Plants Distinguish Different Photoperiods to Independently Regulate Post-Flowering Vegetative Growth and Reproductive Growth
Abstract
1. Introduction
2. Results
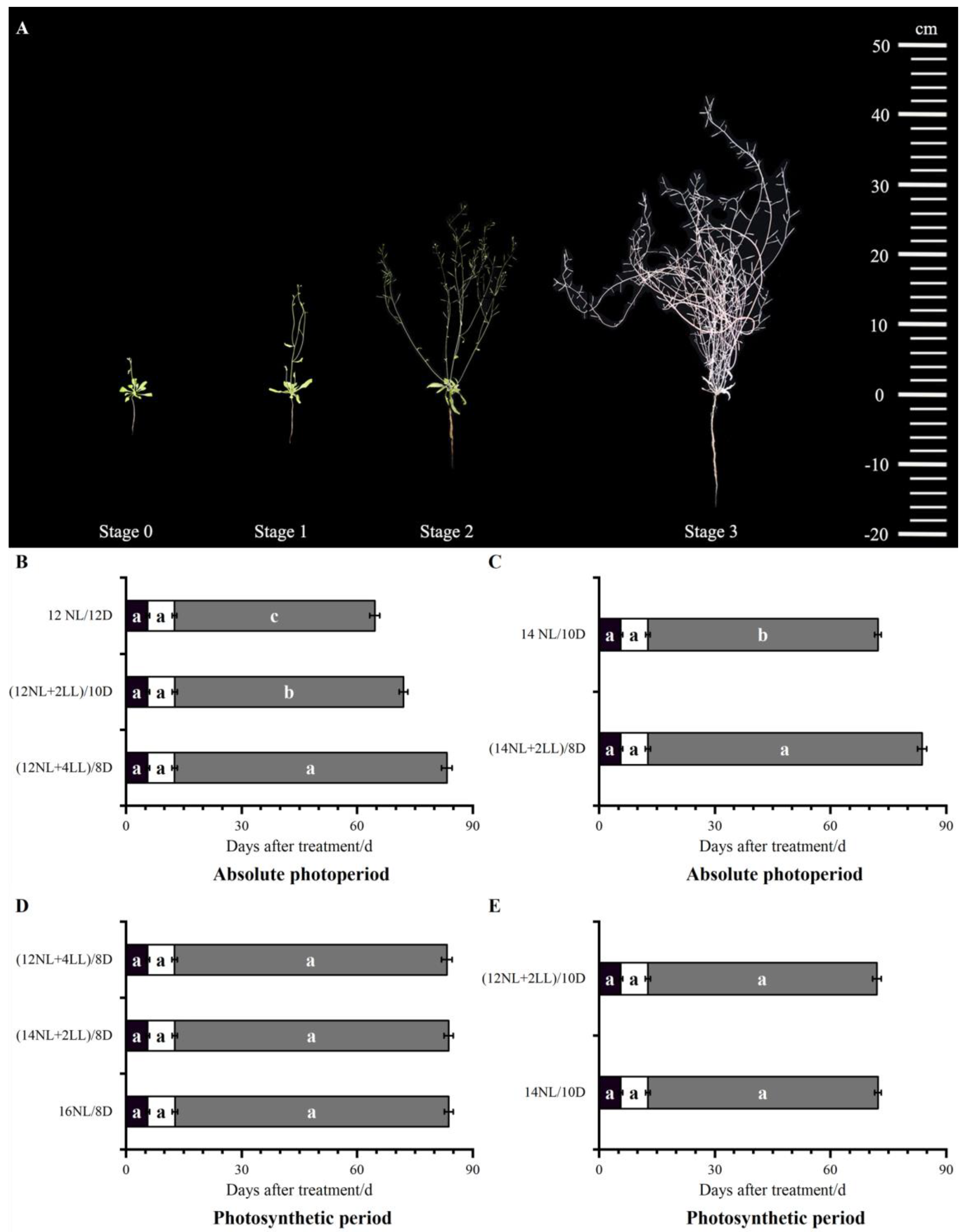
2.1. Photoperiod Had a Significant Effect on Post-Flowering
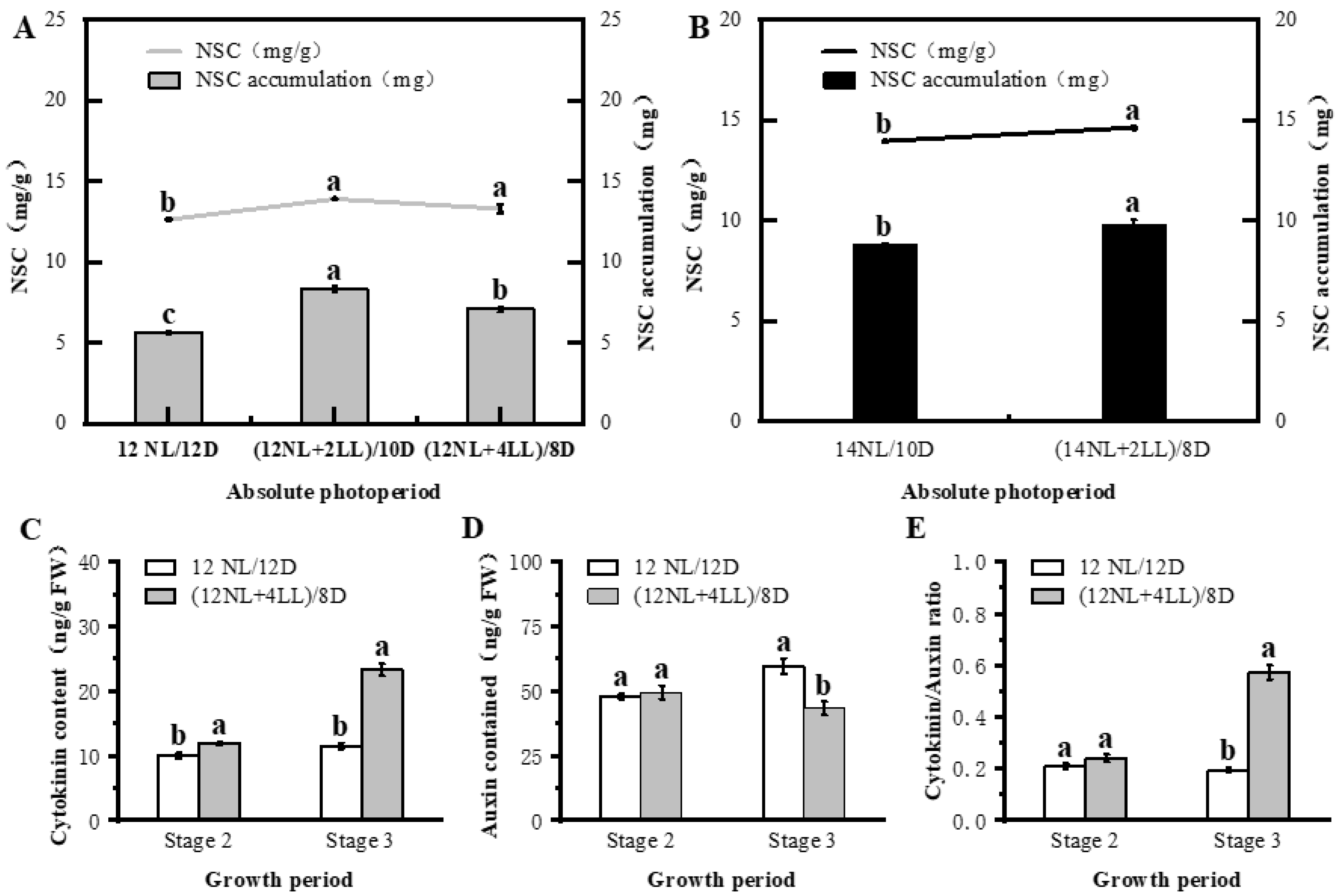
2.2. Influence of Absolute Photoperiod on Post-Flowering
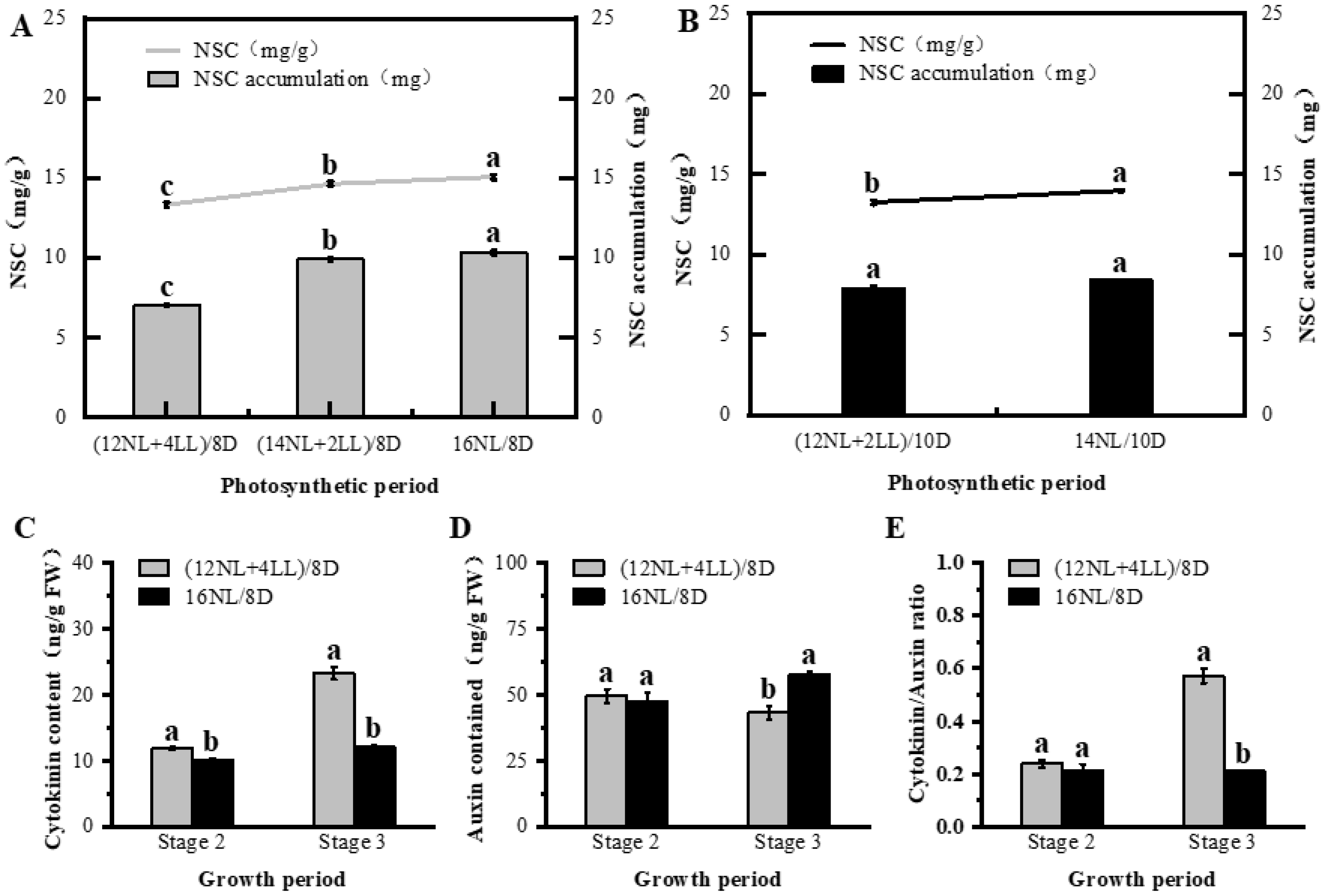
2.3. Influence of Photosynthetic Photoperiod on Post-Flowering
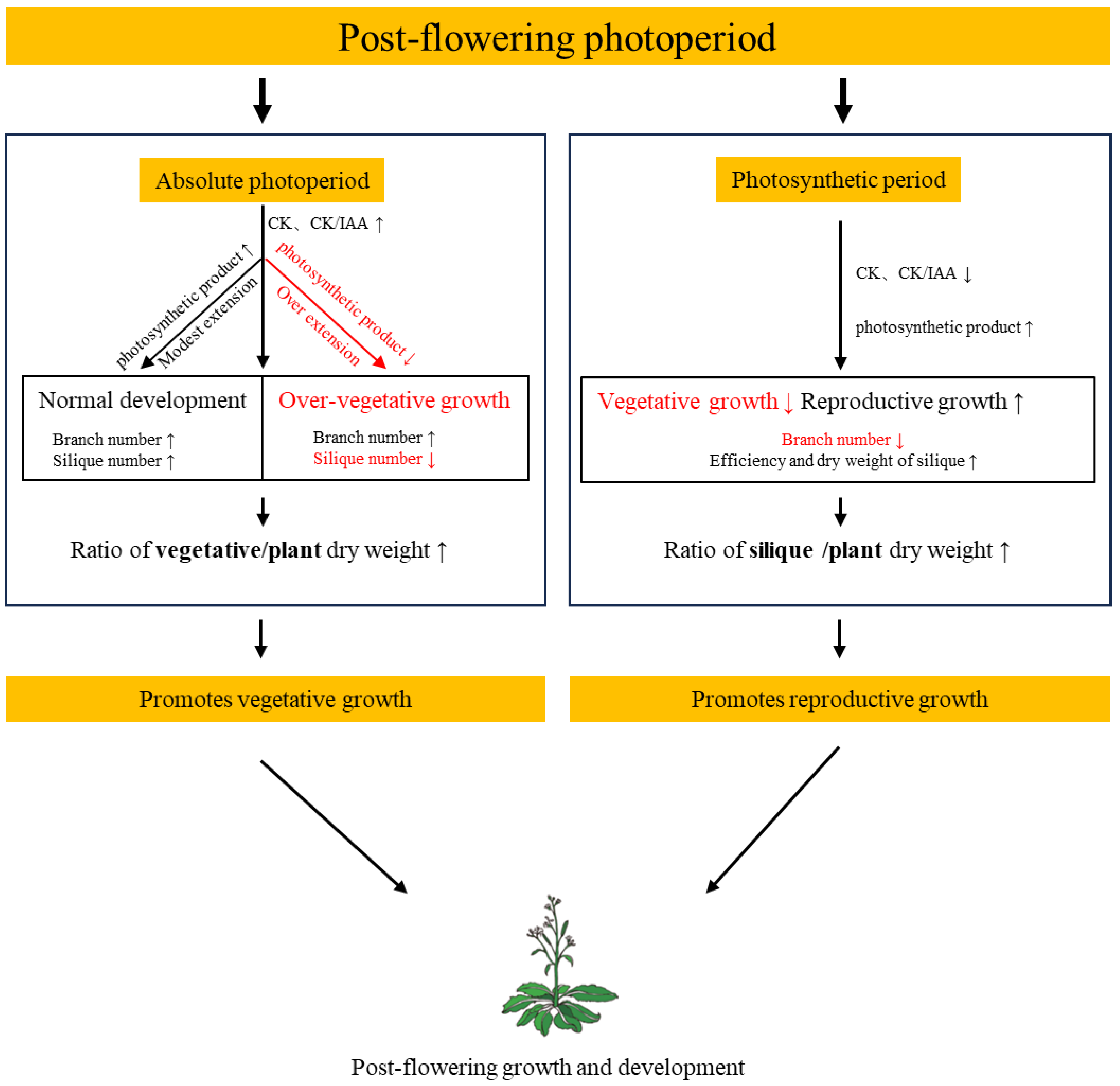
3. Discussion
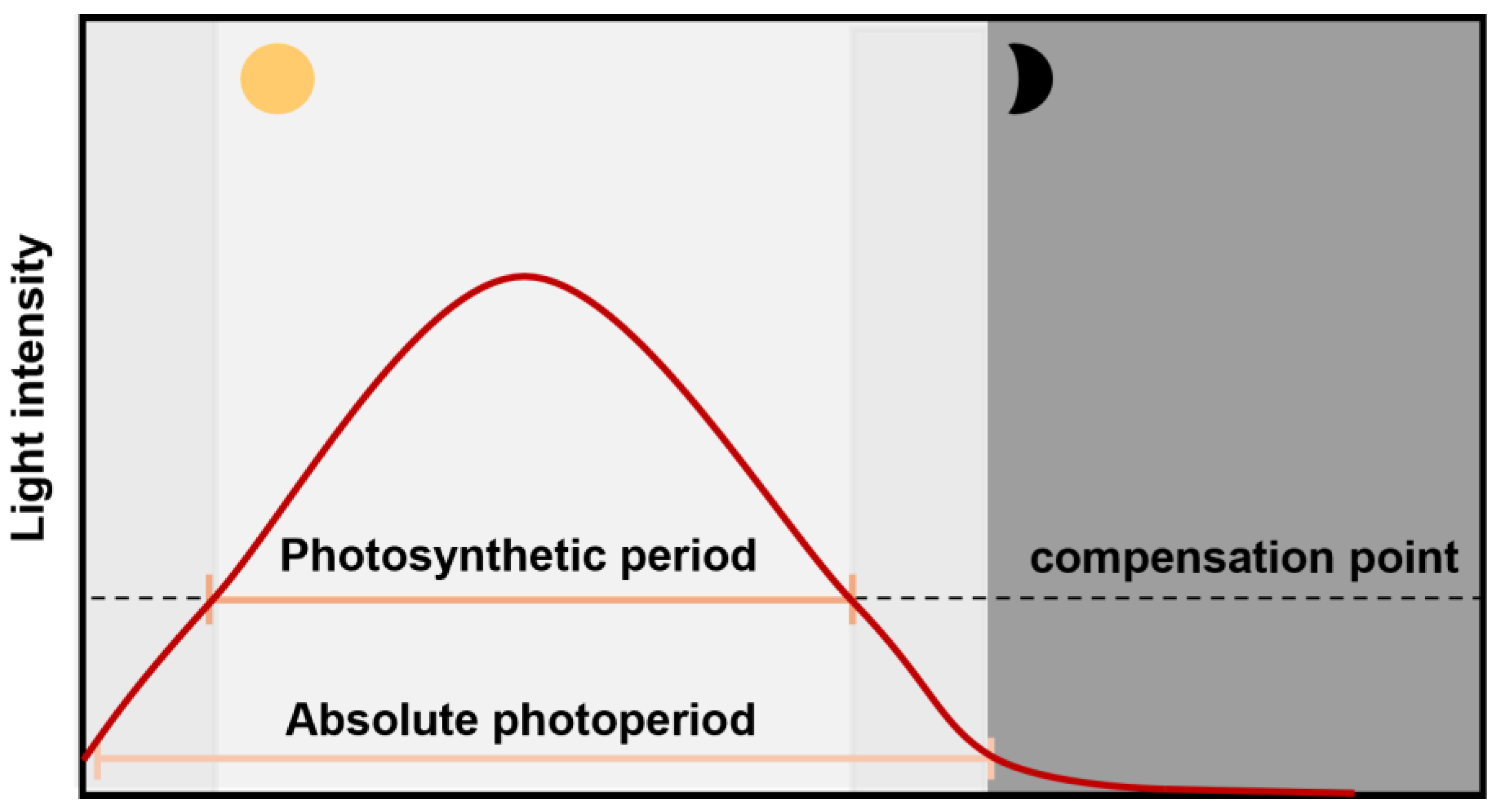
3.1. Absolute Photoperiod (Signaling Effect of Photoperiod)
3.2. Photosynthetic Period (Energetic Effect of Photoperiod)
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growing Conditions
5.2. Experimental Treatments
5.3. Morphological Indicators
5.4. Chlorophyll Content
5.5. Determination of Photosynthetic Gas Exchange Parameters
5.6. Extraction and Determination of Soluble Sugar and Starch
5.7. Phytohormone Content
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Mon. Weather Rev. 1920, 48, 415. [Google Scholar] [CrossRef]
- Putterill, J.; Stockum, C.; Warman, G. Photoperiodic flowering in the long-day plant Arabidopsis thaliana. In Photoperiodism: The Biological Calendar; Nelson, R.J., Denlinger, D.L., Somers, D.E., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 9–11. [Google Scholar]
- Adams, S.R.; Langton, F.A. Photoperiod and plant growth: A review. J. Hortic. Sci. Biotech. 2005, 80, 2–10. [Google Scholar] [CrossRef]
- Devlin, P. Photocontrol of flowering. In Light and Plant Development; Whitelam, G.C., Halliday, K.J., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 185–186. [Google Scholar]
