The Involvement of Glycerophospholipids in Susceptibility of Maize to Gibberella Root Rot Revealed by Comparative Metabolomics and Mass Spectrometry Imaging Joint Analysis
Abstract
1. Introduction
2. Results
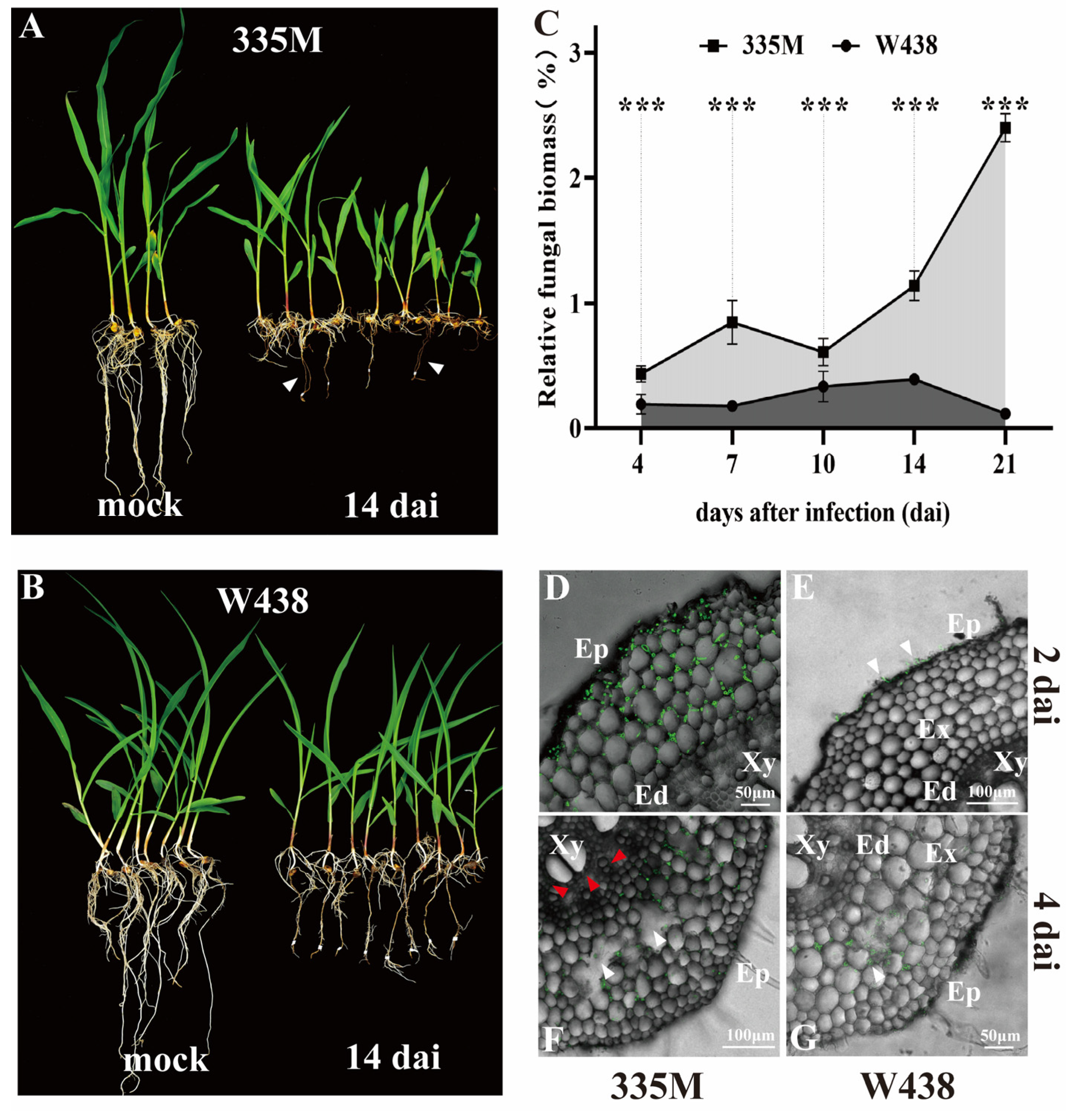
2.1. Morphological and Pathological Differences Between Maize Inbred Lines to GRR
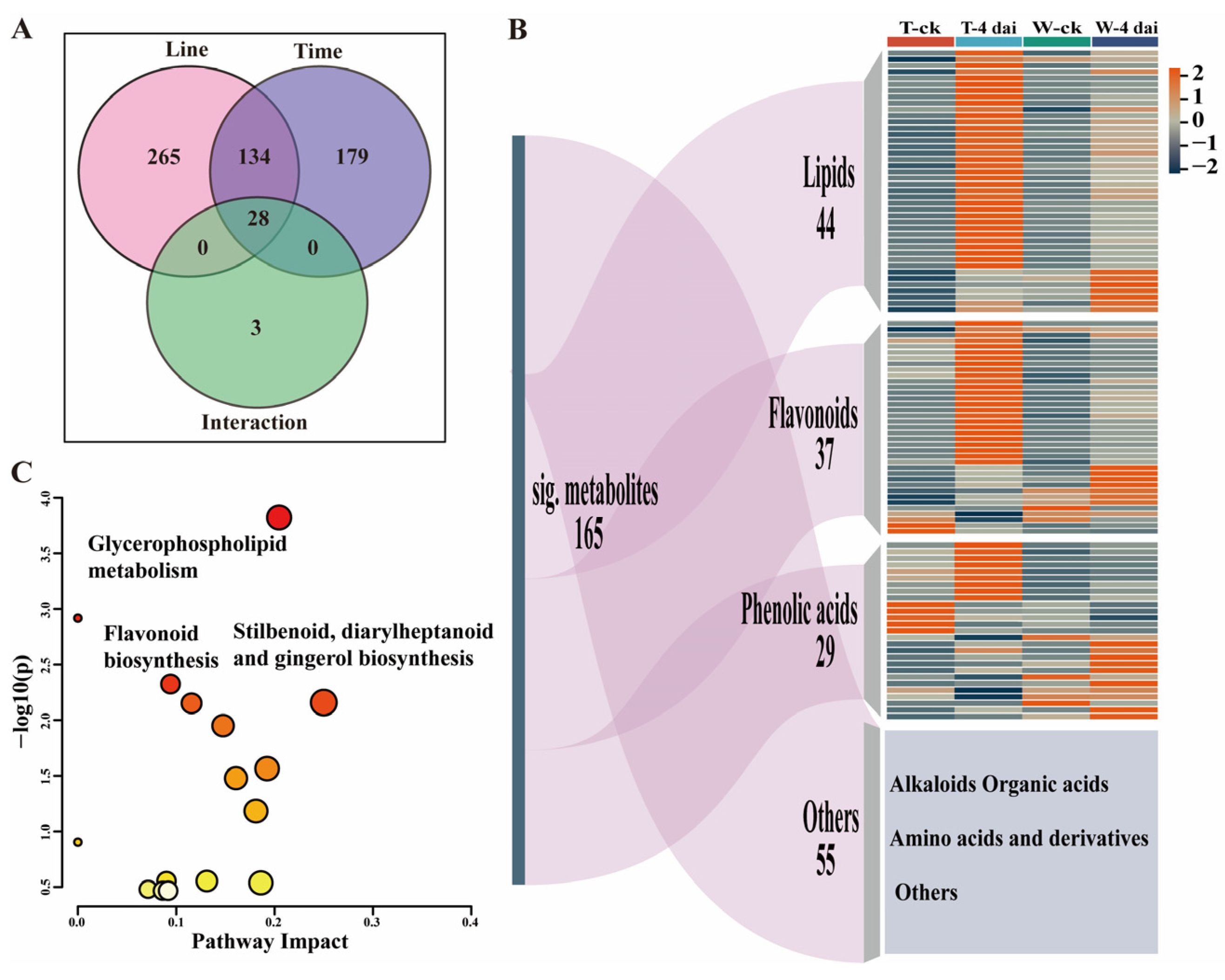
2.2. Metabolomic Changes Between GRR-Resistant and Susceptible Lines upon F. graminearum Infection
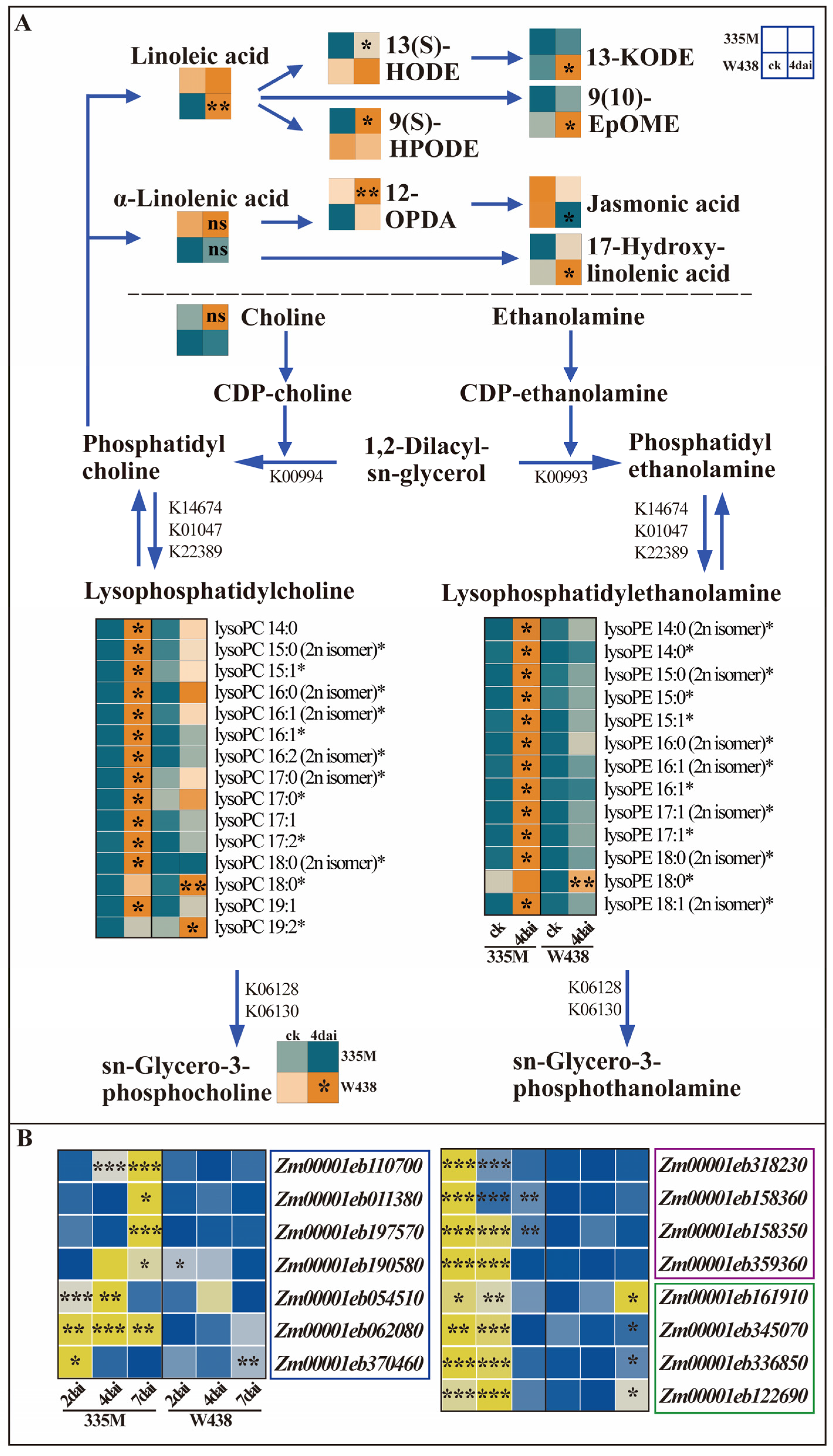
2.3. Differential Accumulation of Lysophospholipids and Expression of Related Genes
2.4. Temporal and Spatial Distribution of GRR Induced Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth
4.2. Fungal Strain and Preparation of Inoculum
4.3. Kernel–Sand Mixture Inoculation and Sampling
4.4. DNA Isolation from Maize Roots and Fungal Mycelia
4.5. qPCR Measurement of Fungal and Root gDNA
4.6. Tissue Preparation for Microscopy Analyses
4.7. Metabolomics Analysis Using UPLC-ESI-Q TRAP-MS/MS
4.8. RNA Extraction and Gene Expression Analysis
4.9. Sample Preparation for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging (MALDI MSI)
4.10. MALDI MSI and Data Analysis
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GRR | Gibberella root rot |
| PE | phosphatidylethanolamine |
| PC | phosphatidylcholine |
| lysoPE | lysophosphatidylethanolamine |
| lysoPC | lysophosphatidylcholine |
| PLA | phospholipase |
| LPLA | lysophospholipase |
| MSI | mass spectrometry imaging |
References
- Duan, C.X.; Dong, H.Y.; Li, X.; Li, H.; Li, C.H.; Sun, S.L.; Zhu, Z.D.; Wang, X.M. A large-scale screening of maize germplasm for resistance to multiple diseases in multi-plot demonstration for several years under natural condition. Acta Agron. Sin. 2020, 46, 1135–1145. [Google Scholar] [CrossRef]
- Xu, F.; Yang, G.Q.; Wang, J.M.; Song, Y.L.; Liu, L.L.; Zhao, K.; Li, Y.H.; Han, Z.H. Spatial distribution of root and crown rot fungi associated with winter wheat in the North China plain and its relationship with climate variables. Front. Microbiol. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhao, J.J.; Zhao, S.Q.; Li, M.Y.; Pang, S.Y.; Kang, Z.S.; Zhen, W.C.; Chen, S.S.; Chen, F.; Wang, X.D. Genetics of resistance to common root rot (spot blotch), Fusarium crown rot, and sharp eyespot in wheat. Front. Genet. 