Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography
Abstract
1. Introduction
2. Results
2.1. Proximate Phytochemical Composition
2.2. UHPLC-QToF-MS Analysis of Phenolic Compounds
2.3. UHPLC Q-ToF MS Analysis of Other (Non-Phenolic) Bioactive Compounds
2.4. Antioxidant Properties
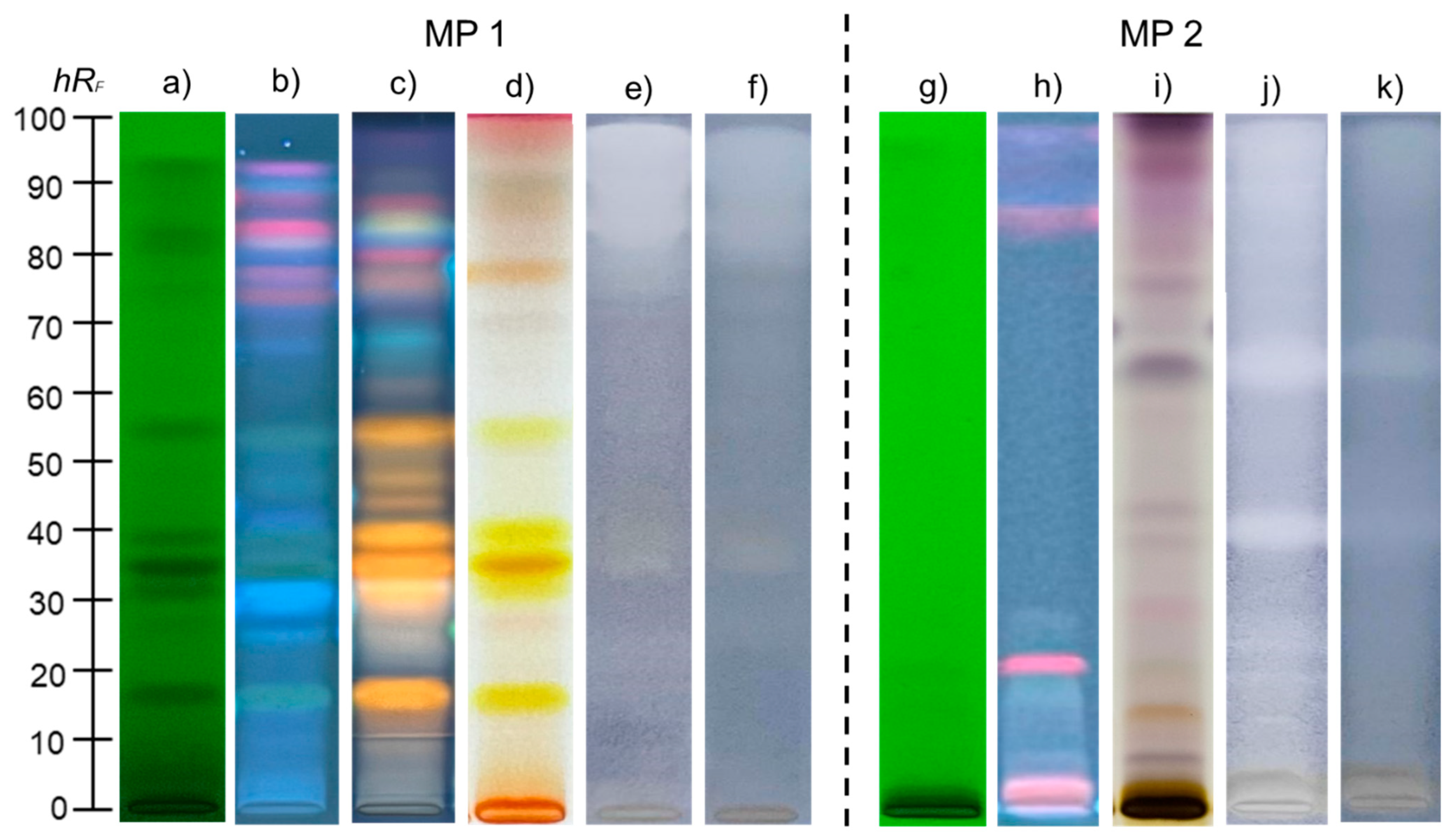
2.5. HPTLC Fingerprinting and Chemical Profile of H. Perforatum Methanolic Extract
2.6. HPTLC Antibacterial Activity of H. perforatum Methanolic Extract
3. Discussion
3.1. Spectrophotometric Characterization of H. perforatum Tea Infusion and Methanolic Extract
3.2. UHPLC-QToF Characterization of Bioactive Compounds Derived from H. perforatum
3.3. HPTLC Phytochemical Fingerprinting of Methanolic H. perforatum Extract
3.4. HPTLC Antibacterial Activity of Methanolic H. perforatum Extract
4. Materials and Methods
4.1. Chemicals and Materials Used for Analyses
4.2. Plant Material and Extraction Protocol for Spectrophotometric Analysis
4.3. Spectrophotometric Determination of Proximate Phytochemical Composition and Antioxidant Activity
4.4. UHPLC Q-ToF MS Analysis of Bioactive Compounds
4.5. HPTLC Analysis
4.6. Antibacterial Assays
4.7. Image Processing and Data Acquisition
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| TPC | Total phenolic content |
| HCAs | Total dyhydroxicinnamic acid derivative content |
| GAE | Gallic acid |
| CGAE | Chlorogenic acid equivalent |
| TAE | Tannic acid equivalent |
| ABTS⦁+ | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation |
| DPPH⦁ | 2,2-diphenylpicrylhydrazyl radical |
| TAC | Total antioxidant capacity determined via in vitro phosphomolybdenum assay |
| FRP | Ferric reducing power |
| CUPRAC | Cupric reducing antioxidant capacity |
| AAE | Ascorbic acid equivalent |
| StrpE | Streptomycin equivalent |
Appendix A
| Bacterial Strain | Regression Equation | R2 | Linear Range (μg) | LOD (μg) | LOQ (μg) |
|---|---|---|---|---|---|
| K. pneumoniae | y = 406141x + 3222862 | 0.993 | 10–45 | 3.8 | 11.4 |
| S. aureus | y = 623224x − 650809 | 0.994 | 10–45 | 3.4 | 10.2 |
References
- Ranđelović, N.; Avramović, D. Protected nature areas, flora, and vegetation in the vicinity of Sokobanja (Serbia and Montenegro). Nat. Montenegrina 2004, 3, 379–386. [Google Scholar]
- Zlatković, B.K.; Bogosavljević, S.S.; Radivojević, A.R.; Pavlović, M.A. Traditional use of the native medicinal plant resource of Mt. Rtanj (Eastern Serbia): Ethnobotanical evaluation and comparison. J. Ethnopharmacol. 2014, 151, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Stanković Jeremić, J.; Miljković, A.; Rat, M.; Lončar, B. Screening of volatile compounds, traditional and modern phytotherapy approaches of selected non-aromatic medicinal plants (Lamiaceae, Lamioideae) from Rtanj Mountain, Eastern Serbia. Molecules 2023, 28, 4611. [Google Scholar] [CrossRef]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional uses of autochthonous medicinal and ritual plants and other remedies for health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef] [PubMed]
- Simić, M.N. Ethnopharmacological use of St. John’s wort (Hypericum perforatum) on Rujan Mountain (southeastern Serbia). Etnobotanika 2024, 4, 31–58. [Google Scholar] [CrossRef]
- Dajić Stevanović, Z.; Petrović, M.; Aćić, S. Ethnobotanical knowledge and traditional use of plants in Serbia in relation to sustainable rural development. In Ethnobotany and Biocultural Diversities in the Balkans; Springer: New York, NY, USA, 2014; pp. 229–252. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef]
- Sharpley, A.L.; McGavin, C.L.; Whale, R.; Cowen, P.J. Antidepressant-like effect of Hypericum perforatum (St John’s wort) on the sleep polysomnogram. Psychopharmacology 1998, 139, 286–287. [Google Scholar] [CrossRef]
- Brondz, I.; Brondz, A. Recent enhancement of the immunity in AIDS and other immunocompromised patients by hyperforin, an antibiotic from Hypericum perforatum L. (in vitro model) part I. J. Biophys. Chem. 2012, 03, 304–310. [Google Scholar] [CrossRef]
