Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of LEO
2.2. Determination of the Antioxidant Activity of LEO
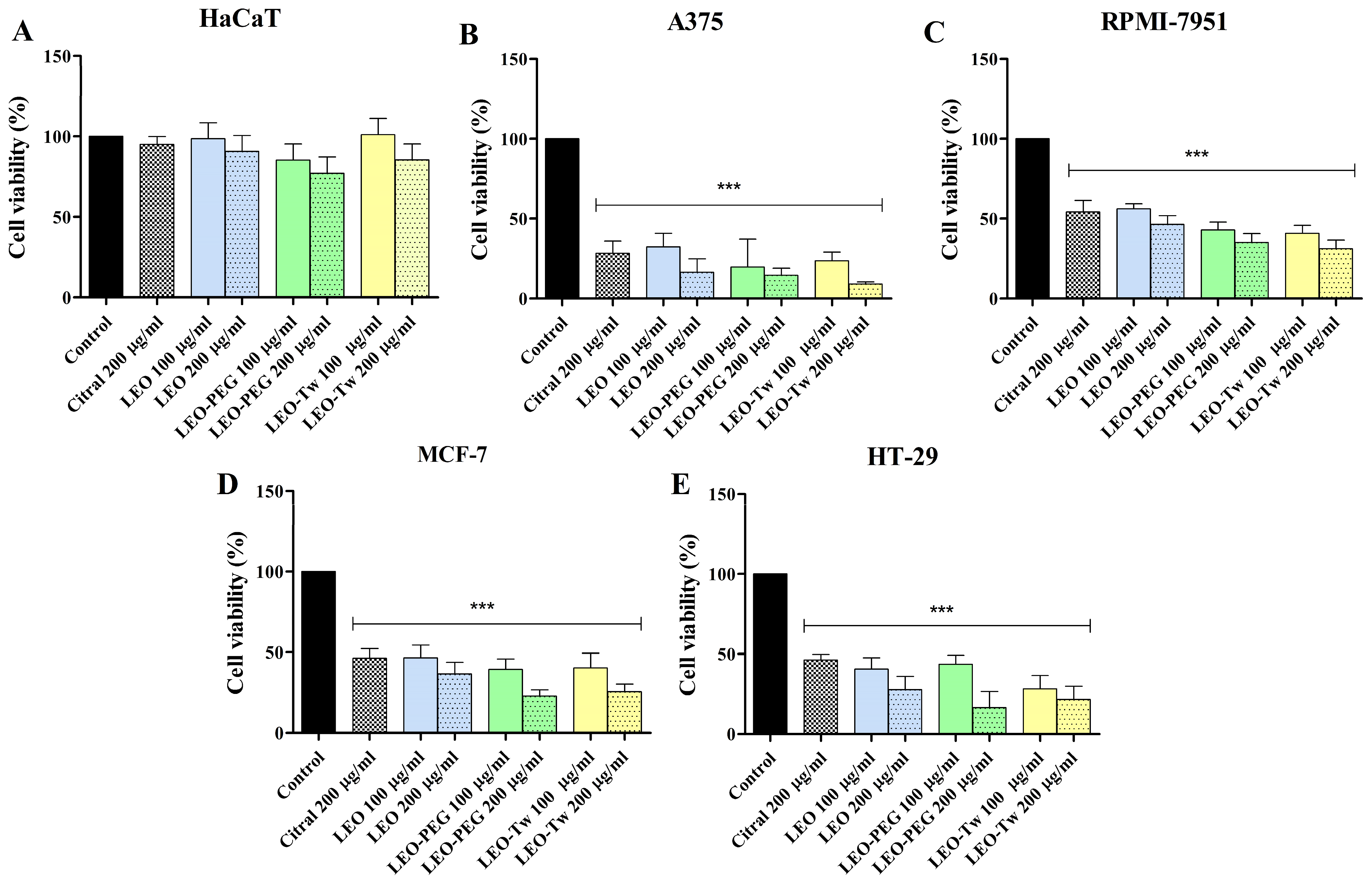
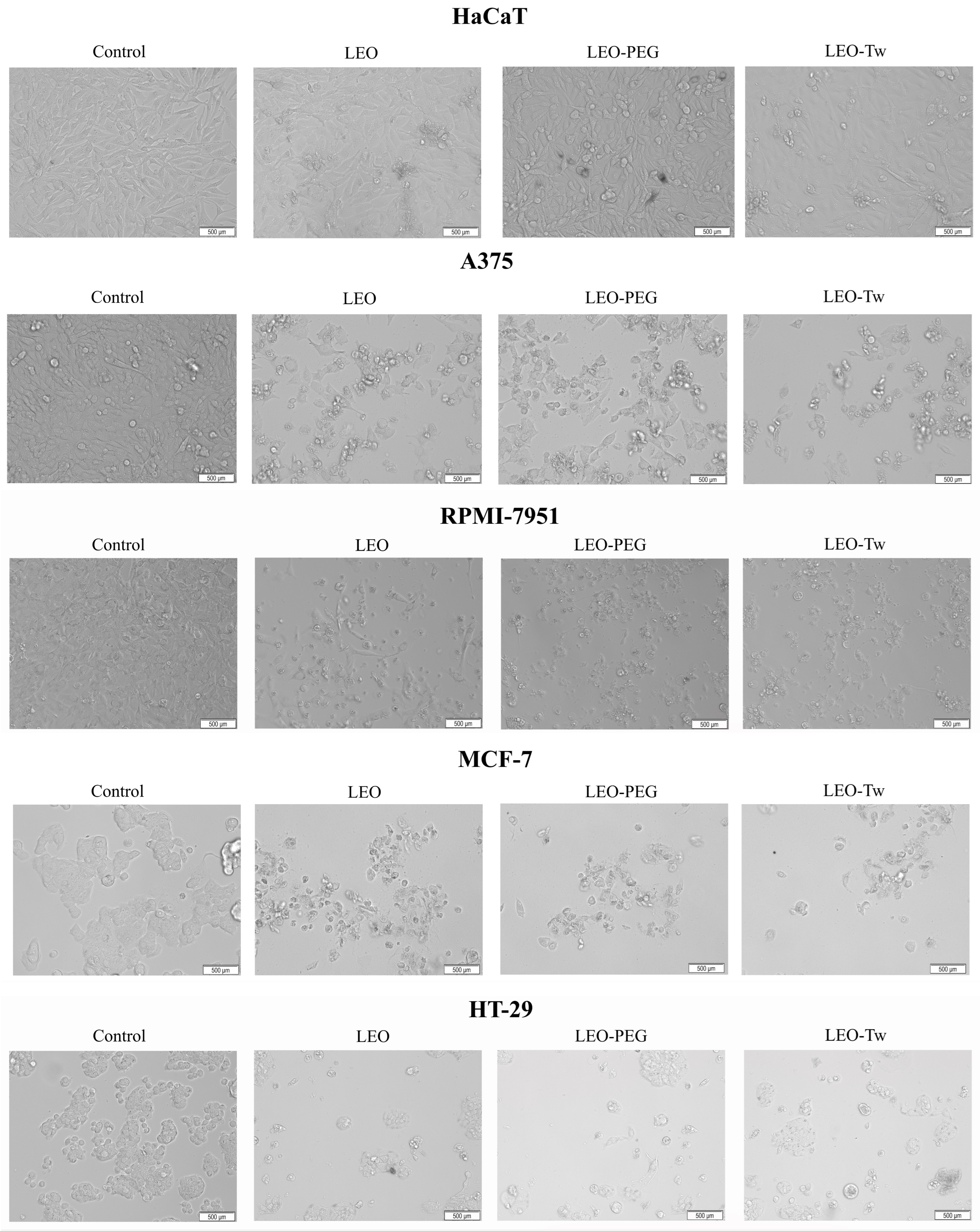
2.3. LEO Effect on Cell Viability
2.4. LEO Increases ROS Production
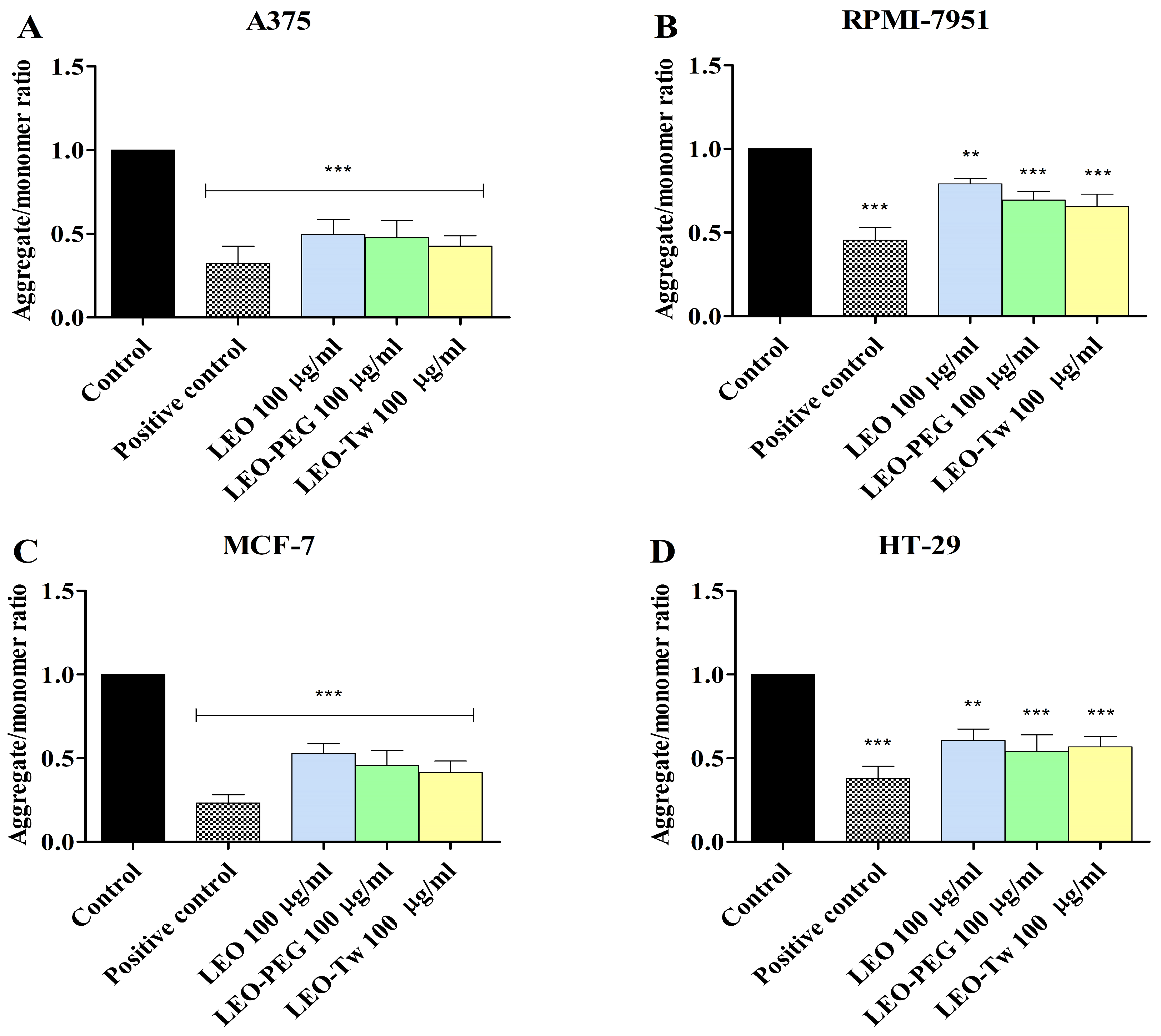
2.5. LEO Decreases Mitochondrial Membrane Potential
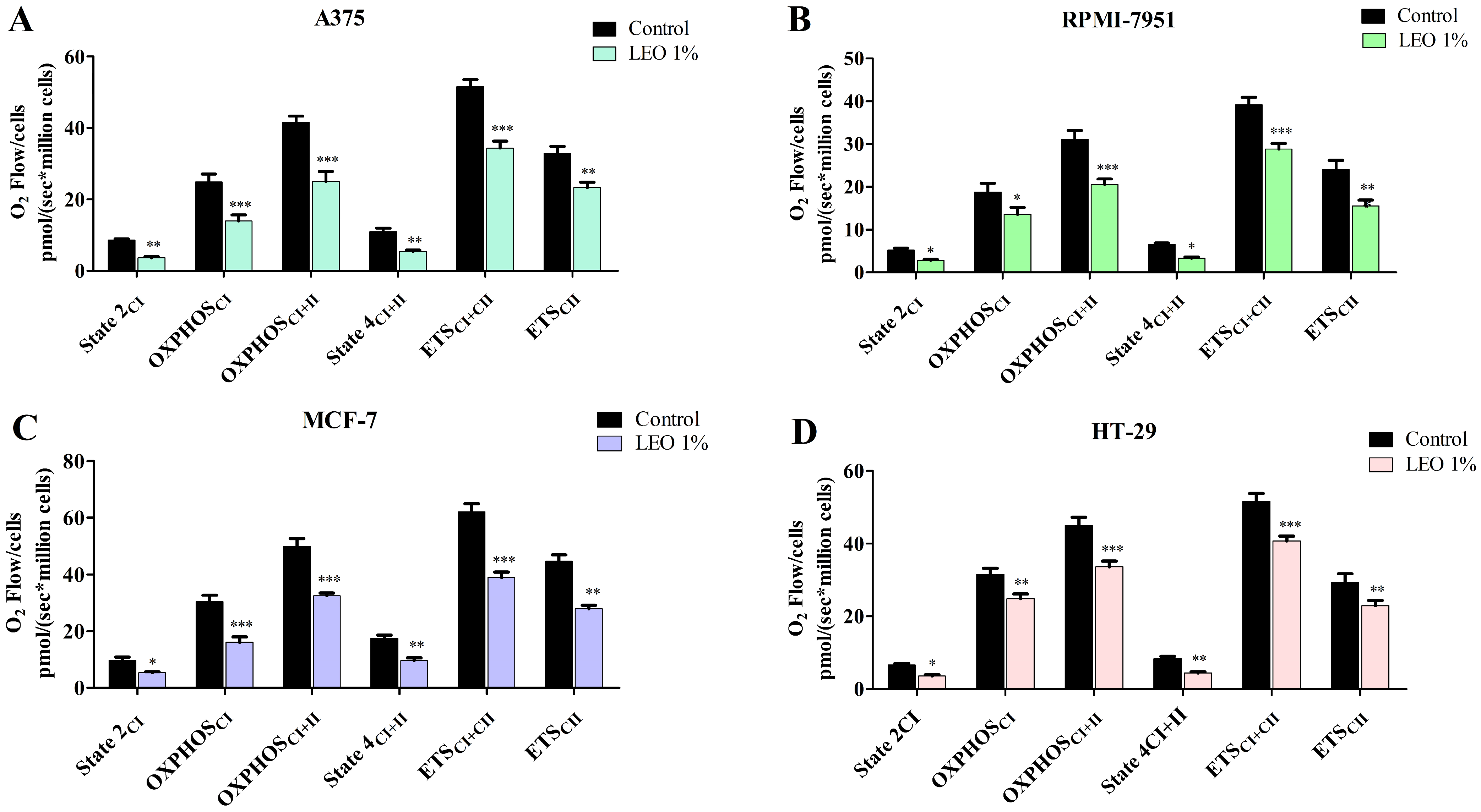
2.6. LEO Effect on Mitochondrial Function
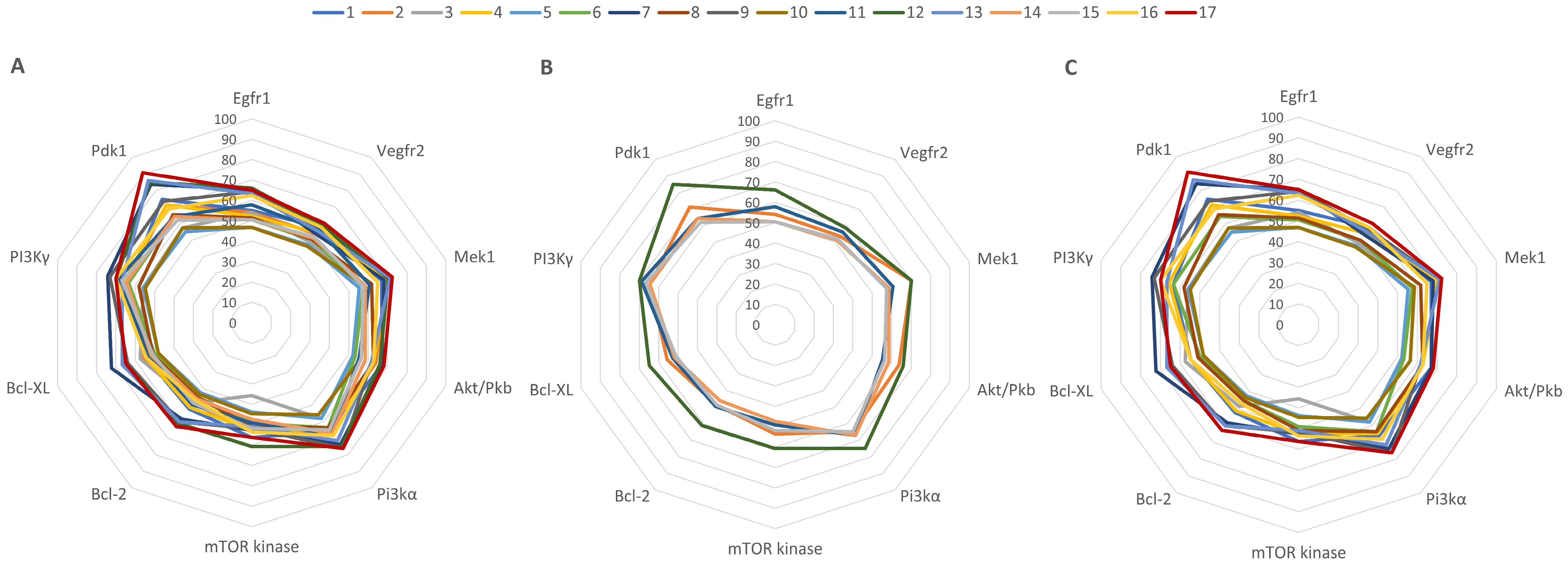
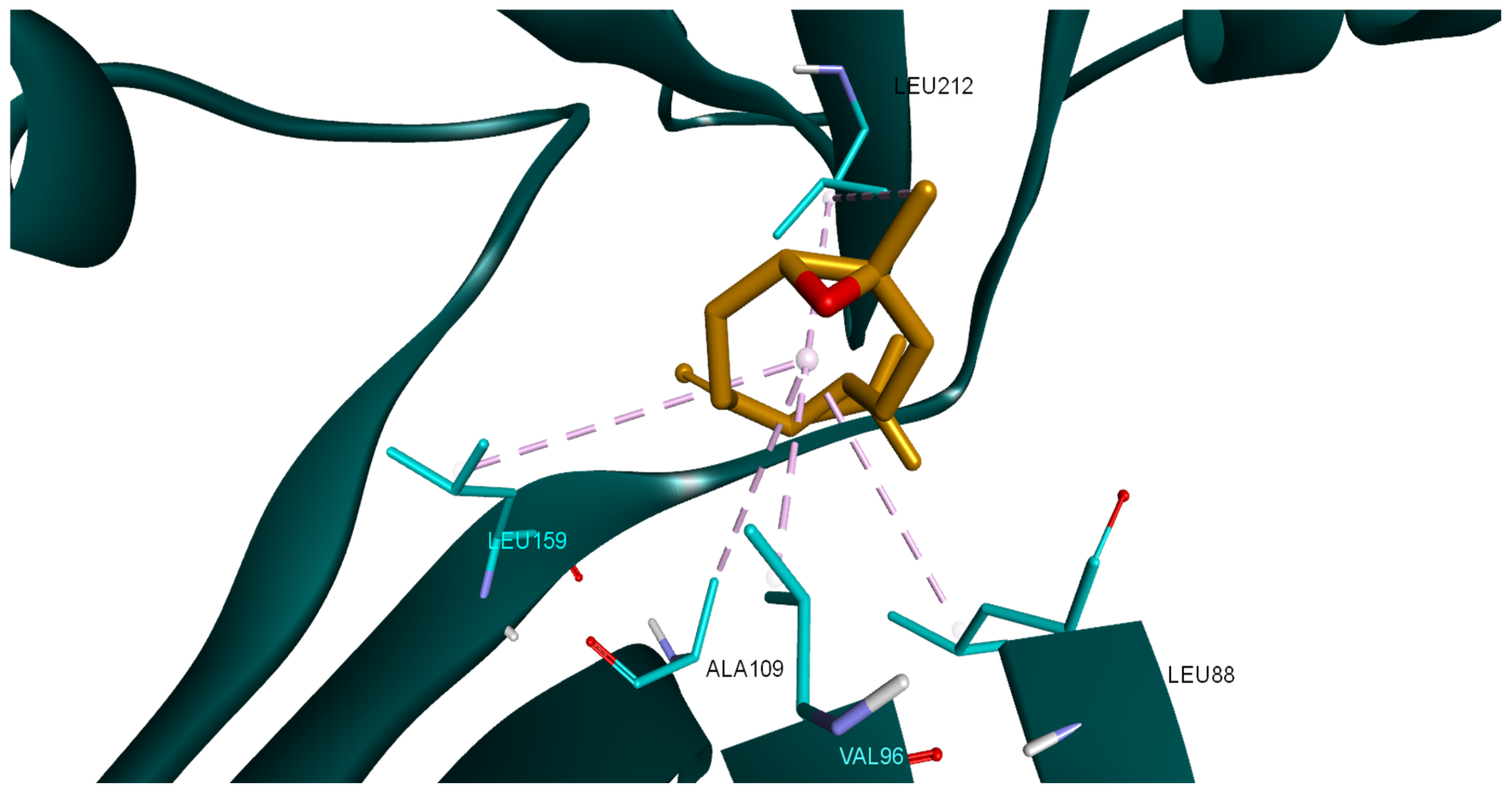
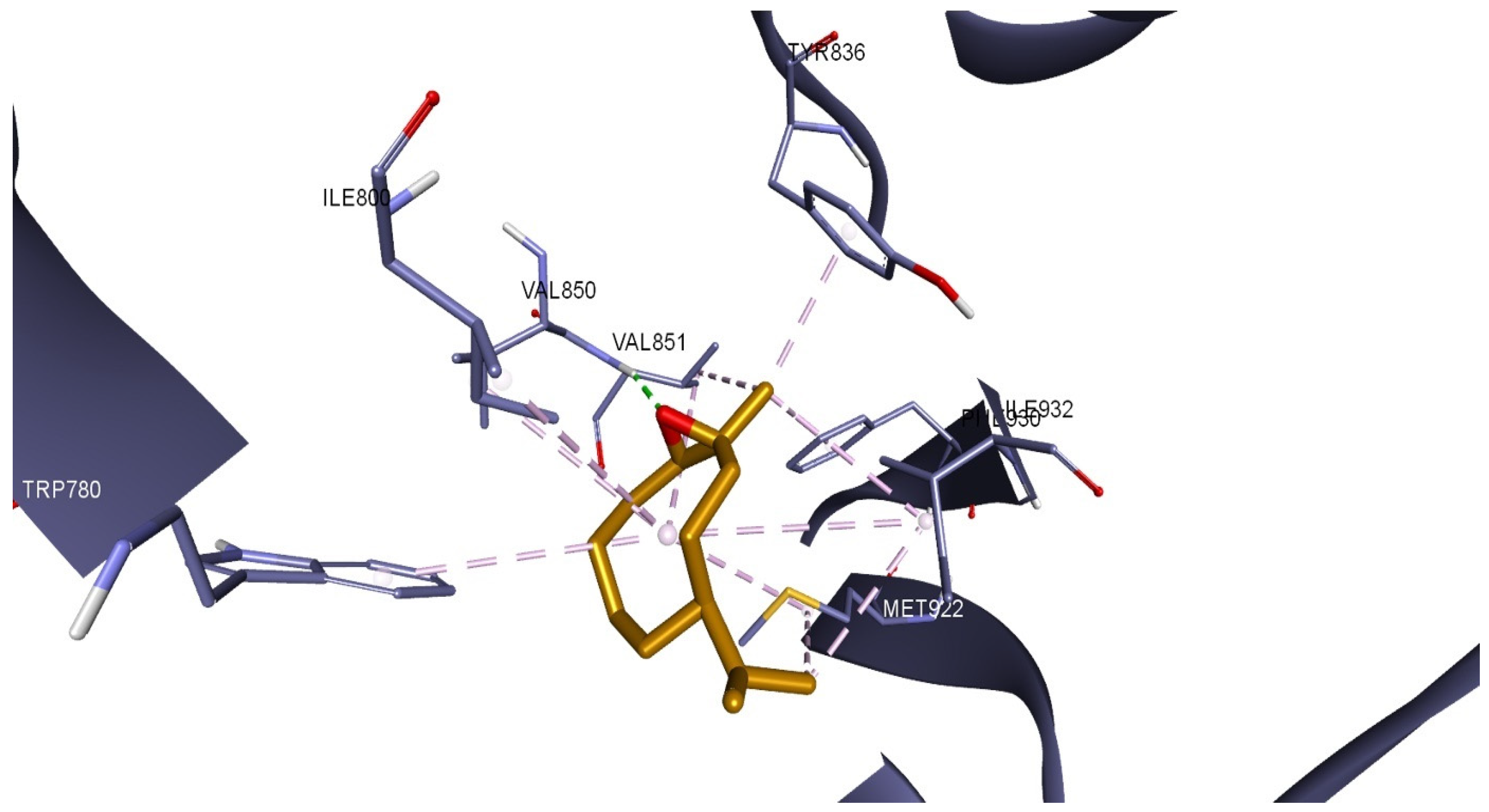
2.7. Molecular Docking of LEO Components
3. Discussion
4. Materials and Methods
4.1. LEO Extraction and GC-MS Analysis
4.2. DPPH Antioxidant Scavenging Activity Determination
4.3. ABTS Radical Scavenging Assay
4.4. Cell Culture
4.5. Cell Viability Assessment
4.6. Assessment of Cellular ROS Production
4.7. JC-1 Assay
4.8. High-Resolution Respirometry
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-drug Resistance (MDR): Reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.; Abarikwu, S.O.; Mmema, L.; Jibril, A.T.; Rahmah, L.; Ivanovska, M.; Rahimi, A.M.; Joya, M.; Hashem, F.; Essouma, M.; et al. A Global Perspective of Cancer Prevalence: The Causative Agent, the Environment, or the Genes? In Handbook of Cancer and Immunology; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–21. [Google Scholar]
- Lin, S.; Chang, C.; Hsu, C.; Tsai, M.; Cheng, H.; Leong, M.K.; Sung, P.; Chen, J.; Weng, C. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009, 179, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, R.; Shiralgi, Y.; Lakkappa, D.B.; Hegde, G. Essential oil from Cymbopogon flexuosus as the potential inhibitor for HSP90. Toxicol. Rep. 2018, 5, 489–496. [Google Scholar] [CrossRef]