2021, 12, 699342. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chand, R.; Vasistha, N.K.; Pandey, S.P.; Kumar, U.; Mishra, V.K.; Joshi, A.K. Spot blotch disease of wheat: The current status of research on genetics and breeding. Plant Pathol. 2018, 67, 508–531. [Google Scholar] [CrossRef]
- Hamada, M.S.; Yin, Y.; Chen, H.; Ma, Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 2011, 67, 1411–1419. [Google Scholar] [CrossRef]
- Sikora, E.; Faske, T.; Meyer, R.; Betts, A.; Kemerait, B.; Camiletti, B.; Telenko, D.; Robertson, A.; Mueller, D.; Sisson, A.; et al. Corn Disease Loss Estimates from the United States and Ontario, Canada—2024; Crop Protection Network: Ames, IA, USA, 2025. [Google Scholar] [CrossRef]
- Feng, C.; Xu, F.; Li, L.; Zhang, J.; Wang, J.; Li, Y.; Liu, L.; Han, Z.; Shi, R.; Wan, X.; et al. Biological control of Fusarium crown rot of wheat with Chaetomium globosum 12XP1-2-3 and its effects on rhizosphere microorganisms. Front. Microbiol 2023, 14, 1133025. [Google Scholar] [CrossRef]
- Ye, J.; Guo, Y.; Zhang, D.; Zhang, N.; Wang, C.; Xu, M. Cytological and molecular characterization of quantitative trait locus qRfg1, which confers resistance to gibberella stalk rot in maize. Mol. Plant Microbe Interact. 2013, 26, 1417–1428. [Google Scholar] [CrossRef]
- Ye, J.; Zhong, T.; Zhang, D.; Ma, C.; Wang, L.; Yao, L.; Zhang, Q.; Zhu, M.; Xu, M. The Auxin-Regulated Protein ZmAuxRP1 Coordinates the Balance between Root Growth and Stalk Rot Disease Resistance in Maize. Mol. Plant 2019, 12, 360–373. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.K.; Kremling, K.A.; Ding, Y.; Bennett, J.S.; Bae, J.S.; Kim, D.K.; Ackerman, H.H.; Kolomiets, M.V.; Schmelz, E.A.; et al. Ethylene signaling regulates natural variation in the abundance of antifungal acetylated diferuloylsucroses and Fusarium graminearum resistance in maize seedling roots. New Phytol. 2019, 221, 2096–2111. [Google Scholar] [CrossRef]
- Forster, C.; Handrick, V.; Ding, Y.; Nakamura, Y.; Paetz, C.; Schneider, B.; Castro-Falcon, G.; Hughes, C.C.; Luck, K.; Poosapati, S.; et al. Biosynthesis and antifungal activity of fungus-induced O-methylated flavonoids in maize. Plant Physiol. 2022, 188, 167–190. [Google Scholar] [CrossRef]