- Gendron, J.M.; Staiger, D. New horizons in plant photoperiodism. Annu. Rev. Plant Biol. 2023, 74, 481–509. [Google Scholar] [CrossRef]
- Nico, M.; Mantese, A.I.; Miralles, D.J.; Kantolic, A.G. Soybean fruit development and set at the node level under combined photoperiod and radiation conditions. J. Exp. Bot. 2016, 67, 365–377. [Google Scholar] [CrossRef]
- Yu, B.; He, X.M.; Tang, Y.; Chen, Z.H.; Zhou, L.M.; Li, X.M.; Zhang, C.Y.; Huang, X.; Yang, Y.H.; Zhang, W.B.; et al. Photoperiod controls plant seed size in a CONSTANS-dependent manner. Nat. Plants 2023, 9, 343–354. [Google Scholar] [CrossRef]
- Criswell, J.G.; Hume, D.J. Variation in sensitivity to photoperiod among early maturing soybean strains. Crop Sci. 1972, 12, 657–660. [Google Scholar] [CrossRef]
- Komatsu, K.; Hwang, T.Y.; Takahashi, M.; Sayama, T.; Funatsuki, H.; Oki, N.; Ishimoto, M. Identification of QTL controlling post-flowering period in soybean. Breeding Sci. 2012, 61, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Xu, Z.H.; Liu, B.H.; Kong, F.J.; Tsubokura, Y.; Watanabe, S.; Xia, Z.J.; Harada, K.; Kanazawa, A.; Yamada, T.; et al. Genetic variation in four maturity genes affects photoperiod insensitivity and PHYA-regulated post-flowering responses of soybean. BMC Plant Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Takeshima, R.; Yabe, S.; Matsui, K. Genetic basis of maturity time is independent from that of flowering time and contributes to ecotype differentiation in common buckwheat (Fagopyrum esculentum Moench). BMC Plant Biol. 2022, 22, 353. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, S.S. Effects of photoperiod on wheat growth, development and yield in CELSS. Acta Astronaut. 2014, 105, 24–29. [Google Scholar]