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest. J. Breast Cancer 2015, 18, 225. [Google Scholar] [CrossRef]
- Bailly, C. Targets and pathways involved in the antitumor activity of citral and its stereo-isomers. Eur. J. Pharmacol. 2020, 871, 172945. [Google Scholar] [CrossRef]
- Viktorová, J.; Stupák, M.; Řehořová, K.; Dobiasová, S.; Hoang, L.; Hajšlová, J.; Van Thanh, T.; Van Tri, L.; Van Tuan, N.; Ruml, T. Lemon Grass Essential Oil does not Modulate Cancer Cells Multidrug Resistance by Citral—Its Dominant and Strongly Antimicrobial Compound. Foods 2020, 9, 585. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.Y.; Sarker, M.M.R.; Kamarudin, M.N.A.; Mohan, G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed. Pharmacother. 2017, 96, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Sanches, L.J.; Marinello, P.C.; Panis, C.; Fagundes, T.R.; Morgado-Díaz, J.A.; De-Freitas-Junior, J.C.M.; Cecchini, R.; Cecchini, A.L.; Luiz, R.C. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumor Biol. 2017, 39, 101042831769591. [Google Scholar] [CrossRef]
- Younis, N.K.; Roumieh, R.; Bassil, E.P.; Ghoubaira, J.A.; Kobeissy, F.; Eid, A.H. Nanoparticles: Attractive tools to treat colorectal cancer. Semin. Cancer Biol. 2022, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lupea-Chilom, D.-S.; Solovan, C.S.; Farcas, S.S.; Gogulescu, A.; Andreescu, N.I. Latent Tuberculosis in Psoriasis Patients on Biologic Therapies: Real-World Data from a Care Center in Romania. Medicina 2023, 59, 1015. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Cruz-Martins, N. Advances in Plants-Derived Bioactives for Cancer Treatment. Cells 2023, 12, 1112. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- Rahimi, G.; Yousefnia, S.; Angnes, L.; Negahdary, M. Design a PEGylated nanocarrier containing lemongrass essential oil (LEO), a drug delivery system: Application as a cytotoxic agent against breast cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104183. [Google Scholar] [CrossRef]
- Basiouny, E.; Abdakaway, A.; Asker, M.; Abozid, M. A comparative study between three essential oils in terms of their chemical composition and antioxidant activity. Menoufia J. Agric. Biotechnol. 2023, 8, 81–91. [Google Scholar] [CrossRef]
- Al Weshahi, H.; Akhtar, M.S.; Al Tobi, S.S.; Hossain, A.; Khan, S.A.; Akhtar, A.B.; Said, S.A. Evaluation of acute plant toxicity, antioxidant activity, molecular docking and bioactive compounds of lemongrass oil isolated from Omani cultivar. Toxicol. Reports 2025, 14, 101888. [Google Scholar] [CrossRef]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil—A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Cieśla, Ł.M.; Waksmundzka-Hajnos, M. Approach to Determination a Structure—Antioxidant Activity Relationship of Selected Common Terpenoids Evaluated by ABTS•+ Radical Cation Assay. Nat. Prod. Commun. 2018, 13, 295–299. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Gopakumar, V.; Nagarajan, R. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomed. 2019, 14, 6439–6450. [Google Scholar] [CrossRef] [PubMed]