- de Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef]
- Colovic, M.; Caccia, S. Liquid chromatography–tandem mass spectrometry of I3,II8-biapigenin, the major biflavone in Hypericum perforatum extracts. J. Chromatogr. B 2008, 863, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [PubMed]
- Piperopoulos, G.; Lotz, R.; Wixforth, A.; Schmierer, T.; Zeller, K.-P. Determination of naphthodianthrones in plant extracts from Hypericum perforatum L. by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1997, 695, 309–316. [Google Scholar] [CrossRef]
- Hecka, A.; Maunit, B.; Aubriet, F.; Muller, J. Study of active naphthodianthrone St. John’s Wort compounds by electrospray ionization Fourier transform ion cyclotron resonance and multi-stage mass spectrometry in sustained off-resonance irradiation collision-induced dissociation and infrared multiphoton dissociation modes. Rapid Commun. Mass Spectrom. 2009, 23, 885–898. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Butterweck, V. Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry 1997, 30, 129–134. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Todorovska, I.; Stanoeva, J.P.; Simic, S.G. Photoperiod modulates the production of biologically active compounds in Hypericum perforatum L. hairy roots: An in vitro and in silico approach. Plant Cell Tissue Organ Cult. 2024, 156, 96. [Google Scholar] [CrossRef]
- Kucharíková, A.; Kimáková, K.; Janfelt, C.; Čellárová, E. Interspecific variation in localization of hypericins and phloroglucinols in the genus Hypericum as revealed by desorption electrospray ionization mass spectrometry imaging. Physiol. Plant 2016, 157, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Cvejić, J.; Stilinović, N.; Goločorbin-Kon, S.; Vukmirović, S.; Mimica-Dukić, N.; Mikov, M. Interaction between different extracts of Hypericum perforatum L. from Serbia and pentobarbital, diazepam, and paracetamol. Molecules 2014, 19, 3869–3882. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Qi, C.; Tong, Q.; Chen, C.; Yang, J.; Zhu, H.; Zhang, Y. Polycyclic polyprenylated acylphloroglucinols with immunosuppressive activity from Hypericum perforatum and absolute configurations assignment of previously reported analogues. Bioorg. Chem. 2021, 114, 105144. [Google Scholar] [CrossRef]
- China National Intellectual Property Administration; Zhang, W.; Xu, J.; He, J.; Pan, X.; Ding, K.; Li, X. Novel Antidepressant Phloroglucinol Compounds, Their Preparation Method, and Application. Patent Number CN 118754864 A, 11 October 2024. [Google Scholar]
- Lou, H.; Yi, P.; Hu, Z.; Li, Y.; Zeng, Y.; Gu, W.; Huang, L.; Yuan, C.; Hao, X. Polycyclic polyprenylated acylphloroglucinols with acetylcholinesterase inhibitory activities from Hypericum perforatum. Fitoterapia 2020, 143, 104550. [Google Scholar] [CrossRef]
- Pan, X.-G.; Li, X.-X.; Xia, C.-Y.; Yin, W.-F.; Ding, K.; Zuo, G.-Y.; Wang, M.-N.; Zhang, W.-K.; He, J.; Xu, J.-K. New polycyclic polyprenylated acylphloroglucinols with antidepressant activities from Hypericum perforatum L. Bioorg. Chem. 2024, 151, 107657. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Gao, L.-L.; Zhou, W.; Ma, Y.-N.; Tian, J.-M.; Gao, J.-M. Hypertums A−J, bioactive polycyclic polyprenylated acylphloroglucinols with diverse skeletons from Hypericum perforatum L. (St. John’s wort). Phytochemistry 2025, 235, 114450. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.; Stefova, M.; Kungulovski, D.; Pancevska, N.; Sekulovski, N.; Panov, S.; Simic, S. Hairy roots of Hypericum perforatum L. : A promising system for xanthone production. Open Life Sci. 2013, 8, 1010–1022. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Nedialkov, P.T. Mangiferin and isomangiferin in some Hypericum species. Syst. Ecol. 1998, 26, 647–653. [Google Scholar] [CrossRef]