- Bhandari, D.R.; Wang, Q.; Friedt, W.; Spengler, B.; Gottwald, S.; Römpp, A. High resolution mass spectrometry imaging of plant tissues: Towards a plant metabolite atlas. Analyst 2015, 140, 7696–7709. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.R.; Wang, Q.; Li, B.; Friedt, W.; Römpp, A.; Spengler, B.; Gottwald, S. Histology-guided high-resolution AP-SMALDI mass spectrometry imaging of wheat-Fusarium graminearum interaction at the root–shoot junction. Plant Methods 2018, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, D.; Dhakshinamoorthy, S.; Alexandrov, T.; Becker, M.; Bretschneider, T.; Buerkert, A.; Crecelius, A.C.; De Waele, D.; Elsen, A.; Heckel, D.G.; et al. Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis. Proc. Natl. Acad. Sci. USA 2014, 111, 105–110. [Google Scholar] [CrossRef]
- Righetti, L.; Gottwald, S.; Tortorella, S.; Spengler, B.; Bhandari, D.R. Mass Spectrometry Imaging Disclosed Spatial Distribution of Defense-Related Metabolites in Triticum spp. Metabolites 2022, 12, 48. [Google Scholar] [CrossRef]
- Righetti, L.; Bhandari, D.R.; Rolli, E.; Tortorella, S.; Bruni, R.; Dall’Asta, C.; Spengler, B. Unveiling the spatial distribution of aflatoxin B1 and plant defense metabolites in maize using AP-SMALDI mass spectrometry imaging. Plant J. 2021, 106, 185–199. [Google Scholar] [CrossRef]
- Kalmar, J.G.; Oh, Y.; Dean, R.A.; Muddiman, D.C. Investigating host-pathogen meta-metabolic interactions of Magnaporthe oryzae infected barley using infrared matrix-assisted laser desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 139–147. [Google Scholar] [CrossRef]
- Mehta, S.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Fight Hard or Die Trying: Current Status of Lipid Signaling during Plant-Pathogen Interaction. Plants 2021, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K.; Dordschbal, B.; Roos, W. Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell 2002, 14, 1509–1525. [Google Scholar] [CrossRef]
- Völz, R.; Kim, K.T.; Alazem, M.; Harris, W.; Hwang, S.; Lee, Y.H. Lyso-phosphatidylethanolamine triggers immunity against necrotrophs by promoting JA-signaling and ROS-homeostasis. Plant Mol. Biol. 2023, 113, 237–247. [Google Scholar] [CrossRef]
