Full-Tree Biomass, Root Carbon Stock, and Nutrient Use Efficiency Across Ages in Eucalyptus Stands Under Seedling and Coppice Systems
Abstract
1. Introduction
2. Results
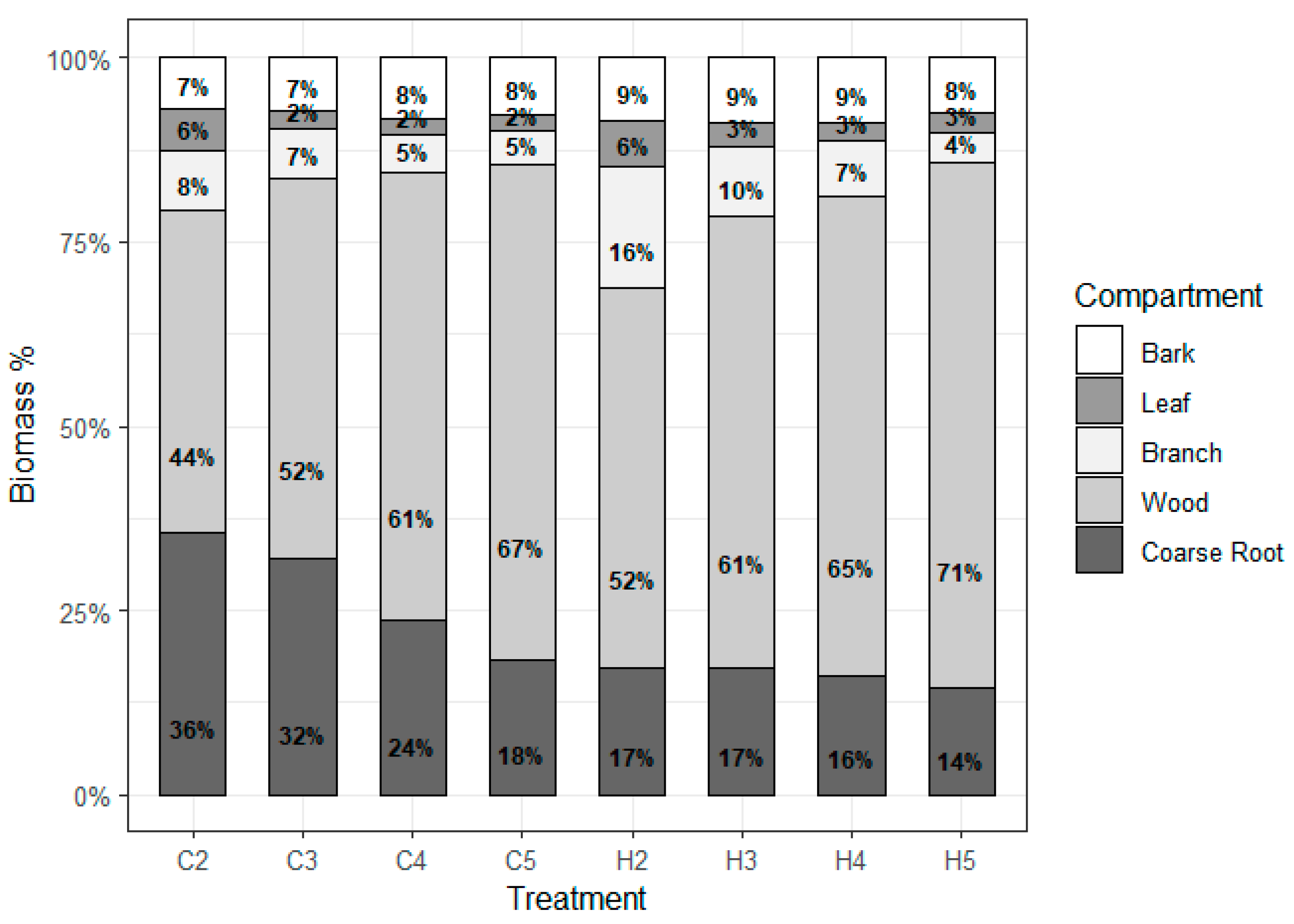