- Jürgenliemk, G.; Nahrstedt, A. Phenolic compounds from Hypericum perforatum. Planta Med. 2002, 68, 88–91. [Google Scholar] [CrossRef]
- Kosuge, T.; Ishida, H.; Satoh, T. Studies on antihemorrhagic substances in herbs classified as hemostatics in Chinese medicine. IV. On antihemorrhagic principles in Hypericum erectum Thunb. Chem. Pharm. Bull. 1985, 33, 202–205. [Google Scholar] [CrossRef]
- Fuzzati, N.; Gabetta, B.; Strepponi, I.; Villa, F. High-performance liquid chromatography–electrospray ionization mass spectrometry and multiple mass spectrometry studies of hyperforin degradation products. J. Chromatogr. A 2001, 926, 187–198. [Google Scholar] [CrossRef]
- Liu, F.; Pan, C.; Drumm, P.; Ang, C.Y.W. Liquid chromatography–mass spectrometry studies of St. John’s wort methanol extraction: Active constituents and their transformation. J. Pharm. Biomed. Anal. 2005, 37, 303–312. [Google Scholar] [CrossRef]
- Sleno, L.; Daneshfar, R.; Eckert, G.P.; Müller, W.E.; Volmer, D.A. Mass spectral characterization of phloroglucinol derivatives hyperforin and adhyperforin. Rapid Commun. Mass Spectrom. 2006, 20, 2641–2648. [Google Scholar] [CrossRef]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 2022, 16, 64. [Google Scholar] [CrossRef]
- Tahirović, I.; Kožljak, M.; Toromanović, J.; Čopra-Janićijević, A.; Klepo, J.; Topčagić, A.; Demirović, H. Total phenolic content and antioxidant capacity in infusions of various herbal teas. Bull. Chem. Technol. Bosnia Herzegovina 2014, 42, 51–55. [Google Scholar]
- Yousefi, L. Impact of ultrasound pretreatment with different solvents on the antioxidant activity, phenolic, and flavonoid compounds of the St. John’s wort (Hypericum perforatum L.) extract. J. Food Sci. Technol. 2024, 20, 126–139. [Google Scholar] [CrossRef]
- Kovačević, S.; Popović, T. Effect of the vegetation cycle on total phenolic and flavonoid compounds in Hypericum perforatum L. and Melissa officinalis L. collected in Montenegro. Agric. Forest. 2021, 67, 15. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of major constituents of Hypericum perforatum L. extracts in Syria by development of a rapid, simple, and reproducible HPLC-ESI-Q-TOF MS analysis and their antioxidant activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities, and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.; Barreiro, M.; Ferreira, I. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges, and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Maleš, Ž.; Brantner, A.H.; Sović, K.; Hazler Pilepić, I.; Plazibat, M. Comparative phytochemical and antimicrobial investigations of Hypericum perforatum L. subsp. perforatum and H. perforatum subsp. angustifolium (DC.). Gaudin. Acta Pharm. 2006, 56, 259–367. [Google Scholar]
- Germ, M.; Stibilj, V.; Kreft, S.; Gaberščik, A.; Kreft, I. Flavonoid, tannin, and hypericin concentrations in the leaves of St. John’s wort (Hypericum perforatum L. ) are affected by UV-B radiation levels. Food Chem. 2010, 122, 471–474. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant activity and protecting health effects of common medicinal plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical characterization and antioxidant activity of nine Hypericum species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s Wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Yılmazoğlu, E.; Metin Hasdemir, İ.; Hasdemir, B.; Yaşa, H. Investigation of essential oil composition, hypericin content, and antioxidant capacity of different extracts from flowers and leaves of Hypericum perforatum L. growing wild in Turkey. J. Essent. Oil Bear. Plants 2023, 26, 1350–1370. [Google Scholar] [CrossRef]
- Ersoy, E.; Eroglu Ozkan, E.; Boga, M.; Mat, A. Evaluation of in vitro biological activities of three Hypericum species (H. calycinum, H. confertum, and H. perforatum) from Turkey. S. Afr. J. Bot. 2020, 130, 141–147. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Ion, V.; Ielciu, I.; Cârje, A.-G.; Muntean, D.L.; Crişan, G.; Păltinean, R. Hypericum spp.—An overview of the extraction methods and analysis of compounds. Separations 2022, 9, 17. [Google Scholar] [CrossRef]