- Roozitalab, G.; Yousefpoor, Y.; Abdollahi, A.; Safari, M.; Rasti, F.; Osanloo, M. Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem. Pap. 2022, 76, 4261–4271. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Al-Hamoud, G.A.; Al-Dbass, A.; El-Ansary, A.; Ali, M.A. Prospective of biosynthesized L. satiVum oil/PEG/Ag-MgO bionanocomposite film for its antibacterial and anticancer potential. Saudi J. Biol. Sci. 2021, 28, 5971–5985. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, Q.; Wang, Q.-L.; Adu-Frimpong, M.; Wei, C.-M.; Weng, W.; Bao, R.; Wang, Y.-P.; Yu, J.-N.; Xu, X.M. Improvement of Oral Bioavailability and Anti-Tumor Effect of Zingerone Self-Microemulsion Drug Delivery System. J. Pharm. Sci. 2021, 110, 2718–2727. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Bruschi, M.L. Self-Emulsifying Systems for Delivery of Bioactive Compounds from Natural Origin. AAPS PharmSciTech 2022, 23, 134. [Google Scholar] [CrossRef]
- Milan, A.; Mioc, M.; Mioc, A.; Gogulescu, A.; Mardale, G.; Avram, Ș.; Maksimović, T.; Mara, B.; Șoica, C. Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines. Molecules 2024, 29, 3399. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanayem, A.A. Antifungal Activity of Cymbopogon flexuosus Essential Oil and its Effect on Biofilm Formed by Candida parapsilosis and Candida tropicalis on Polystyrene and Polyvinyl Plastic Surfaces. Indian J. Pharm. Educ. Res. 2023, 57, 113–119. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, F.; Bhushan, S.; Sethi, V.K.; Shahi, A.K.; Kaur, J.; Taneja, S.C.; Qazi, G.N.; Singh, J. An essential oil and its major constituent isointermedeol induce apoptosis by increased expression of mitochondrial cytochrome c and apical death receptors in human leukaemia HL-60 cells. Chem. Biol. Interact. 