2.1. Biomass Stock
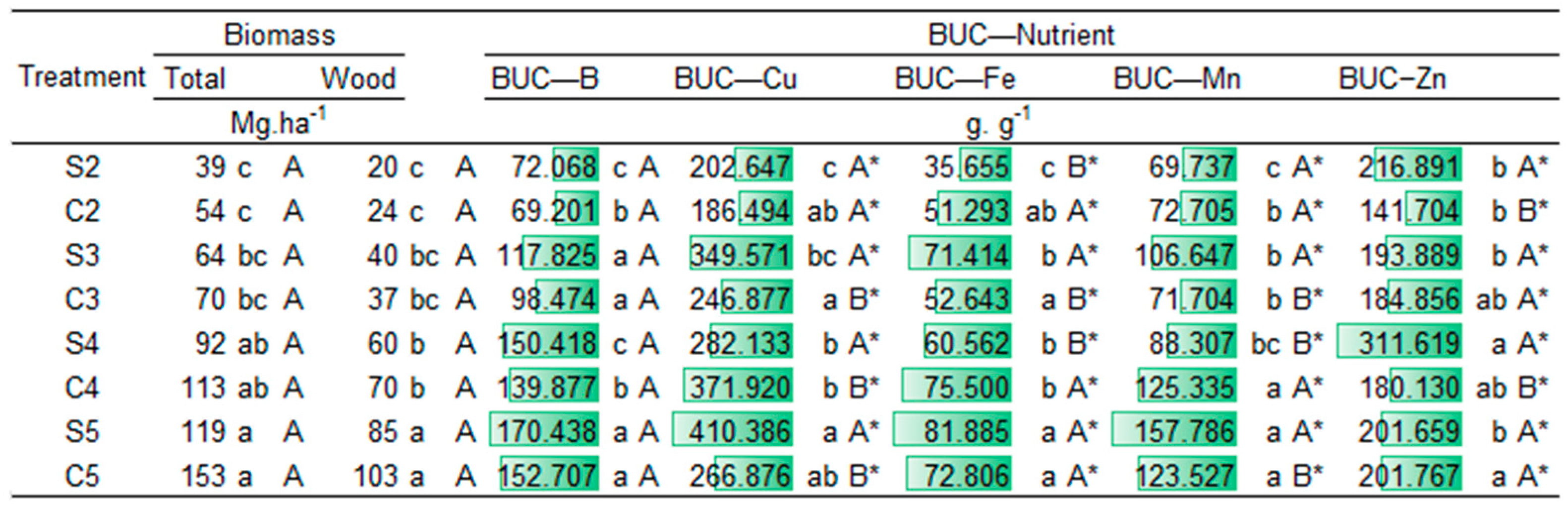
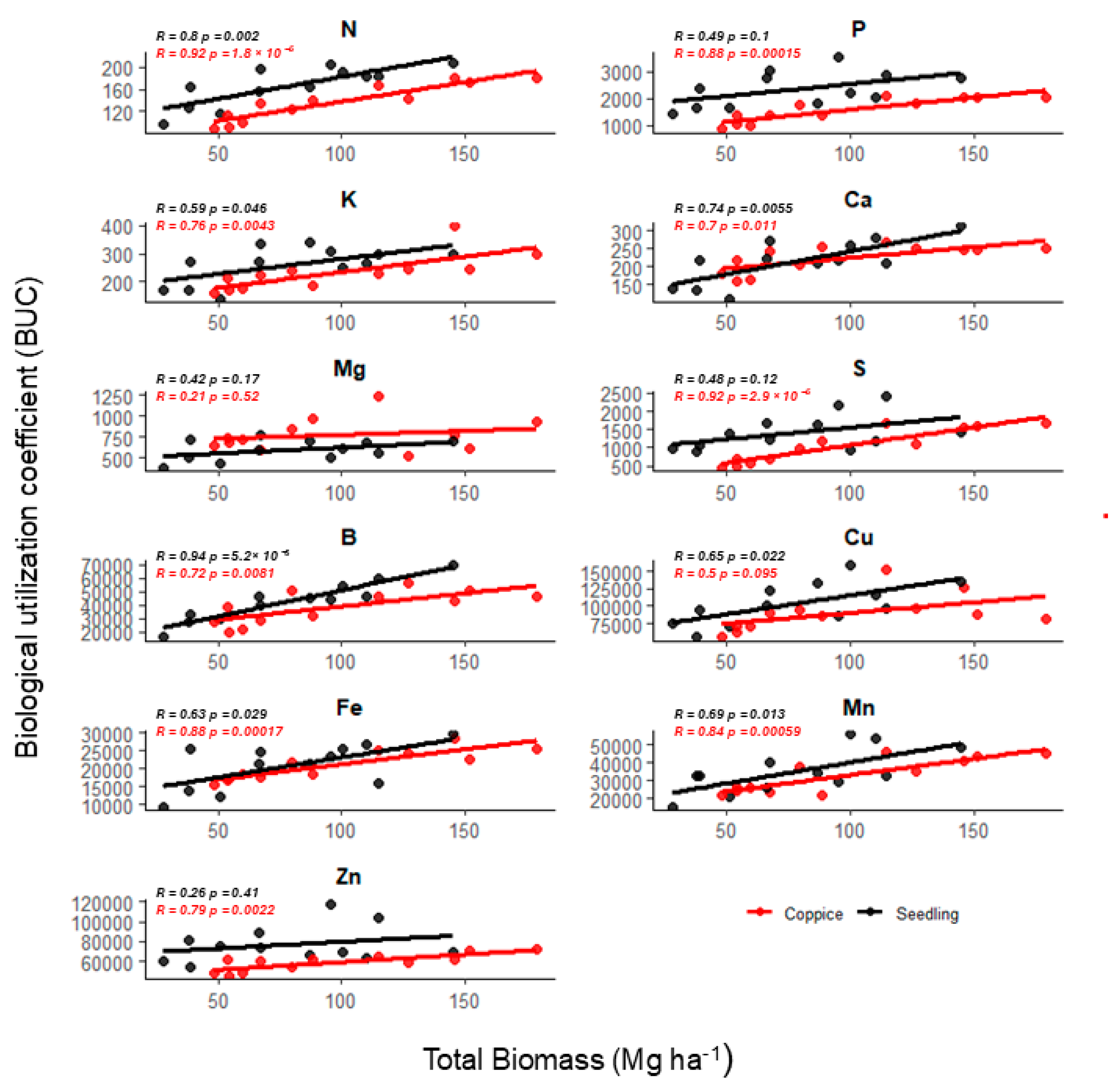
2.2. Nutritional Demand
2.3. Carbon Stock
3. Discussion
4. Materials and Methods
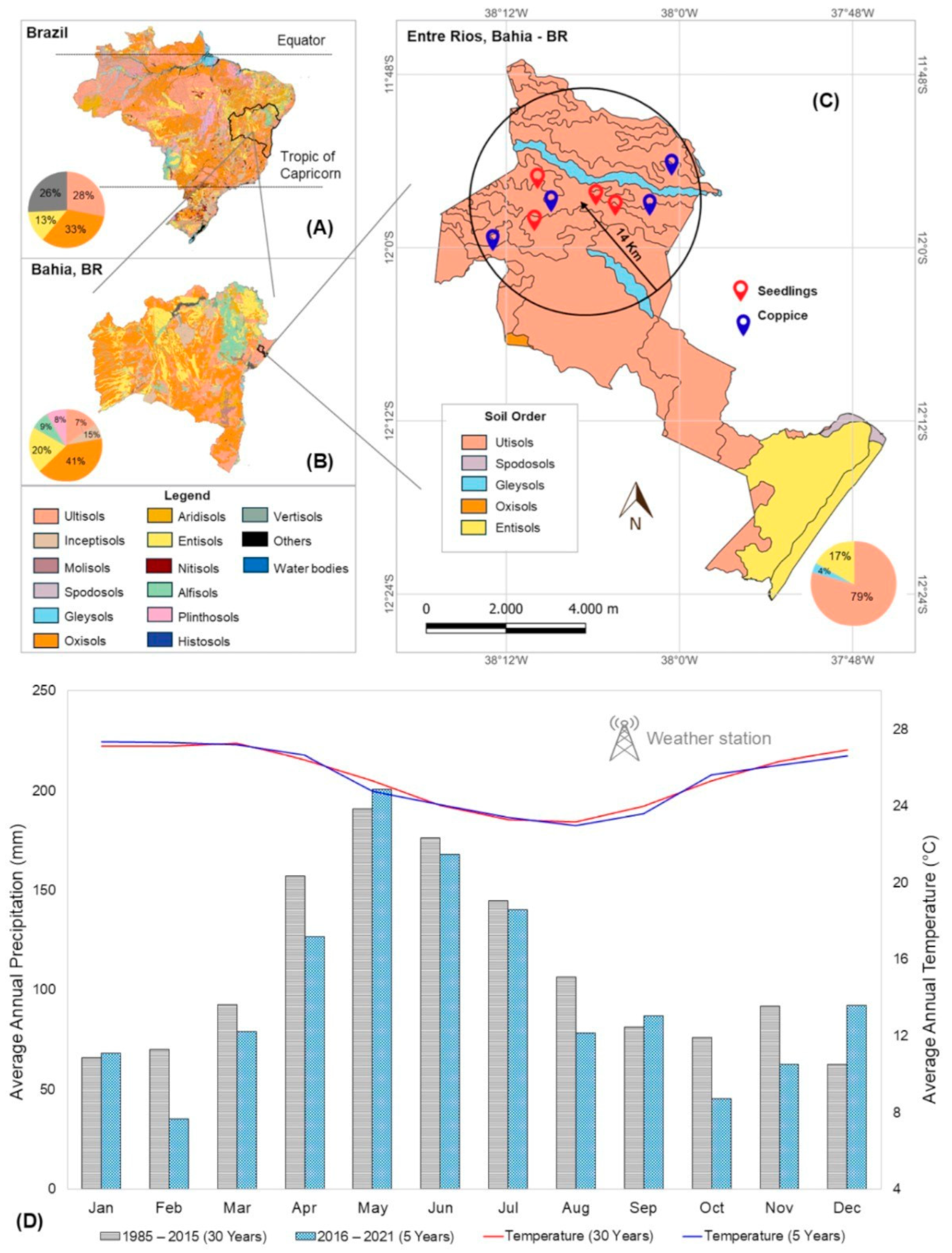
4.1. Study Area
4.2. Experimental Design and Treatments
4.3. Aerial Biomass
4.4. Root System Biomass
- Y = biomass, in Kg
- β0 and β1 = coefficients of the models.
- h = height of the individuals.