- Velingkar, V.S.; Gupta, G.L.; Hegde, N.B. A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem. Rev. 2017, 16, 725–744. [Google Scholar] [CrossRef]
- Belwal, T.; Devkota, H.P.; Singh, M.K.; Sharma, R.; Upadhayay, S.; Joshi, C.; Bisht, K.; Gour, J.K.; Bhatt, I.D.; Rawal, R.S.; et al. St. John’s Wort (Hypericum perforatum). In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 415–432. [Google Scholar]
- Tusevski, O.; Krstikj, M.; Stanoeva, J.P.; Stefova, M.; Gadzovska Simic, S. Phenolic compounds composition of Hypericum perforatum L. wild-growing plants from the Republic of Macedonia. Agric. Conspec. Sci. 2019, 84, 89–94. [Google Scholar]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Zvezdanović, J. UHPLC–DAD–ESI–MS/MS characterization of St. John’s wort infusions from Serbia origin. Chem. Pap. 2022, 76, 1329–1347. [Google Scholar] [CrossRef]
- Beschasnyi, S.; Hasiuk, O. Carbon monoxide and their donor (CORM-2) change the healing rate of skin wound healing in mice through reduced expression of aquaporin-3. Fabad J. Pharm. Sci. 2023, 48, 1–10. [Google Scholar] [CrossRef]
- Ang, C.Y.W.; Hu, L.; Heinze, T.M.; Cui, Y.; Freeman, J.P.; Kozak, K.; Luo, W.; Liu, F.F.; Mattia, A.; DiNovi, M. Instability of St. John’s Wort (Hypericum perforatum L.) and degradation of hyperforin in aqueous solutions and functional beverages. J. Agric. Food Chem. 2004, 52, 6156–6164. [Google Scholar] [CrossRef] [PubMed]
- Koyu, H.; Haznedaroglu, M.Z. Investigation of impact of storage conditions on Hypericum perforatum L. dried total extract. J. Food Drug Anal. 2015, 23, 545–551. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Campone, L.; Dal Piaz, F.; Cuesta-Rubio, O.; Rastrelli, L. Fragmentation pathways of polycyclic polyisoprenylated benzophenones and degradation profile of nemorosone by multiple-stage tandem mass spectrometry. J. Am. Soc. Mass. Spectrom. 2009, 20, 1688–1698. [Google Scholar] [CrossRef][Green Version]
- Fotie, J.; Bohle, D. Pharmacological and biological activities of xanthones. Antiinfect. Agents Med. Chem. 2006, 5, 15–31. [Google Scholar] [CrossRef]
- Ha, N.M.; Hop, N.Q.; Son, N.T. Wedelolactone: A molecule of interest. Fitoterapia 2023, 164, 105355. [Google Scholar] [CrossRef] [PubMed]
- Romero Rocamora, C.; Ramasamy, K.; Meng Lim, S.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC-based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef]
- Jović, M.D.; Agatonovic-Kustrin, S.; Ristivojević, P.M.; Trifković, J.Đ.; Morton, D.W. Bioassay-Guided Assessment of Antioxidative, Anti-Inflammatory and Antimicrobial Activities of Extracts from Medicinal Plants via High-Performance Thin-Layer Chromatography. Molecules 2023, 28, 7346. [Google Scholar] [CrossRef]
- Lawag, I.L.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. The Development and Application of a HPTLC-Derived Database for the Identification of Phenolics in Honey. Molecules 2022, 27, 6651. [Google Scholar] [CrossRef]
- Khan, I.A.; Jadhav, A.N. High Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants, Eike Reich, Anne Schibli (Eds.), Thieme Medical Publishers Inc., New York (2007), 264pp., US$149.95, Hard cover, ISBN: 978-1-58890-409-6. Phytomedicine 2008, 15, 781. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Wong, S.; Dolzhenko, A.V.; Gegechkori, V.; Ku, H.; Tucci, J.; Morton, D.W. Evaluation of bioactive compounds from Ficus carica L. leaf extracts via high-performance thin-layer chromatography combined with effect-directed analysis. J. Chromatogr. A 2023, 1706, 464241. [Google Scholar] [CrossRef] [PubMed]
- Raclariu, A.C.; Paltinean, R.; Vlase, L.; Labarre, A.; Manzanilla, V.; Ichim, M.C.; Crisan, G.; Brysting, A.K.; de Boer, H. Comparative authentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS. Sci. Rep. 2017, 7, 1291. [Google Scholar] [CrossRef]