- Han, T.F.; Wu, C.X.; Mentreddy, R.S.; Zhao, J.; Xu, X.; Gai, J.Y. Post-flowering photoperiod effects on reproductive development and agronomic traits of long-day and short-day crops. J. Agron. Crop Sci. 2005, 191, 255–262. [Google Scholar] [CrossRef]
- Shanmugasundaran, S.; Tsou, S.C. Photoperiod and critical duration for flower induction in soybean. Crop Sci. 1978, 18, 598–601. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Kumar, A.; Patil, N.S.; Malankar, N.N.; Saha, K.; Banerjee, A.K. Development of aerial and belowground tubers in potato is governed by photoperiod and epigenetic mechanism. Plant Physiol. 2021, 187, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Board, J.E.; Settimi, J.R. Photoperiod effect before and after flowering on branch development in determinate soybean. Agron. J. 1986, 78, 995–1002. [Google Scholar] [CrossRef]
- Caffaro, S.V.; Martigmone, R.A.; Torres, R.; Nakayama, F. Photoperiod regulation of vegetative growth and meristem behavior toward flower initiation of an indeterminate soybean. Bot. Gaz. 1988, 149, 311–316. [Google Scholar] [CrossRef]
- Kelly, S.J.; Cano, M.G.; Fanello, D.D.; Tambussi, E.A.; Guiamet, J.J. Extended photoperiods after flowering increase the rate of dry matter production and nitrogen assimilation in mid maturing soybean cultivars. Field Crops Res. 2021, 265, 108104. [Google Scholar] [CrossRef]