2008, 171, 332–347. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Zheng, Y.; Cha, Y.-Y.; Feng, Y.; Dai, S.-X.; Zhao, S.; Chen, H.; Xu, M. Essential oil of lemon myrtle (Backhousia citriodora) induces S-phase cell cycle arrest and apoptosis in HepG2 cells. J. Ethnopharmacol. 2023, 312, 116493. [Google Scholar] [CrossRef]
- Luang-In, V.; Saengha, W.; Karirat, T.; Senakun, C.; Siriamornpun, S. Phytochemical Profile of Cymbopogon citratus (DC.) Stapf Lemongrass Essential Oil from Northeastern Thailand and Its Antioxidant and Antimicrobial Attributes and Cytotoxic Effects on HT-29 Human Colorectal Adenocarcinoma Cells. Foods 2024, 13, 2928. [Google Scholar] [CrossRef]
- Srivastava, G.; Mukherjee, E.; Mittal, R.; Ganjewala, D. Geraniol and citral: Recent developments in their anticancer credentials opening new vistas in complementary cancer therapy. Z. Für Naturforsch. C 2024, 79, 163–177. [Google Scholar] [CrossRef]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer activity of monoterpenes: A systematic review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Lee, J.-E.; Seo, S.-M.; Huh, M.-J.; Lee, S.-C.; Park, I.-K. Reactive oxygen species mediated-antifungal activity of cinnamon bark (Cinnamomum verum) and lemongrass (Cymbopogon citratus) essential oils and their constituents against two phytopathogenic fungi. Pestic. Biochem. Physiol. 