- Scotti, F.; Löbel, K.; Booker, A.; Heinrich, M. St. John’s Wort (Hypericum perforatum) products—How variable is the primary material? Front. Plant Sci. 2019, 9, 1973. [Google Scholar] [CrossRef]
- Rychlewski, P.; Kamgar, E.; Mildner-Szkudlarz, S.; Kowalczewski, P.Ł.; Zembrzuska, J. Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples. Open Chem. 2023, 21, 20220347. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae biofilms and their role in disease pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Mironova, A.V.; Karimova, A.V.; Bogachev, M.I.; Kayumov, A.R.; Trizna, E.Y. Alterations in antibiotic susceptibility of Staphylococcus aureus and Klebsiella pneumoniae in dual species biofilms. Int. J. Mol. Sci. 2023, 24, 8475. [Google Scholar] [CrossRef]
- Jović, M.; Ristivojević, P.; Živković-Radovanović, V.; Andrić, F.; Dimkić, I.; Milojković-Opsenica, D.; Trifković, J. Statistical analysis-based green planar chromatographic methodology for the quality assessment of food supplements: A case study on Origanum vulgare L. commercial products. JPC–J. Planar Chromatogr.–Mod. TLC 2023, 36, 493–502. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, antimicrobial evaluation, and structure-activity relationship of α-pinene derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Spiteller, M.; Ligon, A.P.; Smelcerovic, Z.; Raabe, N. Essential oil composition of Hypericum L. species from Southeastern Serbia and their chemotaxonomy. Biochem. Syst. Ecol. 2007, 35, 99–113. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Kakouri, E.; Daferera, D.; Trigas, P.; Charalambous, D.; Pantelidou, M.; Tarantilis, P.A.; Kanakis, C.D. Comparative study of the antibacterial activity, total phenolic and total flavonoid content of nine Hypericum species grown in Greece. Appl. Sci. 2023, 13, 3305. [Google Scholar] [CrossRef]
- Avato, P.; Raffo, F.; Guglielmi, G.; Vitali, C.; Rosato, A. Extracts from St John’s wort and their antimicrobial activity. Phytother. Res. 2004, 18, 230–232. [Google Scholar] [CrossRef]
- Kilibarda, S.N.; Vuković, S.Z.; Milinčić, D.D.; Mačukanović-Jocić, M.P.; Jarić, S.; Kostić, A.Ž. Phytochemical and antioxidant properties of Athamanta turbith (L.) Brot collected from Serbia. Biol. Life Sci. Forum 2022, 11, 30. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification, and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, C.; Jin, Z.; Xu, X.; Cai, Y.; Bai, Y. HPTLC-bioautography/SERS screening nifedipine adulteration in food supplement based on Ginkgo biloba. Microchem. J. 2020, 154, 104647. [Google Scholar] [CrossRef]

| Analysis | Infusion Tea (ATI) | Methanolic Extract (MW) |
|---|---|---|
| TPC (mg/g GAE DW) * | 26.48 ± 0.96 b | 31.38 ± 0.52 a |
| HCAs (mg/g CGAE DW) | 3.28 ± 0.24 a | 4.22 ± 0.20 a |
| Total tannin content (mg/g TAE DW) | 12.83 ± 2.50 b | 7.51 ± 2.72 a |
| No. | RT | Compounds | Formulas | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment) | Extracts | |
|---|---|---|---|---|---|---|---|---|---|
| MW | ATI | ||||||||
| Hydroxybenzoic acid and derivatives | |||||||||
| 1 | 4.33 | Hydroxybenzoic acid | C7H5O3− | 137.0239 | 137.0242 | 0.33 | 108.0205(100) | + | + |
| 2 | 2.37 | Dihydroxybenzoic acid (Protocatehuic acid)* | C7H5O4− | 153.0188 | 153.0187 | −0.08 | 108.0206(100), 109.0281 | + | + |
| 3 | 2.02 | Gallic acid * | C7H5O5− | 169.0137 | 169.0132 | −0.5 | 107.0127(100), 151.0019, 125.0224 | + | + |
| 4 | 1.84 | Dihydroxybenzoic acid hexoside is. I | C13H15O9− | 315.0716 | 315.0716 | −0.01 | 108.0205(100), 152.0100, 109.0276 | + | − |
| 5 | 2.44 | Dihydroxybenzoic acid hexoside is. II | C13H15O9− | 315.0716 | 315.0716 | −0.01 | 108.0206(100), 152.0103, 109.0274, 153.0168 | + | + |
| 6 | 4.12 | Dihydroxybenzoic acid hexoside is. III | C13H15O9− | 315.0716 | 315.0717 | 0.09 | 109.0283(100), 153.0181, 152.0099, 108.0204 | − | + |