4.5. Biological Utilization Coefficient
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Eucalipto en la Repoblación Forestal; Organização das Nações Unidas Para a Agricultura e a Alimentação: Roma, Italy, 1981. [Google Scholar]
- Stape, J.L. Planejamento global e normatização de procedimentos operacionais da talhadia simples em Eucalyptus. Série Téc. IPEF 1997, 11, 51–62. [Google Scholar]
- Bouvet, J.M.; Saya, A.; Vigneron, P. Performance of coppices of Eucalyptus hybrid clones compared to seedling plantations in Congo. Ann. For. Sci. 2005, 62, 681–688. [Google Scholar]
- de Moraes Gonçalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; de Barros Ferraz, S.F.; de Paula Lima, W.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Zanetti, S.S.; Silva, L.D.; Silva, J.C.G.; Gonçalves, J.L.M.; Moreira, R.M.; Hubner, A. Productivity and nutrient cycling in a second rotation Eucalyptus plantation in Brazil managed by coppice. J. For. Res. 2017, 28, 1277–1287. [Google Scholar]
- Reis, G.G.; Reis, M.G.F. Fisiologia da brotação de eucalipto com ênfase nas suas relações hídricas. Série Téc. IPEF 1997, 11, 9–22. [Google Scholar]
- Arthur Junior, J.C.; Bazani, J.H.; Hakamada, R.E.; Rocha, J.H.T.; Melo, E.A.S.C.; Gonçalves, J.L.M. Considerações finais: Avanços nas práticas silviculturais no manejo da brotação com enfoque no aumento da produtividade e na redução de custos. Série Téc. IPEF 2015, 21, 75–79. [Google Scholar]
- Rocha, J.H.T.; Venturin, N.; Gonçalves, J.L.M.; Couto, H.T.Z.; Souza, A.N.; Albarelo, C.L. Relação entre fertilidade do solo e produtividade de Eucalyptus spp. em áreas de talhadia. Rev. Árvore 2019, 43, e430105. [Google Scholar]
- Santana, R.C.; Alvares, C.A.; Gonçalves, J.L.M.; Moreira, A.M.; Silva, L.D.; Rocha, J.H.T. Impactos da colheita operacional na rebrota e na produtividade de talhadia em plantações de eucalipto. New For. 2022, 53, 249–265. [Google Scholar]
- Binkley, D.; Stape, J.L.; Ryan, M.G.; Resende, G.D.; Eubanks, S. Crescimento e nutrição de plantações de eucalipto em uma ampla gama de solos no Brasil. Ecol. Manag. Florest. 1997, 83, 91–97. [Google Scholar]
- Gonçalves, J.L.M.; Stape, J.L.; Benedetti, V.; Fessel, V.A.G.; Gava, J. Reflexos do cultivo mínimo e intensivo do solo em sua fertilidade e na nutrição das árvores. In Nutrição e Fertilização Florestal, 1st ed.; Gonçalves, J.L.M., Benedetti, V., Eds.; IPEF: Piracicaba, Brazil, 2000; pp. 1–58. [Google Scholar]
- Gonçalves, J.L.M.; Alvares, C.A.; Rocha, J.H.T.; Gava, J.L.; Leite, F.P.; Souza, V.C.; Hubner, A.; Bouillet, J.P. Produtividade e ciclagem de nutrientes em plantações de eucalipto estabelecidas em diferentes solos tropicais. Rev. Bras. Ciência Solo 2014, 38, 1216–1226. [Google Scholar] [CrossRef]
- Barros, N.F. Sustentabilidade da produtividade em plantações de eucalipto. Rev. Madeira 2022, 33, 24–32. [Google Scholar]
- Camargo, M.L.P.; Moraes, C.B.; Mori, E.S.; Guerrini, I.A.; Mello, E.J.; Oda, S. Considerações sobre eficiência nutricional em Eucalyptus. Científica 2004, 32, 191–196. [Google Scholar]
- Queiroz, T.B.; Campoe, O.C.; Montes, C.R.; Alvares, C.A.; Cuartas, M.Z.; Guerrini, I.A. Temperature thresholds for Eucalyptus genotypes growth across tropical and subtropical ranges in South America. For. Ecol. Manag. 2020, 472, 118248. [Google Scholar] [CrossRef]
- Laclau, J.P.; Bouillet, J.P.; Gonçalves, J.L.M.; Silva, E.V.; Jourdan, C.; Cunha, M.C.S.; Moreira, R.M.; Saint-André, L.; Maquère, V.; Nouvellon, Y.; et al. Alterações na alocação de carbono e nutrientes durante a fase inicial do estabelecimento de povoamentos de eucalipto de crescimento rápido no Brasil. Planta Solo 2010, 330, 3–18. [Google Scholar]