- Washburn, C.F.; Thomas, J.F. Reversion of flowering in Glycine max (Fabaceae). Am. J. Bot. 2000, 87, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Han, T.F.; Wu, C.X.; Tong, Z.; Mentreddy, R.S.; Tan, K.H.; Gai, J.Y. Postflowering photoperiod regulates vegetative growth and reproductive development of soybean. Environ. Exp. Bot. 2006, 55, 120–129. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Photoperiodic control of flower initiation. In Photoperiodism in Plants; Academic Press: London, UK, 1997; pp. 3–26. [Google Scholar]
- Mazumdar, B.C. Physical aspects of light and its role in plants. In Photoperiodism and Vernalization in Plants; Daya Publishing House: Delhi, India, 2025; pp. 3–17. [Google Scholar]
- Wang, Q.Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants distinguish different photoperiods to independently control seasonal flowering and growth. Science 2024, 383, eadg9196. [Google Scholar] [CrossRef]
- Nico, M.; Miralles, D.J.; Kantolic, A.G. Post-flowering photoperiod and radiation interaction in soybean yield determination: Direct and indirect photoperiodic effects. Field Crops Res. 2015, 176, 45–55. [Google Scholar] [CrossRef]
- Benner, B.L. Effects of apex removal and nutrient supplementation on branching and seed production in Thlaspi arvense (Brassicaceae). Am. J. Bot. 1988, 75, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Nico, M.; Miralles, D.J.; Kantolic, A.G. Natural post-flowering photoperiod and photoperiod sensitivity: Roles in yield-determining processes in soybean. Field Crops Research. 2019, 231, 141–152. [Google Scholar] [CrossRef]