2020, 168, 104644. [Google Scholar] [CrossRef]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical Composition and In Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.A. Lemongrass Extract and Anticancer Impact: mRNA Levels of Expressing Apoptosis and Mitochondrial Fission in MCF7 Cells. Pak. J. Zool. 2024, 56, 1–7. [Google Scholar] [CrossRef]
- Alwaili, M.A. Protective effects of lemongrass (Cymbopogon citratus STAPF) extract mediated mitochondrial fission and glucose uptake inhibition in SW1417. Food Sci. Technol. 2023, 43, e94522. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Pelicano, H.; Lu, W.; Zhou, Y.; Zhang, W.; Chen, Z.; Hu, Y.; Huang, P. Mitochondrial Dysfunction and Reactive Oxygen Species Imbalance Promote Breast Cancer Cell Motility through a CXCL14-Mediated Mechanism. Cancer Res. 2009, 69, 2375–2383. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green Tea Component, Catechin, Induces Apoptosis of Human Malignant B Cells via Production of Reactive Oxygen Species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef]
- Du, J.; Daniels, D.H.; Asbury, C.; Venkataraman, S.; Liu, J.; Spitz, D.R.; Oberley, L.W.; Cullen, J.J. Mitochondrial Production of Reactive Oxygen Species Mediate Dicumarol-induced Cytotoxicity in Cancer Cells. J. Biol. Chem. 2006, 281, 37416–37426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- OuYang, Q.; Tao, N.; Zhang, M. A Damaged Oxidative Phosphorylation Mechanism Is Involved in the Antifungal Activity of Citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.; Militão, G.; De Morais, M.; De Sousa, D. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ouvry, G.; Clary, L.; Tomas, L.; Aurelly, M.; Bonnary, L.; Borde, E.; Bouix-Peter, C.; Chantalat, L.; Defoin-Platel, C.; Deret, S.; et al. Impact of Minor Structural Modifications on Properties of a Series of mTOR Inhibitors. ACS Med. Chem. Lett. 2019, 10, 1561–1567. [Google Scholar] [CrossRef]