- Barros, W.T. Crescimento, Biomassa e Eficiência Nutricional de Híbridos de Eucalipto na Região Sudoeste da Bahia. Master’s Thesis, Universidade Estadual do Sudoeste da Bahia, Campus de Vitória da Conquista, Bahia, Brazil, 2021. [Google Scholar]
- Laclau, J.P.; Le Maire, G.; Bernard, F.; Laroche, B.; Brevet, J.L. Dynamics of eucalyptus plantations and carbon sequestration in the soil and in plant compartments. For. Ecol. Manag. 2013, 310, 166–177. [Google Scholar]
- Gonçalves, J.L.M.; Mattos, P.P.; Santos, J.A.F.; Souza, M.P.; Silva, F.F. Biomassa de raízes grossas em plantação de Eucalyptus grandis em Itatinga, São Paulo. Rev. Árvore 2012, 36, 453–461. [Google Scholar]
- Eufrade-Junior, J.P.; Gomes, F.P.; Basso, A.M. Qualidade do combustível da biomassa de eucalipto em sistemas de rotação curta. Sci. For. 2018, 46, 13–20. [Google Scholar]
- Silva, E.P.; Pereira, M.G.; Carvalho, L.A. Efeito do manejo de talhadia sobre a permeabilidade do solo e o crescimento das raízes de eucalipto. Rev. Bras. Ciênc. Solo 2017, 41, 157–167. [Google Scholar]
- Gatto, D.A.; Lima, J.S.; Carvalho, G.S. Distribuição de biomassa em uma plantação de híbrido de Eucalyptus urophylla x E. grandis no Distrito Federal. Rev. Bras. Ciênc. Ambient. 2014, 47, 23–34. [Google Scholar]
- Reis, R.S.; Barros, N.F. Distribuição de biomassa em plantação de Eucalyptus grandis. Rev. Árvore 1990, 14, 74–80. [Google Scholar]
- Wang, F.; Liu, Y.; Wang, X. Impact of climate variability on biomass production in eucalyptus plantations. Glob. Change Biol. 2008, 14, 92–104. [Google Scholar]
- Resquin, F.; Laclau, J.P.; Martinez, J.F. Impact of soil and climate on biomass production in Eucalyptus forests: A regional perspective. Tree Physiol. 2019, 39, 963–974. [Google Scholar]
- Cavalli, R.M.; Oliveira, P.P.; Silva, T.M. Effects of climatic and soil conditions on biomass accumulation in Eucalyptus plantations. For. Ecol. Manag. 2020, 477, 118–127. [Google Scholar]
- Rocha, D.M.; Martins, S.J.; Santos, R.F. Biomass accumulation in relation to soil characteristics in Eucalyptus plantations. For. Syst. 2020, 29, eFS3036. [Google Scholar]
- Gonçalves, J.L.M. Root biomass production in Eucalyptus plantations under varying nutritional and water stress conditions. For. Ecol. Manag. 1994, 64, 143–152. [Google Scholar]
- Hakamada, R.I.; Souza, R.M.; Pereira, C.S. Influence of light use efficiency on tree uniformity and productivity in Eucalyptus plantations. For. Syst. 2022, 31, eFS4012. [Google Scholar]
- Schumacher, T.; Müller, U.; Weiss, M. Biomass partitioning and growth patterns in the early stages of tree development. For. Ecol. Manag. 2011, 261, 789–798. [Google Scholar]
- Frantz, D.A. Biomass accumulation and carbon partitioning in young tree plantations: Implications for forest management. For. Sci. 2016, 62, 345–353. [Google Scholar]
- Witschoreck, D.A. The biological utilization coefficient in wood biomass production systems. For. Stud. 2008, 41, 112–124. [Google Scholar]
- Bazani, J.H. Eficiência de Fertilizantes Fosfatados Solúveis e Pouco Solúveis, Com ou Sem Complexação Com Substâncias Húmicas, Em Plantações de Eucalipto. Master’s Thesis, Escola Superior de Agricultura “Luiz de Queiroz”, University of São Paulo (USP), Piracicaba, Brazil, 2014. [Google Scholar]
- Santana, M.M.; Barros, M.F.; Neves, L.M. Nutrient utilization efficiency and biomass production in forest systems: A comparative analysis. For. Ecosyst. Stud. 2022, 51, 77–89. [Google Scholar]
- Dias, A.S.; Silva, D.T.; Nascimento, D.O. Coppice systems as a more efficient nutrient recycling model: Evidence from magnesium uptake. Environ. For. Stud. 2023, 60, 23–32. [Google Scholar]
- Andrade, J.A.; Silva, L.A.; Silva, P.A. The role of potassium and boron in nutrient dynamics and growth of forest plantations. For. Ecol. Manag. 2023, 541, 118–130. [Google Scholar]