- Wi, S.J.; Seo, S.; Cho, K.; Nam, M.H.; Park, K.Y. Lysophosphatidylcholine enhances susceptibility in signaling pathway against pathogen infection through biphasic production of reactive oxygen species and ethylene in tobacco plants. Phytochemistry 2014, 104, 48–59. [Google Scholar] [CrossRef]
- Wi, S.J.; Ji, N.R.; Park, K.Y. Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol. 2012, 159, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Bi, M.; Zhang, H.; He, Y.; Cui, Y.; Qi, M. Membrane lipid metabolism and scavenging of reactive oxygen species are essential for cold resistance of Solanum habrochaites (LA1777): Lipidomics and transcriptomes analysis. Environ. Exp. Bot. 2023, 209, 105290. [Google Scholar] [CrossRef]
- Völz, R.; Park, J.-Y.; Harris, W.; Hwang, S.; Lee, Y.H. Lyso-phosphatidylethanolamine primes the plant immune system and promotes basal resistance against hemibiotrophic pathogens. BMC Biotechnol. 2021, 21, 12. [Google Scholar] [CrossRef]
- Zhou, Q.; Jayawardhane, K.N.; Strelkov, S.E.; Hwang, S.F.; Chen, G.Q. Identification of Arabidopsis phospholipase A mutants with increased susceptibility to plasmodiophora brassicae. Front. Plant Sci. 2022, 13, 799142. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Song, J.; Wang, H.; Ruan, C.; Zhang, W.; Guo, Z.; Li, W.; Guo, W. Cotton peroxisome-localized lysophospholipase counteracts the toxic effects of Verticillium dahliae NLP1 and confers wilt resistance. Plant J. 2023, 115, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Wang, F.; Huang, P.-C.; Wang, Y.; Ruan, X.; Ma, L.; Li, X.; Kolomiets, M.V.; Gao, X. Transcriptome and Oxylipin Profiling Joint Analysis Reveals Opposite Roles of 9-Oxylipins and Jasmonic Acid in Maize Resistance to Gibberella Stalk Rot. Front. Plant Sci. 2021, 12, 699146. [Google Scholar] [CrossRef] [PubMed]
- Gorzolka, K.; Perino, E.H.B.; Lederer, S.; Smolka, U.; Rosahl, S. Lysophosphatidylcholine 17:1 from the Leaf Surface of the Wild Potato Species Solanum bulbocastanum Inhibits Phytophthora infestans. J. Agric. Food Chem. 2021, 69, 5607–5617. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeun, Y.; Hwang, B.K. The pepper patatin-like phospholipase CaPLP1 functions in plant cell death and defense signaling. Plant Mol. Biol. 2014, 84, 329–344. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Jia, Y.; Gao, L.; Pei, Y.; Ge, Z.; Ge, X.; Li, F.; Hou, Y. The cotton Ghplp2 positively regulates plant defense against Verticillium dahliae by modulating fatty acid accumulation and jasmonic acid signaling pathway. arXiv 2021. [Google Scholar] [CrossRef]
- Camera, S.L.; Balagué, C.; Göbel, C.; Geoffroy, P.; Legrand, M.; Feussner, I.; Roby, D.; Heitz, T. The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol. PlantMicrobe Interact. 2009, 22, 469–481. [Google Scholar] [CrossRef]
- La Camera, S.; Geoffroy, P.; Samaha, H.; Ndiaye, A.; Rahim, G.; Legrand, M.; Heitz, T. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 2005, 44, 810–825. [Google Scholar] [CrossRef]
- Cho, K.; Kim, Y.; Wi, S.J.; Seo, J.B.; Kwon, J.; Chung, J.H.; Park, K.Y.; Nam, M.H. Nontargeted metabolite profiling in compatible pathogen-inoculated tobacco (Nicotiana tabacum L. cv. Wisconsin 38) using UPLC-Q-TOF/MS. J. Agric. Food Chem. 2012, 60, 11015–11028. [Google Scholar] [CrossRef]
- Schwartze, W.; Roos, W.J.P. The signal molecule lysophosphatidylcholine in Eschscholzia californica is rapidly metabolized by reacylation. Planta 2008, 229, 183–191. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.Y.; Xiao, S.; Chye, M.L. Acyl-CoA-binding protein 2 binds lysophospholipase 2 and lysoPC to promote tolerance to cadmium-induced oxidative stress in transgenic Arabidopsis. Plant J. 2010, 62, 989–1003. [Google Scholar] [CrossRef]
- Scherer, G.F.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010, 15, 693–700. [Google Scholar] [CrossRef]
- Guo, T.; Liu, C.; Meng, F.; Hu, L.; Fu, X.; Yang, Z.; Wang, N.; Jiang, Q.; Zhang, X.; Ma, F.W. The m6A reader MhYTP2 regulates MdMLO19 mRNA stability and antioxidant genes translation efficiency conferring powdery mildew resistance in apple. Plant Biotechnol. J. 2022, 20, 511–525. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Pampori, A.; Droutsa, E.; Laskari, M.; Karakostas, P.; Tsalikis, L.; Barmpalexis, P.; Dordas, C.; Assimopoulou, A.N. Occurrence of Luteolin in the Greek Flora, Isolation of Luteolin and Its Action for the Treatment of Periodontal Diseases. Molecules 2023, 28, 7720. [Google Scholar] [CrossRef]