| 7 | 3.10 | Vanillic acid hexoside | C14H17O9− | 329.0873 | 329.0876 | 0.34 | 108.0207(100), 152.0103, 123.0437, 167.0336 | + | + |
| 8 | 1.81 | Gallic acid hexoside is. I | C13H15O10− | 331.0665 | 331.0662 | −0.32 | 168.0053(100), 125.0233, 149.9945, 124.0151, 313.0544 | − | + |
| 9 | 2.85 | Gallic acid hexoside is. II | C13H15O10− | 331.0665 | 331.0662 | −0.32 | 125.0232(100), 169.0125, 124.0151, 168.0059 | − | + |
| 10 | 5.21 | Syringic acid hexoside | C15H19O10− | 359.0978 | 359.0985 | 0.68 | 138.0309(100), 182.0204, 123.0072, 153.0539, 166.9970, 197.0446 | + | − |
| Hydroxycinnamic acid derivatives | |||||||||
| 11 | 6.44 | Coumaric acid hexoside | C15H17O8− | 325.0923 | 325.0911 | −1.24 | 119.0485(100), 163.0385, 145.0286 | − | + |
| 12 | 5.87 | p-coumaroylquinic acid is. I | C16H17O8− | 337.0923 | 337.0927 | 0.36 | 119.0488(100), 163.0394, 191.0551, 155.0337, 173.0443 | + | + |
| 13 | 7.06 | p-coumaroylquinic acid is. II | C16H17O8− | 337.0923 | 337.0927 | 0.36 | 173.0441(100), 119.0495, 163.0386, 191.0507, 155.0374, 127.037 | + | + |
| 14 | 5.58 | Caffeic acid hexoside | C15H17O9− | 341.0873 | 341.0884 | 1.14 | 135.0436(100), 179.0337, 161.0234 | + | − |
| 15 | 3.71 | Caffeoylquinic acid is. I | C16H17O9− | 353.0873 | 353.0873 | 0.04 | 191.0546(100), 135.0442, 179.0341, 161.0231, 173.0443 | + | + |
| 16 | 4.79 | Caffeoylquinic acid is. II | C16H17O9− | 353.0873 | 353.0873 | 0.04 | 191.0546(100), 135.0434, 179.0344, 161.0234, 173.0441, 127.039 | + | + |
| 17 | 6.41 | Caffeoylquinic acid is. III (Chlorogenic acid)* | C16H17O9− | 353.0873 | 353.0873 | 0.04 | 191.0545(100), 135.0444, 173.0448, 179.0342, 161.023, 127.0388 | + | + |
| 18 | 6.93 | Caffeoylquinic acid is. IV | C16H17O9− | 353.0873 | 353.0873 | 0.04 | 191.0546(100), 135.0438, 161.0249, 173.0446, 179.0351, 127.0424 | + | − |
| 19 | 8.67 | Rosmarinic acid * | C18H15O8− | 359.0767 | 359.078 | 1.31 | 161.0230(100), 135.044, 179.0333, 123.0452, 197.0429 | − | + |
| 20 | 6.46 | Feruloylquinic acid | C17H19O9− | 367.1029 | 367.1039 | 0.99 | 134.0364(100), 193.0494, 191.0536, 149.0593, 155.0335, 173.0443 | + | − |
| 21 | 4.00 | Coumaroylquinic acid hexoside | C22H27O13− | 499.1452 | 499.1443 | −0.87 | 163.0386(100), 119.0488, 173.0432, 155.0331 | − | + |
| 22 | 8.55 | Dicaffeoylquinic acid | C25H23O12− | 515.119 | 515.119 | 0.05 | 173.0448(100), 179.0338, 191.0547, 353.0858, 135.0435, 161.0250, 155.033, 209.0774 | + | − |
| 23 | 5.48 | Caffeoylquinic acid hexoside | C22H27O14− | 515.1401 | 515.1386 | −1.48 | 179.0334(100), 191.0542, 341.0845, 135.0436, 515.1403, 323.0764, 353.0866, 161.0238, 155.0320, 173.0445 | − | + |
| Flavan-3-ols and procyanidins | |||||||||
| 24 | 6.22 | Catechin * | C15H13O6− | 289.0712 | 289.0708 | −0.41 | 123.044(100), 109.0283, 125.0235, 151.0388, 137.0232, 203.0701, 149.0246, 161.0584, 221.0802, 245.0814 | + | − |
| 25 | 6.98 | Epicatechin * | C15H13O6− | 289.0712 | 289.0708 | −0.41 | 123.0440(100), 109.0286, 125.0235, 151.039, 121.0285, 137.0233, 203.0703, 149.0242, 161.0583, 221.0810, 245.0798 | + | − |
| 26 | 6.81 | Procyanidin B-type dimer (Procyanidin B2) * | C30H25O12− | 577.1346 | 577.1337 | −0.9 | 289.0702(100), 407.0761, 125.0233, 245.0781, 161.024, 137.023, 273.0403, 205.0472, 425.0861, 451.1014, 109.0277, 179.0334 | + | + |
| Flavonol aglycones and glycosides | |||||||||
| 27 | 10.37 | Kaempferol * | C15H9O6− | 285.0399 | 285.0395 | −0.41 | 285.0390(100), 185.058, 187.0391, 239.0339, 229.0476, 159.0432, 211.0389, 143.0504, 257.0286, 151.0019, 267.0296 | + | − |
| 28 | 9.83 | Dehydroquercetin | C15H7O7− | 299.0192 | 299.0203 | 1.12 | 151.0026(100), 121.0284, 107.0127, 271.0236, 299.0173, 178.9966, 227.034, 243.0274 | + | − |
| 29 | 9.64 | Quercetin * | C15H9O7− | 301.0348 | 301.0356 | 0.77 | 151.0028(100), 121.0284, 107.0129, 178.9974, 149.0233, 245.0438, 229.0490, 273.0379, 301.0339 | + | + |