- Rocha, M.F.; Silva, G.F.; Silva, R.J. Efficiency of nutrient use in biomass accumulation under different environmental conditions. Agrofor. Syst. 2015, 89, 657–668. [Google Scholar]
- Ryan, M.G.; Binkley, D.; Fownes, J.H. Age-Related Decline in Forest Productivity: Pattern and Process. Adv. Ecol. Res. 1997, 27, 213–262. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Eucalyptus Production and the Supply, Use and Efficiency of Use of Water, Light and Nitrogen Across a Geographic Gradient in Brazil. For. Ecol. Manag. 2004, 193, 17–31. [Google Scholar] [CrossRef]
- Laclau, J.P.; Bouillet, J.P.; Gonçalves, J.L.M.; Silva, E.V.; Jourdan, C.; Cunha, M.C.S.; Moreira, R.M.; Saint-André, L.; Maquère, V.; Nouvellon, Y. Mixed-species plantations can enhance resource capture and use efficiency. Funct. Ecol. 2010, 24, 479–492. [Google Scholar] [CrossRef]
- Bouvet, A.; Laclau, J.P.; Arnaud, M.; Bouillet, J.P. Nutrient Uptake and Dynamics in Eucalyptus Plantations. In Eucalyptus Plantations: Research, Management and Development; da Silva, P.H.M., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 97–126. [Google Scholar]
- Almeida, A.C.; Soares, J.V.; Landsberg, J.J.; Rezende, G.D. Growth and Water Balance of Eucalyptus Grandis Plantations in Brazil under Different Water and Nutrient Availability. For. Ecol. Manag. 2007, 252, 425–436. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 978-0123849052. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Almeida, F.A. Genotype-environment interactions and their impact on forest productivity. For. Genet. Breed. 2009, 15, 455–465. [Google Scholar]
- Saidelles, E.E.; Silva, M.F.; Pereira, A.C. Estimating nutrient requirements for sustainable forest productivity: The role of nutrient utilization efficiency. For. J. 2010, 79, 811–824. [Google Scholar]
- Binkley, D.; Gower, S.T.; Ryan, M.G.; Butnor, J.R.; Powers, R.F.; Fenn, M.E.; Jenkins, J.C.; Zak, D.R.; Mitchell, R.J.; Raich, J.W. Carbon storage in eucalyptus coppices: Implications for forest management and climate change mitigation. Glob. Change Biol. 2013, 19, 1054–1065. [Google Scholar]
- O’Brien, P.S.; Thornley, J.H.M.; McMurtrie, R.E.; Zhang, X.; van Oijen, M.; O’Neill, P.; King, D.; Whitehead, D.; Valinger, E.; Kessler, W. Coppicing systems for long-term carbon sequestration and forest resilience. Environ. Res. Lett. 2017, 12, 064018. [Google Scholar]
- Bouillet, J.P.; Dufour, J.; Dreyer, E.; Gross, P.; Roux, F.; Giguère, P.; Frossard, E.; Lemoine, D.; Vennetier, M.; Minnebo, P. Root biomass and its role in carbon sequestration in temperate forest ecosystems. For. Ecol. Manag. 2008, 255, 2244–2251. [Google Scholar]
- O’Connell, A.M.; Rutter, M.; Honnay, O.; McMullin, R.; Bozzi, F.; Taylor, R.; Jackson, R.; Villanueva, S.; Rojas, M.; Messier, C. Root diversity and soil stability in coppice-managed forests: Implications for ecosystem resilience. Soil Biol. Biochem. 2013, 59, 99–110. [Google Scholar]
- Ryan, M.G.; Binkley, D.; Green, D.W.; Simard, S.W.; Houghton, R.A.; Hwang, T. The role of root biomass in carbon storage in temperate forests. J. For. Ecol. Manag. 2010, 259, 1156–1167. [Google Scholar]
- Van der Werf, G.R.; Leip, A.; Sander, M.; Gregor, R.; Monni, S.; Pitkänen, E.; Ockenden, M.; Pignotti, R.; Deryng, D.; Van den Hoven, S. Forest management and carbon sequestration: Assessing the role of coppicing in the context of climate change. Glob. Change Biol. 2014, 20, 3532–3544. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M. Modeling monthly mean air temperature for Brazil. Theor. Appl. Clim. 2013, 113, 407–427. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análise de Solo, Plantas e Outros Materiais, 2nd ed.; Boletim Técnico, 5; Departamento de Solos, UFRGS: Porto Alegre, Brazil, 1995; p. 174. [Google Scholar]