- Biswas, S.; Kundu, A.; Suby, S.B.; Kushwah, A.S.; Patanjali, N.; Shasany, A.K.; Verma, R.; Saha, S.; Mandal, A.; Banerjee, T.; et al. Lippia alba—A potential bioresource for the management of Spodoptera frugiperda (Lepidoptera: Noctuidae). Front. Plant Sci. 2024, 15, 1422578. [Google Scholar] [CrossRef]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Balmer, D.; de Papajewski, D.V.; Planchamp, C.; Glauser, G.; Mauch-Mani, B. Induced resistance in maize is based on organ-specific defence responses. Plant J. 2013, 74, 213–225. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, X.; Wang, W.; Sun, Y.; Tian, X.; Chen, Z.; Mou, X.; Zhang, Y.; Wei, Y.; Fang, Z.; et al. Metabolomics reveals the response of hydroprimed maize to mitigate the impact of soil salinization. Front. Plant Sci. 2023, 14, 1109460. [Google Scholar] [CrossRef]
- Koutouan, C.; Clerc, V.L.; Baltenweck, R.; Claudel, P.; Halter, D.; Hugueney, P.; Hamama, L.; Suel, A.; Huet, S.; Merlet, M.B.; et al. Link between carrot leaf secondary metabolites and resistance to Alternaria dauci. Sci. Rep. 2018, 8, 13746. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, X.; Meng, J.; Xu, H.; Zhou, X. WRKY Transcription Factors Modulate the Flavonoid Pathway of Rhododendron chrysanthum Pall. Under UV-B Stress. Plants 2025, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Riboni, L.; Giussani, P.; Viani, P. Sphingolipid Transport. In Sphingolipids as Signaling and Regulatory Molecules, 1st ed.; Chalfant, C., Poeta, M.D., Eds.; Springer: New York, NY, USA, 2010; Volume 688, pp. 24–25. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Ernst, A.M.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bienias, K.; Fiedorowicz, A.; Sadowska, A.; Prokopiuk, S.; Car, H. Regulation of sphingomyelin metabolism. Pharmacol. Rep. 2016, 68, 570–581. [Google Scholar] [CrossRef]
- Lachaud, C.; Da Silva, D.; Amelot, N.; Béziat, C.; Brière, C.; Cotelle, V.; Graziana, A.; Grat, S.; Mazars, C.; Thuleau, P. Dihydrosphingosine-induced programmed cell death in tobacco BY-2 cells is independent of H2O2 production. Mol. Plant 2011, 4, 310–318. [Google Scholar] [CrossRef]