- Hay, R.K.M. The influence of photoperiod on the dry matter production of grasses and cereals. New Phytol. 1990, 116, 233–254. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Q.M.; Guo, Y.P.; Guo, D.P.; Shah, G.A.; Liu, H.L.; Mao, A. Stem-swelling and photosynthate partitioning in stem mustard are regulated by photoperiod and plant hormones. Environ. Exp. Bot. 2008, 62, 160–167. [Google Scholar] [CrossRef]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [CrossRef]
- Sang, D.J.; Chen, D.Q.; Liu, G.F.; Liang, Y.; Huang, L.Z.; Meng, X.B.; Chu, J.F.; Sun, X.H.; Dong, G.J.; Yuan, Y.D.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef]
- Emery, R.N.; Longnecker, N.E.; Atkins, C.A. Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J. Exp. Bot. 1998, 49, 555–562. [Google Scholar]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. How to grow Arabidopsis. In Arabidopsis: A Laboratory Manual; Spring Harbor Laboratory Press: New York, NY, USA, 2002; pp. 1–16. [Google Scholar]
- Donahue, R.A.; Poulson, M.E.; Edwards, G.E. A method for measuring whole plant photosynthesis in Arabidopsis thaliana. Photosynth. Res. 1997, 52, 263–269. [Google Scholar] [CrossRef]
- Van Brenk, J.B.; Courbier, S.; Kleijweg, C.L.; Verdonk, J.C.; Marcelis, L.F. Paradise by the far-red light: Far-red and red: Blue ratios independently affect yield, pigments, and carbohydrate production in lettuce, Lactuca sativa. Front. Plant Sci. 2024, 15, 1383100. [Google Scholar]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Gorlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]
- Tian, H.Q.; Zhou, Q.P.; Liu, W.H.; Zhang, J.; Chen, Y.J.; Jia, Z.F.; Shao, Y.Q.; Wang, H. Responses of photosynthetic characteristics of oat flag leaf and spike to drought stress. Front. Plant Sci. 2022, 13, 917528. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Wang, F.B.; Lin, W.W.; Zhao, Q.; Liu, J.C.; Cheng, F.M. Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiol. Plant. 2017, 39, 1–14. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, K.H.; Li, G.H.; Pan, J.F.; Huang, J.L.; Peng, S.B. Stem small vascular bundles have greater accumulation and translocation of non-structural carbohydrates than large vascular bundles in rice. Physiol. Plant. 2022, 174, e13695. [Google Scholar] [CrossRef] [PubMed]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- Savi, T.; García González, A.; Herrera, J.C.; Forneck, A. Gas exchange, biomass and non-structural carbohydrates dynamics in vines under combined drought and biotic stress. BMC Plant Biol. 2019, 19, 1–11. [Google Scholar] [CrossRef]
| Photoperiod | Morphological and Developmental Parameter | ||||
|---|---|---|---|---|---|
| Plant Height (mm) | Total Branch Number | Total Branch Length (mm) | Silique Number | Post-Flowering Duration (Days) | |
| 12NL/12D | 399.4 ± 15.4 a | 51.2 ± 3.5 c | 6325.6 ± 351.4 c | 477.2 ± 25.7 c | 64.6 ± 2.1 c |
| 14NL/10D | 397.0 ± 13.0 a | 58.9 ± 4.3 b | 6751.0 ± 467.0 b | 555.4 ± 19.0 b | 72.3 ± 1.7 b |
| 16NL/8D | 396.0 ± 13.8 a | 62.4 ± 5.5 a | 7308.0 ± 387.5 a | 583.1 ± 19.7 a | 83.8 ± 2.0 a |
| Group | Illumination Treatment | Morphological Parameter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Height (mm) | Total Branches | Total Branches Length (mm) | Silique Number | Rate of Effective Silique (%) | Silique Length (mm) | Seed Number per Silique | 100-Seed Weight (mg) | ||
| I | 12 NL/12D | 399.4 ± 15.4 a | 51.2 ± 3.5 c | 6325.6 ± 351.4 c | 477.2 ± 25.7 c | 63.7 ± 1.9 c | 7.4 ± 2.1 a | 18.6 ± 9.2 a | 1.74 ± 011 b |