- Schumacher, F.X.; Hall, F.A. Logarithmic expression of timber tree volume. J. Agric. Res. 1933, 47, 719–734. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; De Pascoal, M.M. Manual de Nutrição Mineral de Plantas, 2nd ed.; Ceres: São Paulo, Brazil, 1997; p. 374. [Google Scholar]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories. Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Corte, A.P.D.; Higashi, E.N.; Schumacher, M.V.; Vieira, M. Carbon stock estimation in the roots of Eucalyptus spp. plantations in southern Brazil. For. Ecol. Manag. 2012, 282, 122–130. [Google Scholar]
- Barros, N.F.; Kubota, T.; Vitti, G.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Ceres: São Paulo, Brazil, 1986; p. 274. [Google Scholar]


| Treatment | BEF | R | Wtunk | Waboveground | Wroot | Carbon in the Roots | ||
|---|---|---|---|---|---|---|---|---|
| kg | Mg ha−1 | |||||||
| S2 | 1.37 | 0.20 | 23,629 | 32,475 | 6588 | 5.12 | c | B |
| S3 | 1.18 | 0.20 | 45,340 | 53,716 | 10,760 | 7.19 | b | B |
| S4 | 1.14 | 0.19 | 68,491 | 77,776 | 14,461 | 9.15 | a | B |
| S5 | 1.08 | 0.17 | 93,780 | 101,714 | 16,820 | 9.99 | a | B |
| C2 | 1.27 | 0.55 | 27,520 | 35,072 | 19,120 | 17.70 | a | A |
| C3 | 1.16 | 0.45 | 41,678 | 48,209 | 21,785 | 17.20 | a | A |
| C4 | 1.10 | 0.29 | 79,601 | 87,948 | 25,498 | 17.08 | a | A |
| C5 | 1.09 | 0.22 | 114,701 | 124,972 | 27,560 | 17.23 | a | A |
| Treatment | Depth | Coarse Sand | Thin Sand | Silt | Clay | M.O | pH | Al | Ca | Mg | K | Al + H | SB | V | m | P | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | g kg−1 | g dm−3 | mmolc dm−3 | % | mg dm−3 | ||||||||||||||||
| S2 | 0–20 | 420 | 194 | 111 | 275 | 23 | 5.0 | 0.23 | 26 | 3.5 | 0.68 | 28 | 30 | 52 | 0.7 | 8 | 0.41 | 1.0 | 40 | 1.67 | 0.47 |
| 20–40 | 421 | 184 | 111 | 283 | 18 | 4.9 | 0.85 | 21 | 3.2 | 0.54 | 31 | 24 | 43 | 3.8 | 6 | 0.47 | 1.0 | 31 | 0.97 | 0.27 | |
| C2 | 0–20 | 445 | 244 | 95 | 217 | 20 | 4.9 | 1.38 | 12 | 4.1 | 0.67 | 35 | 17 | 33 | 7.2 | 9 | 0.50 | 0.7 | 35 | 0.47 | 0.53 |
| 20–40 | 435 | 211 | 104 | 250 | 16 | 4.6 | 4.07 | 8 | 3.9 | 0.39 | 44 | 13 | 22 | 23.8 | 5 | 0.47 | 0.9 | 21 | 0.20 | 0.27 | |
| S3 | 0–20 | 483 | 276 | 74 | 167 | 15 | 5.0 | 0.98 | 10 | 2.9 | 0.55 | 32 | 13 | 29 | 6.9 | 6 | 0.44 | 0.7 | 21 | 0.53 | 0.27 |
| 20–40 | 459 | 210 | 97 | 233 | 14 | 4.8 | 2.03 | 11 | 4.6 | 0.63 | 44 | 16 | 27 | 11.6 | 5 | 0.36 | 0.8 | 18 | 0.27 | 0.17 | |
| C3 | 0–20 | 522 | 273 | 71 | 133 | 13 | 4.9 | 0.98 | 12 | 3.5 | 0.53 | 41 | 16 | 28 | 6.4 | 5 | 0.27 | 0.7 | 31 | 0.93 | 0.40 |
| 20–40 | 516 | 264 | 70 | 150 | 8 | 4.8 | 1.61 | 9 | 3.7 | 0.41 | 41 | 13 | 24 | 9.9 | 4 | 0.31 | 0.8 | 27 | 0.37 | 0.17 | |
| S4 | 0–20 | 344 | 205 | 126 | 325 | 20 | 5.1 | 0.63 | 22 | 5.6 | 1.07 | 30 | 29 | 49 | 2.8 | 21 | 0.71 | 1.2 | 38 | 1.17 | 0.80 |
| 20–40 | 347 | 192 | 120 | 342 | 13 | 4.8 | 2.53 | 16 | 4.0 | 0.78 | 38 | 21 | 35 | 13.0 | 11 | 0.67 | 1.0 | 24 | 0.50 | 0.37 | |
| C4 | 0–20 | 448 | 237 | 98 | 217 | 17 | 5.2 | 0.00 | 20 | 6.2 | 0.48 | 24 | 27 | 52 | 0.0 | 8 | 0.34 | 0.9 | 21 | 1.13 | 0.33 |
| 20–40 | 427 | 218 | 105 | 250 | 11 | 4.8 | 2.33 | 9 | 3.7 | 0.47 | 46 | 13 | 22 | 15.1 | 7 | 0.46 | 0.7 | 14 | 0.30 | 0.13 | |
| S5 | 0–20 | 338 | 179 | 141 | 342 | 23 | 4.9 | 0.69 | 25 | 6.5 | 1.60 | 34 | 33 | 49 | 2.2 | 16 | 0.49 | 1.5 | 38 | 0.83 | 0.60 |
| 20–40 | 364 | 122 | 139 | 375 | 18 | 4.8 | 1.81 | 15 | 4.5 | 1.29 | 37 | 21 | 36 | 8.1 | 11 | 0.64 | 1.1 | 24 | 0.67 | 0.23 | |
| C5 | 0–20 | 445 | 238 | 92 | 225 | 17 | 4.9 | 0.92 | 21 | 5.0 | 0.65 | 32 | 27 | 45 | 3.6 | 10 | 0.33 | 0.7 | 24 | 0.60 | 0.33 |