- Liang, H.; Yao, N.; Song, J.T.; Luo, S.; Lu, H.; Greenberg, J.T. Ceramides modulate programmed cell death in plants. Genes Dev. 2003, 17, 2636–2641. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Le Bourse, D.; Markham, J.; Dorey, S.; Clément, C.; Baillieul, F.; Dhondt-Cordelier, S. Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiol. 2015, 169, 2255–2274. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, C.; Pang, X.; Wang, Z.; Guo, Y.; Du, F.; Wu, R.L. Functional mapping of ontogeny in flowering plants. Brief. Bioinform. 2012, 13, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vera Buxa, S.; Furch, A.; Friedt, W.; Gottwald, S. Insights into Triticum aestivum seedling root rot caused by Fusarium graminearum. Mol. Plant Microbe Interact. 2015, 28, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Kovalsky Paris, M.P.; Paolino, G.; Bürstmayr, H.; Lemmens, M.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Mach, R.L. A reference-gene-based quantitative PCR method as a tool to determine Fusarium resistance in wheat. Anal. Bioanal. Chem. 2009, 395, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhao, Z.; Li, X.; Gao, X. The Involvement of Glycerophospholipids in Susceptibility of Maize to Gibberella Root Rot Revealed by Comparative Metabolomics and Mass Spectrometry Imaging Joint Analysis. Plants 2025, 14, 1376. https://doi.org/10.3390/plants14091376
Wang Q, Zhao Z, Li X, Gao X. The Involvement of Glycerophospholipids in Susceptibility of Maize to Gibberella Root Rot Revealed by Comparative Metabolomics and Mass Spectrometry Imaging Joint Analysis. Plants. 2025; 14(9):1376. https://doi.org/10.3390/plants14091376
Chicago/Turabian StyleWang, Qing, Zi’an Zhao, Xin Li, and Xiquan Gao. 2025. "The Involvement of Glycerophospholipids in Susceptibility of Maize to Gibberella Root Rot Revealed by Comparative Metabolomics and Mass Spectrometry Imaging Joint Analysis" Plants 14, no. 9: 1376. https://doi.org/10.3390/plants14091376
APA StyleWang, Q., Zhao, Z., Li, X., & Gao, X. (2025). The Involvement of Glycerophospholipids in Susceptibility of Maize to Gibberella Root Rot Revealed by Comparative Metabolomics and Mass Spectrometry Imaging Joint Analysis. Plants, 14(9), 1376. https://doi.org/10.3390/plants14091376