| (12NL + 2LL)/10D | 397.0 ± 14.2 a | 62.4 ± 1.8 b | 6763.8 ± 424.8 a | 560.1 ± 21.4 a | 69.3 ± 1.8 a | 7.9 ± 2.1 a | 19.5 ± 7.6 a | 1.86 ± 0.05 b | |
| (12NL + 4LL)/8D | 385.1 ± 14.5 b | 67.5 ± 5.9 a | 6659.3 ± 304.0 a | 516.8 ± 32.9 b | 67.0 ± 1.5 b | 7.8 ± 2.1 a | 19.1 ± 9.8 a | 2.06 ± 0.11 a | |
| II | 14NL/10D | 397.0 ± 13.0 a | 58.9 ± 4.3 b | 6750.7 ± 466.8 b | 555.4 ± 19.0 b | 72.1 ± 1.4 b | 8.8 ± 1.9 a | 23.4 ± 10.4 a | 1.88 ± 0.08 b |
| (14NL + 2LL)/8D | 397.0 ± 12.3 a | 64.5 ± 2.1 a | 7292.9 ± 312.2 a | 593.2 ± 21.9 a | 74.4 ± 1.6 a | 9.2 ± 2.1 a | 25.6 ± 11.1 a | 2.04 ± 0.09 a | |
| Group | Illumination Treatment | Morphological Parameter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Height (mm) | Total Branches | Total Branches Length (mm) | Silique Number | Rate of Effective Silique | Silique Length (mm) | Seed Number per Silique | 100-Seed Weight (mg) | ||
| I | (12NL + 4LL)/8D | 385.1 ± 14.5 b | 67.5 ± 5.9 a | 6659.3 ± 304.0 b | 516.8 ± 32.9 b | 67.0 ± 1.5 c | 7.8 ± 2.1 b | 19.1 ± 9.8 b | 2.06 ± 0.11 a |
| (14NL + 2LL)/8D | 397.0 ± 12.3 a | 64.5 ± 2.1 b | 7292.9 ± 312.2 a | 593.2 ± 21.9 a | 74.4 ± 1.6 b | 9.2 ± 2.1 a | 25.6 ± 11.1 a | 2.04 ± 0.09 a | |
| 16NL/8D | 396.0 ± 13.8 a | 62.4 ± 5.5 b | 7307.8 ± 387.5 a | 583.1 ± 20.0 a | 77.2 ± 1.3 a | 9.3 ± 2.0 a | 27.9 ± 13.0 a | 2.06 ± 0.11 a | |
| II | (12NL + 2LL)/10D | 397.0 ± 14.2 a | 62.4 ± 1.8 a | 6763.8 ± 424.8 a | 560.1 ± 21.4 a | 69.3 ± 1.8 b | 7.9 ± 2.1 a | 19.5 ± 7.6 a | 1.86 ± 0.05 a |
| 14NL/10D | 397.0 ± 13.0 a | 58.9 ± 4.3 b | 6750.7 ± 466.8 a | 555.4 ± 19.0 a | 72.1 ± 1.4 a | 8.8 ± 1.9 a | 23.4 ± 10.4 a | 1.88 ± 0.08 a | |