| 30 | 7.20 | Myricetin * | C15H9O8− | 317.0297 | 317.031 | 1.26 | 109.0287(100), 151.0034, 243.1227, 163.0029, 125.0218, 179.0043, 107.0122, 227.0327, 257.1385, 271.0259 | + | − |
| 31 | 7.54 | Dihydromyricetin | C15H11O8− | 319.0454 | 319.0448 | −0.59 | 139.0386(100), 109.0291, 183.0273, 153.0196, 258.0154, 165.0193, 201.0100, 214.0265, 242.0161 | + | − |
| 32 | 8.19 | Quercetin 3-O-pentoside (Guaijaverin) | C20H17O11− | 433.0771 | 433.0775 | 0.41 | 300.0264(100), 301.0313, 271.0238, 255.0285, 151.0024, 179.0004 | + | − |
| 33 | 8.30 | Quercetin 3-O-rhamnoside (Quercitrin) * | C21H19O11− | 447.0927 | 447.0933 | 0.56 | 300.0263(100), 301.033, 271.024, 151.0045, 255.0288, 178.9976, 243.0286, 227.0340, 285.0388 | + | + |
| 34 | 7.92 | Quercetin 3-O-hexoside (Hyperoside) * | C21H19O12− | 463.0877 | 463.0879 | 0.25 | 300.0261(100), 301.0313, 271.0237, 255.0284, 151.0027, 178.9973 | + | + |
| 35 | 7.94 | Quercetin 3-O-glucuronide (Miquelianin) | C21H17O13− | 477.0669 | 477.067 | 0.08 | 301.0347(100), 151.0022, 178.9974, 273.0391, 255.0285, 229.049 | + | + |
| 36 | 7.45 | Myricetin 3-O-hexoside * | C21H19O13− | 479.0826 | 479.084 | 1.43 | 316.0196(100), 317.0264, 271.0237, 479.0813, 287.0178, 257.0445, 178.9988, 151.0022 | + | − |
| 37 | 8.26 | Quercetin 3-O-(6”-O-acetyl)hexoside | C23H21O13− | 505.0982 | 505.0988 | 0.58 | 300.0266(100), 301.0308, 271.0233, 255.0288, 243.0281, 151.0007 | + | + |
| 38 | 8.80 | Quercetin 3-O-(6”-O-acetyl)-beta-D-glucopyranoside * | C23H21O13− | 505.0982 | 505.0988 | 0.58 | 300.0269(100), 301.0304, 271.0233, 255.0284, 243.0284, 151.0021 | + | + |
| 39 | 7.79 | Quercetin 3-O-(6”-rhamnosyl)hexoside (Rutin)* | C27H29O16− | 609.1456 | 609.1462 | 0.64 | 300.0264(100), 609.1442, 301.0327, 271.024, 151.003, 178.9977, 255.0303, 243.0288 | + | + |
| Other flavonoids | |||||||||
| 40 | 10.16 | Naringenin * | C15H11O5− | 271.0606 | 271.0611 | 0.45 | 119.0483(100), 151.0036, 107.0124, 187.037, 145.0273 | + | − |
| 41 | 12.88 | Trimethoxyflavone (like Salvigenin) | C18H15O6− | 327.0869 | 327.0869 | 0.04 | 327.0866(100), 297.0396, 328.0895, 311.0548, 283.0241, 312.0599, 298.0422, 271.0253 | + | − |
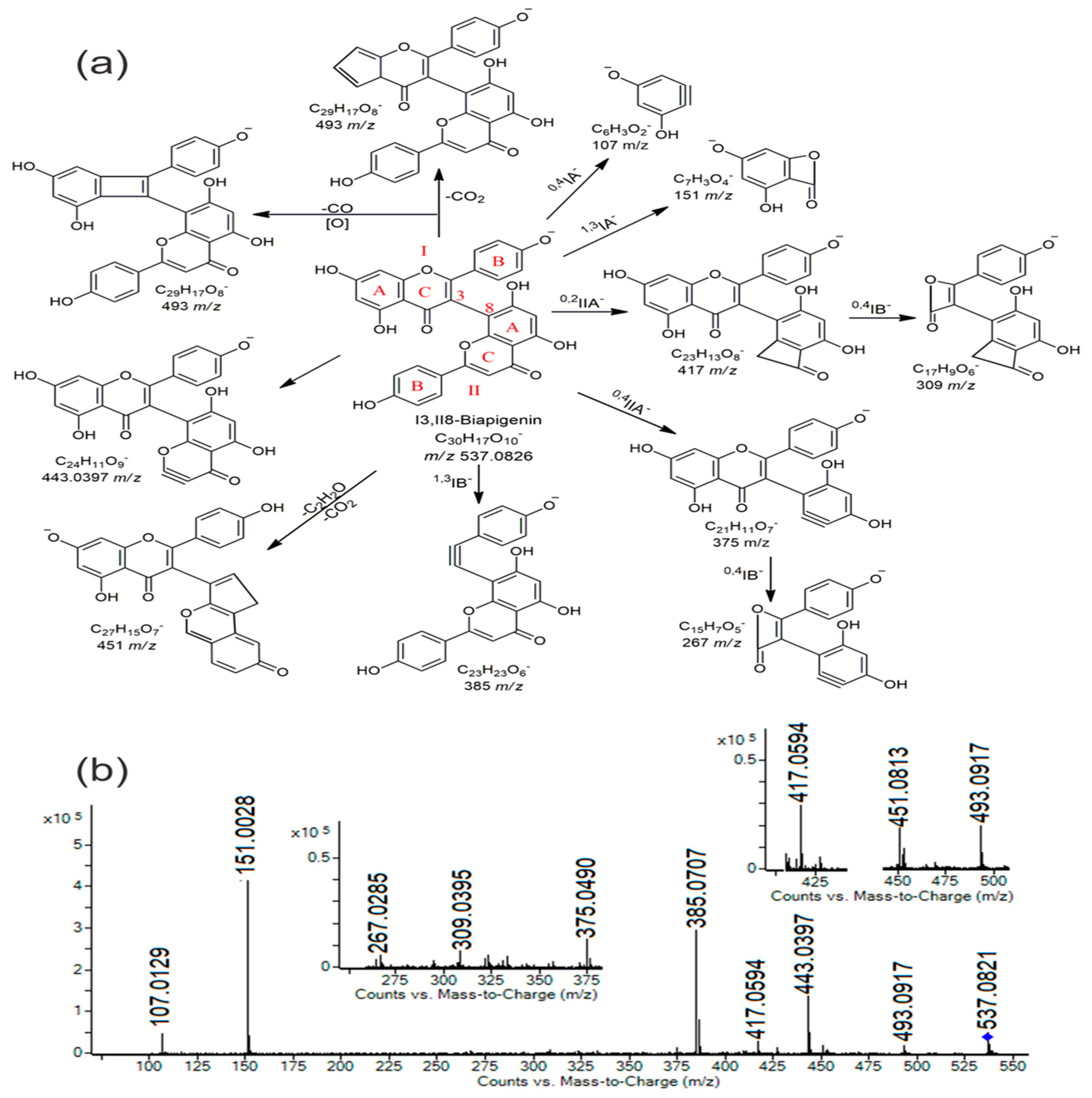
| 42 | 10.69 | I3,II8-Biapigenin | C30H17O10− | 537.0822 | 537.0826 | 0.43 | 151.0028(100), 385.0707, 443.0397, 537.0821, 107.0129, 417.0594, 493.0917, 267.0285, 451.0813, 375.0490, 309.0395 | + | − |
| No. | RT | Tentatively Identified Compounds | Formulas | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment) | Extracts | Previously Reported in Hypericum | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATI | MW | M | |||||||||
| Naphthodianthrones | |||||||||||
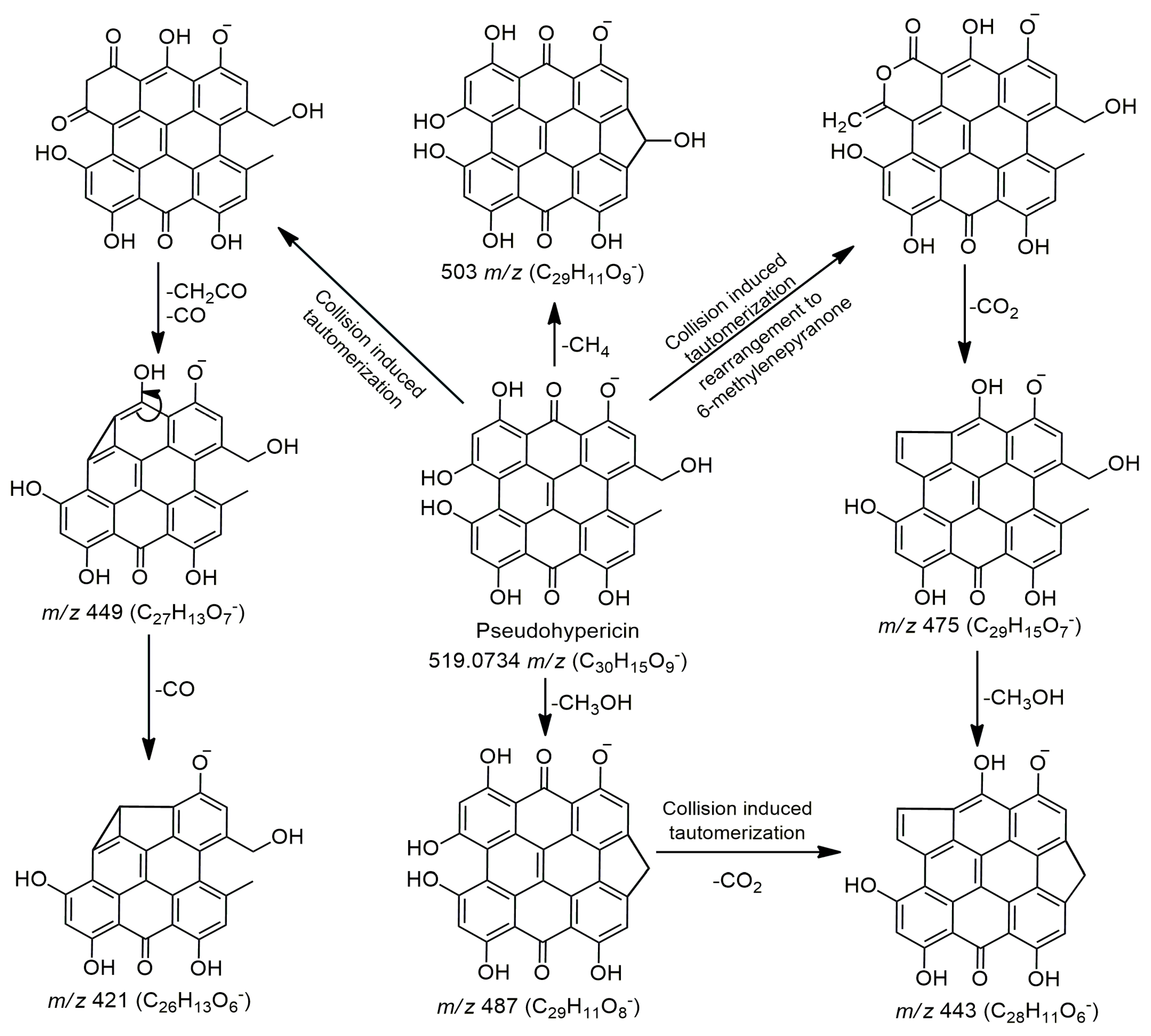
| 43 | 16.65 | Pseudohypericin | C30H15O9− | 519.0716 | 519.0734 | 1.79 | 519.0746(100), 520.0773, 487.0466, 503.044, 475.0752, 449.0711, 443.0575, 421.069 | − | − | + | [14,15,16,17] |
| 44 | 15.90 | Pseudoprotohypricin | C30H17O9− | 521.0873 | 521.0891 | 1.84 | 521.0905(100), 522.0924, 477.0988, 423.0885, 379.0945, 449.1025 | − | − | + | [14,15,16,17] |
| Polycyclic polyprenylated acylphloroglucinols(PPAPs) | |||||||||||
| 45 | 14.69 | Hyperfirin | C30H45O4+ | 469.3318 | 469.3322 | 0.42 | 401.2703(100), 469.3346, 345.2076, 413.2704, 223.0977, 333.2072, 279.1601, 277.1451, 291.1602, 305.1757, 319.1912, 357.2073 | − | + | + | [11,18,19] |
| 46 | 14.93 | Adhyperfirin | C31H47O4+ | 483.3474 | 483.3484 | 0.97 | 415.2856(100), 483.3488, 427.2858, 359.2228, 293.1737, 237.1139, 371.2231, 347.2227 | − | − | + | [11,18,19] |
| Furano-polycyclic polyprenylated acylphloroglucinols(FPPAPs) | |||||||||||
| 47 | 13.13 | FPPAP derivative 1 (like Hyperformitin J, K, L or M) | C30H45O5+ | 485.3267 | 485.3284 | 1.7 | 485.33(100), 467.3182, 399.2547, 411.2547, 385.2388, 333.2074, 331.1917 | − | − | + | [20] |
| 48 | 15.54 | FPPAP derivative 2 (like Hyperioxide D) | C35H51O6+ | 567.3686 | 567.3712 | 2.64 | 293.1401(100), 275.1303, 331.1914, 329.1794, 347.1868, 349.1993, 443.2847, 425.2754, 481.3098, 499.3120, 549.3530 | − | − | + | [21] |