- Craveiro, A.A. Oleos essenciais de plantas do Nordeste. In Coleção Ciência; Edições UFC: Fortaleza, Brazil, 1981; p. 209. [Google Scholar]
- Craveiro, A.A.; Matos, F.J.A.; de Alencar, J.W. A simple and inexpensive steam generator for essential oils extraction. J. Chem. Educ. 1976, 53, 652. [Google Scholar] [CrossRef]
- Jianu, C.; Rusu, L.-C.; Muntean, I.; Cocan, I.; Lukinich-Gruia, A.T.; Goleț, I.; Horhat, D.; Mioc, M.; Mioc, A.; Șoica, C.; et al. In Vitro and In Silico Evaluation of the Antimicrobial and Antioxidant Potential of Thymus pulegioides Essential Oil. Antioxidants 2022, 11, 2472. [Google Scholar] [CrossRef]
- Rădulescu, M.; Jianu, C.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Șoica, C.; Stana, L.G. Chemical Composition, In Vitro and In Silico Antioxidant Potential of Melissa officinalis subsp. officinalis Essential Oil. Antioxidants 2021, 10, 1081. [Google Scholar] [CrossRef]
- Pricop, M.-A.; Lukinich-Gruia, A.T.; Cristea, I.-M.; Păunescu, V.; Tatu, C.A. Aristolochia clematitis L. Ethanolic Extracts: In Vitro Evaluation of Antioxidant Activity and Cytotoxicity on Caco-2 Cell Line. Plants 2024, 13, 2987. [Google Scholar] [CrossRef]
- Abcam, C. (UK): DCFDA/H2DCFDA—Cellular ROS Assay Kit (ab113851). Available online: https://doc.abcam.com/datasheets/active/ab113851/en-us/dcfda-h2dcfda-cellular-ros-assay-kit-ab113851.pdf (accessed on 3 December 2024).