| Photoperiod | Group | Light/Dark Period (h) | Illumination Treatment |
|---|---|---|---|
| Normal photoperiod (Experiment 1) | I | 12L/12D | 12NL/12D |
| 14L/10D | 14NL/10D | ||
| 16L/8D | 16NL/8D | ||
| Absolute photoperiod (Experiment 2) | I | 12L/12D | 12NL/12D |
| 14L/10D | (12NL + 2LL)/10D | ||
| 16L/8D | (12NL + 4LL)/8D | ||
| II | 14L/10D | 14NL/10D | |
| 16L/8D | (14NL + 2LL)/8D | ||
| Photosynthetic period (Experiment 3) | I | 16L/8D | (12NL + 4LL)/8D |
| 16L/8D | (14NL + 2LL)/8D | ||
| 16L/8D | 16NL/8D | ||
| II | 14L/10D | (12NL + 2LL)/10D | |
| 14L/10D | 14NL/10D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, Z.; Jiang, L.; Yimingniyazi, A.; Ren, C. Plants Distinguish Different Photoperiods to Independently Regulate Post-Flowering Vegetative Growth and Reproductive Growth. Plants 2025, 14, 1368. https://doi.org/10.3390/plants14091368
Chen W, Wang Z, Jiang L, Yimingniyazi A, Ren C. Plants Distinguish Different Photoperiods to Independently Regulate Post-Flowering Vegetative Growth and Reproductive Growth. Plants. 2025; 14(9):1368. https://doi.org/10.3390/plants14091368
Chicago/Turabian StyleChen, Weizhi, Ziyi Wang, Lamei Jiang, Amanula Yimingniyazi, and Cai Ren. 2025. "Plants Distinguish Different Photoperiods to Independently Regulate Post-Flowering Vegetative Growth and Reproductive Growth" Plants 14, no. 9: 1368. https://doi.org/10.3390/plants14091368
APA StyleChen, W., Wang, Z., Jiang, L., Yimingniyazi, A., & Ren, C. (2025). Plants Distinguish Different Photoperiods to Independently Regulate Post-Flowering Vegetative Growth and Reproductive Growth. Plants, 14(9), 1368. https://doi.org/10.3390/plants14091368





