 0.0482 | 0.0522 | 0.0215 | 0.0646 |
| Yield 2 (%) | 9.94 | 4.82 | 5.22 | 2.15 | 6.46 |
| Mass 3 (g) | 0.0345 | 0.0189 | 0.0169 | 0.0113 | 0.0319 |
| Yield 3 (%) | 3.45 | 1.89 | 1.69 | 1.13 | 3.19 |
| Final Mass (g) | 0.2549 | 0.1431 | 0.1641 | 0.0978 | 0.1915 |
| Final Yield (%) | 25.49 | 14.31 | 16.41 | 9.78 | 19.15 |
| Samples | TCP (μg GAE/mg) 1 | FC (μg QE/mg) 2 | DPPH IC50 (μg/mL) 3 | FRAP IC50 (μg/mL) 4 |
|---|---|---|---|---|
| T1 | 27.81 ± 0.07 c | 15.41 ± 0.02 b | 178.69 ± 32.87 cd | 25.38 ± 3.26 c |
| T2 | 36.36 ± 0.04 b | 13.14 ± 0.02 b | 235.44 ± 38.77 d | 33.97 ± 5.32 c |
| T4 | 33.73 ± 0.04 b | 8.08 ± 0.01 c | 135.13 ± 10.31 c | 12.26 ± 1.88 b |
| T6 | 25.32 ± 0.03 c | 5.83 ± 0.01 c | 142.56 ± 19.63 c | 14.14 ± 1.75 b |
| ME | 186.73 ± 6.60 a | 26.67 ± 0.33 a | 6.59 ± 0.87 a | 0.88 ± 0.12 a |
| BHT | - | - | 16.36 ± 1.63 b | 1.47 ± 0.18 a |
| Samples | DPPH Free Radical Capture (%) | ||||
| 1 μg/mL | 10 μg/mL | 100 μg/mL | 250 μg/mL | 500 μg/mL | |
| T1 | 36.56 ± 0.38 a | 37.67 ± 0.79 b | 42.89 ± 0.36 c | 46.71 ± 0.38 c | 74.59 ± 0.71 b |
| T2 | 36.17 ± 0.42 a | 36.85 ± 0.25 b | 41.54 ± 0.08 c | 45.60 ± 0.08 c | 62.31 ± 0.79 c |
| T4 | 36.61 ± 0.55 a | 37.62 ± 0.17 b | 46.13 ± 0.25 c | 53.86 ± 0.51 b | 92.61 ± 1.31 a |
| T6 | 36.69 ± 0.54 a | 37.11 ± 0.08 b | 44.53 ± 0.87 c | 56.38 ± 0.79 b | 65.42 ± 1.08 c |
| ME | 34.05 ± 0.63 a | 57.53 ± 0.38 a | 96.28 ± 0.08 a | 97.00 ± 0.51 a | 98.16 ± 0.84 a |
| BHT | 18.50 ± 0.65 b | 25.90 ± 0.64 c | 86.00 ± 0.56 b | 91.40 ± 0.28 a | 94.02 ± 0.51 a |
| Samples | FRAP activity (%) | ||||
| 2 μg/mL | 5 μg/mL | 10 μg/mL | 15 μg/mL | 30 μg/mL | |
| T1 | 0.00 d | 3.99 ± 0.55 c | 21.63 ± 2.55 c | 34.89 ± 1.17 c | 55.40 ± 3.89 c |
| T2 | 0.00 d | 2.08 ± 0.87 c | 16.03 ± 4.14 c | 26.60 ± 3.33 c | 44.52 ± 1.58 d |
| T4 | 9.29 ± 3.21 c | 26.15 ± 0.92 b | 43.50 ± 1.99 b | 57.77 ± 6.75 b | 70.02 ± 0.55 b |
| T6 | 0.00 d | 20.57 ± 0.62 b | 40.07 ± 0.89 b | 51.80 ± 1.04 b | 69.21 ± 0.51 b |
| ME | 74.79 ± 0.24 a | 88.72 ± 0.22 a | 93.11 ± 1.09 a | 95.64 ± 0.17 a | 97.55 ± 0.02 a |
| BHT | 59.37 ± 0.10 b | 81.60 ± 0.33 a | 89.32 ± 0.36 a | 92.43 ± 0.17 a | 94.97 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amado, P.A.; Castro, A.H.F.; Azevedo, L.S.; Aguilar, M.G.d.; Pimenta, L.P.S.; Lima, L.A.R.d.S. In Vitro Production of Smilax brasiliensis Seedlings, Callus Induction, Chemical Profile, and Assessment of Antioxidant Activity. Plants 2025, 14, 1383. https://doi.org/10.3390/plants14091383
Amado PA, Castro AHF, Azevedo LS, Aguilar MGd, Pimenta LPS, Lima LARdS. In Vitro Production of Smilax brasiliensis Seedlings, Callus Induction, Chemical Profile, and Assessment of Antioxidant Activity. Plants. 2025; 14(9):1383. https://doi.org/10.3390/plants14091383
Chicago/Turabian StyleAmado, Paula Avelar, Ana Hortência Fonsêca Castro, Lucas Santos Azevedo, Mariana Guerra de Aguilar, Lúcia Pinheiro Santos Pimenta, and Luciana Alves Rodrigues dos Santos Lima. 2025. "In Vitro Production of Smilax brasiliensis Seedlings, Callus Induction, Chemical Profile, and Assessment of Antioxidant Activity" Plants 14, no. 9: 1383. https://doi.org/10.3390/plants14091383
APA StyleAmado, P. A., Castro, A. H. F., Azevedo, L. S., Aguilar, M. G. d., Pimenta, L. P. S., & Lima, L. A. R. d. S. (2025). In Vitro Production of Smilax brasiliensis Seedlings, Callus Induction, Chemical Profile, and Assessment of Antioxidant Activity. Plants, 14(9), 1383. https://doi.org/10.3390/plants14091383