| 20–40 | 415 | 180 | 114 | 292 | 16 | 4.9 | 1.87 | 14 | 4.4 | 0.50 | 33 | 19 | 36 | 12.1 | 6 | 0.54 | 0.8 | 18 | 0.30 | 0.17 | |
| Average | 0–20 | 431 | 231 | 101 | 238 | 18 | 5.0 | 0.73 | 18 | 4.7 | 0.78 | 32 | 24 | 42 | 3.7 | 10 | 0.44 | 0.9 | 31 | 0.92 | 0.47 |
| 20–40 | 423 | 198 | 108 | 272 | 14 | 4.8 | 2.14 | 13 | 4.0 | 0.63 | 39 | 17 | 31 | 12.2 | 7 | 0.49 | 0.9 | 22 | 0.45 | 0.22 | |
| General Average | 0–40 | 427 | 214 | 104 | 255 | 16 | 4.9 | 1.43 | 16 | 4.3 | 0.70 | 36 | 21 | 36 | 8.0 | 9 | 0.46 | 0.9 | 26 | 0.68 | 0.34 |
| Standard Deviation | 0–40 | 55 | 17 | 21 | 68 | 4.0 | 0.1 | 1.0 | 5.8 | 1.0 | 0.3 | 6.1 | 6.6 | 10.3 | 6.0 | 4.2 | 0.1 | 0.2 | 7.8 | 0.4 | 0.2 |
| CV% | 0–40 | 13% | 8% | 20% | 27% | 24% | 3% | 69% | 37% | 23% | 47% | 17% | 32% | 28% | 75% | 49% | 27% | 24% | 30% | 58% | 51% |
| Treatment | Silvicultural Operations | Fertilizer Formulation | Quantity (Kg) |
|---|---|---|---|
| S2 | Liming | Dolomitic limestone | 1500 |
| base fertilization | NPK 06-30-06 + Micro | 250 | |
| Topdressing fertilization | NPK 10-00-30 + Micro | 250 | |
| Maintenance fertilization | NPK 09-00-30 + 0.75 B | 300 | |
| C2 | Liming | Dolomitic limestone | 1500 |
| Topdressing fertilization | NPK 10-08-22 + Micro | 600 | |
| S3 | Liming | Dolomitic limestone | 2000 |
| base fertilization | NPK 06-30-06 + Micro | 200 | |
| Topdressing fertilization | NPK 10-08-22 + Micro | 250 | |
| Maintenance fertilization | NPK 10-00-30 + Micro | 400 | |
| C3 | Liming | Dolomitic limestone | 1000 |
| Topdressing fertilization | NPK 10-08-22 + Micro | 600 | |
| Additional fertilization | coated urea + 0.5% B | 150 | |
| S4 | Liming | Dolomitic limestone | 1500 |
| base fertilization | NPK 06-30-06 + Micro | 200 | |
| Topdressing fertilization | NPK 10-00-30 + Micro | 200 | |
| Maintenance fertilization | NPK 10-00-30 + Micro | 350 | |
| Additional fertilization | reactive natural phosphate | 300 | |
| Booster fertilization | NPK 06-30-06 + Micro | 30 | |
| C4 | Liming | Dolomitic limestone | 1500 |
| Topdressing fertilization | NPK 08-12-25 + Micro | 600 | |
| S5 | Liming | Dolomitic limestone | 1500 |
| base fertilization | NPK 06-30-06 + Micro | 200 | |
| Topdressing fertilization | NPK 10-00-30 + Micro | 200 | |
| Maintenance fertilization | NPK 10-00-30 + Micro | 300 | |
| C5 | Liming | Dolomitic limestone | 1500 |
| Topdressing fertilization | NPK 10-08-22 + Micro | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, G.G.; Queiroz, T.B.; Bullock, B.P.; Coelho, J.C.; Hakamada, R.E.; Guerrini, I.A. Full-Tree Biomass, Root Carbon Stock, and Nutrient Use Efficiency Across Ages in Eucalyptus Stands Under Seedling and Coppice Systems. Plants 2025, 14, 1382. https://doi.org/10.3390/plants14091382
de Oliveira GG, Queiroz TB, Bullock BP, Coelho JC, Hakamada RE, Guerrini IA. Full-Tree Biomass, Root Carbon Stock, and Nutrient Use Efficiency Across Ages in Eucalyptus Stands Under Seedling and Coppice Systems. Plants. 2025; 14(9):1382. https://doi.org/10.3390/plants14091382
Chicago/Turabian Stylede Oliveira, Gardenia Gonçalves, Túlio Barroso Queiroz, Bronson P. Bullock, José Carlos Coelho, Rodrigo Eiji Hakamada, and Iraê Amaral Guerrini. 2025. "Full-Tree Biomass, Root Carbon Stock, and Nutrient Use Efficiency Across Ages in Eucalyptus Stands Under Seedling and Coppice Systems" Plants 14, no. 9: 1382. https://doi.org/10.3390/plants14091382
APA Stylede Oliveira, G. G., Queiroz, T. B., Bullock, B. P., Coelho, J. C., Hakamada, R. E., & Guerrini, I. A. (2025). Full-Tree Biomass, Root Carbon Stock, and Nutrient Use Efficiency Across Ages in Eucalyptus Stands Under Seedling and Coppice Systems. Plants, 14(9), 1382. https://doi.org/10.3390/plants14091382