| 49 | 16.49 | FPPAP derivative 3 (like Hyperformitin C, Hyperformitin D (Type A PPAPs) or Hyperfol F (Type B PPAPs)) | C35H53O6+ | 569.3842 | 569.3876 | 3.39 | 293.1398(100), 347.1865, 365.1978, 275.1314, 331.1926, 329.1799, 499.3407, 483.3136 | − | − | + | [20,22] |
| 50 | 13.75 | FPPAP derivative 4 (unknown) | C35H53O7+ | 585.3791 | 585.3819 | 2.77 | 293.1396(100), 275.1304, 347.1863, 365.1996, 329.1782, 481.2988, 517.3159, 567.3643 | − | + | + | / |
| 51 | 15.46 | FPPAP derivative 5 (unknown) | C35H53O7+ | 585.3791 | 585.3819 | 2.77 | 293.1395(100), 275.1302, 347.1877, 329.1783, 365.2011, 567.3697, 549.3622, 517.3203, 499.3137, 481.3050 | − | − | + | / |
| 52 | 15.15 | FPPAP derivative 6 (like Hyperidione F) | C35H55O7+ | 587.3948 | 587.3985 | 3.72 | 293.1397(100), 294.1433, 275.1301, 349.2019, 331.1920, 569.3860, 277.1448, 551.3745, 221.0827 | − | + | + | [23,24] |
| 53 | 14.5 | FPPAP derivative 7 (unknown) | C35H55O8+ | 603.3897 | 603.3928 | 3.11 | 293.1399(100), 347.2873, 365.1983, 329.1781, 275.1300, 441.2648, 481.3007, 499.3105, 567.3702, 585.3797 | − | + | + | / |
| 54 | 13.89 | FPPAP derivative 8 (unknown) | C35H55O9+ | 619.3846 | 619.387 | 2.39 | 293.1395(100), 275.1302, 347.1876, 365.1980, 499.3079, 511.3095, 529.3184, 565.3538, 583.3640, 601.3745 | − | + | + | / |
| Xanthones | |||||||||||
| 55 | 8.98 | Tetrahydroxyxanthone (like Norathyriol) | C13H7O6− | 259.0243 | 259.0242 | −0.06 | 259.0236(100), 109.0285, 215.0336, 187.0388, 159.0437, 231.028, 151.0022 | − | + | + | [17,25] |
| 56 | 12.87 | (2 or 8) Prenyl-tetrahydroxyxanthone | C18H15O6− | 327.0869 | 327.0869 | 0.04 | 327.0866(100), 297.0396, 328.0895, 311.0548, 283.0241, 258.0147, 271.0253 | − | + | − | [17,25] |
| 57 | 13.60 | γ-mangostin | C23H23O6− | 395.1495 | 395.1525 | 3.04 | 272.0303(100), 271.0237, 283.0234, 326.0773, 395.1488, 258.0179, 243.0297 | − | + | − | [17,25] |
| 58 | 7.38 | Mangiferin | C19H17O11− | 421.0771 | 421.0773 | 0.21 | 258.0153(100), 259.0206, 301.0362, 331.0448, 271.0235 | − | + | + | [25,26,27] |
| Other compounds (Coumestan) | |||||||||||
| 59 | 10.08 | Wedelolactone | C16H9O7− | 313.0348 | 313.0359 | 1.07 | 269.0441(100), 225.0543, 241.049, 270.0471, 197.0596, 181.0658, 210.0320, 133.0266 | − | + | + | [28] |
| Analysis | Infusion Tea (ATI) | Methanolic Extract (MW) |
|---|---|---|
| ABTS⦁+ (μmol Trolox/g DW) * | 176.48 ± 2.32 a | 130.49 ± 1.89 b |
| DPPH⦁ (μmol Trolox/g DW) | 132.96 ± 0.96 b | 149.99 ± 1.31 a |
| TAC (mg/g AAE DW) | 20.73 ± 2.42 b | 32.31 ± 0.50 a |
| FRP (mg/g AAE DW) | 21.08 ± 0.71 b | 30.58 ± 3.01 a |
| CUPRAC (mg/g AAE DW) | 27.50 ± 1.82 a | 20.06 ± 2.57 b |
| S. aureus Assay | K. pneumoniae Assay | |
|---|---|---|
| StrpE (mg/mL) | StrpE (mg/mL) | |
| MP 1 | 7.16 ± 0.52 | 5.13 ± 0.30 |
| MP 2 | 12.35 ± 0.96 | 9.70 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilibarda, S.; Jović, M.D.; Milinčić, D.D.; Vuković, S.; Trifković, J.Đ.; Pešić, M.B.; Kostić, A.Ž. Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography. Plants 2025, 14, 1377. https://doi.org/10.3390/plants14091377
Kilibarda S, Jović MD, Milinčić DD, Vuković S, Trifković JĐ, Pešić MB, Kostić AŽ. Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography. Plants. 2025; 14(9):1377. https://doi.org/10.3390/plants14091377
Chicago/Turabian StyleKilibarda, Sofija, Marko D. Jović, Danijel D. Milinčić, Sandra Vuković, Jelena Đ. Trifković, Mirjana B. Pešić, and Aleksandar Ž. Kostić. 2025. "Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography" Plants 14, no. 9: 1377. https://doi.org/10.3390/plants14091377
APA StyleKilibarda, S., Jović, M. D., Milinčić, D. D., Vuković, S., Trifković, J. Đ., Pešić, M. B., & Kostić, A. Ž. (2025). Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography. Plants, 14(9), 1377. https://doi.org/10.3390/plants14091377