- Abcam, C. (UK): JC-1 Mitochondrial Membrane Potential Assay Kit (ab113850). Available online: https://www.abcam.com/en-ro/products/assay-kits/jc-1-mitochondrial-membrane-potential-assay-kit-ab113850 (accessed on 3 November 2024).
- Petrus, A.T.; Lighezan, D.L.; Danila, M.D.; Duicu, O.M.; Sturza, A.; Muntean, D.M.; Ionita, I. Assessment of Platelet Respiration as Emerging Biomarker of Disease. Physiol. Res. 2019, 68, 347–363. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology: Methods and Protocols; Springer: New York, NY, USA, 2015; pp. 243–250. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef]
- Wood, E.R.; Truesdale, A.T.; McDonald, O.B.; Yuan, D.; Hassell, A.; Dickerson, S.H.; Ellis, B.; Pennisi, C.; Horne, E.; Lackey, K.; et al. A Unique Structure for Epidermal Growth Factor Receptor Bound to GW572016 (Lapatinib). Cancer Res. 2004, 64, 6652–6659. [Google Scholar] [CrossRef] [PubMed]
- Tecle, H.; Shao, J.; Li, Y.; Kothe, M.; Kazmirski, S.; Penzotti, J.; Ding, Y.-H.; Ohren, J.; Moshinsky, D.; Coli, R.; et al. Beyond the MEK-pocket: Can current MEK kinase inhibitors be utilized to synthesize novel type III NCKIs? Does the MEK-pocket exist in kinases other than MEK? Bioorg. Med. Chem. Lett. 2009, 19, 226–229. [Google Scholar] [CrossRef]
- Islam, I.; Bryant, J.; Chou, Y.-L.; Kochanny, M.J.; Lee, W.; Phillips, G.B.; Yu, H.; Adler, M.; Whitlow, M.; Ho, E.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 1: Design, synthesis and biological activity. Bioorg. Med. Chem. Lett. 2007, 17, 3814–3818. [Google Scholar] [CrossRef]
- Addie, M.; Ballard, P.; Buttar, D.; Crafter, C.; Currie, G.; Davies, B.R.; Debreczeni, J.; Dry, H.; Dudley, P.; Greenwood, R.; et al. Discovery of 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an Orally Bioavailable, Potent Inhibitor of Akt Kinases. J. Med. Chem. 2013, 56, 2059–2073. [Google Scholar] [CrossRef]
- Le, P.T.; Cheng, H.; Ninkovic, S.; Plewe, M.; Huang, X.; Wang, H.; Bagrodia, S.; Sun, S.; Knighton, D.R.; LaFleur Rogers, C.M.; et al. Design and synthesis of a novel pyrrolidinyl pyrido pyrimidinone derivative as a potent inhibitor of PI3Kα and mTOR. Bioorg. Med. Chem. Lett. 2012, 22, 5098–5103. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Lee, E.F.; Czabotar, P.E.; Smith, B.J.; Deshayes, K.; Zobel, K.; Colman, P.M.; Fairlie, W.D. Crystal structure of ABT-737 complexed with Bcl-xL: Implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007, 14, 1711–1713. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]

| No | Compound Name | RT | RI Calc | AI | Area % Calc |
|---|---|---|---|---|---|
| 1 | Terpinolene | 5.61 | 975 | 0.05 | |
| 2 | d-Limonene | 5.96 | 994 | 1024 | 7.05 |
| 3 | Eucalyptol | 6.19 | 1005 | 0.12 | |
| 4 | p-Cymene | 7.28 | 1063 | 1020 | 0.23 |
| 5 | 6-Methyl-5-hepten-2-one | 8.61 | 1133 | 1.20 | |
| 6 | Citronellal | 11.45 | 1283 | 1148 | 2.06 |
| 7 | α-Cubebene | 11.76 | 1299 | 1387 | 0.18 |
| 8 | 1,2,5,5-Tetramethyl-1,3-cyclopentadiene | 12.07 | 1316 | 0.11 | |
| 9 | Triluoroacetyl-lavandulol | 12.22 | 1324 | 0.18 | |
| 10 | Ethenyl-cyclohexane | 12.74 | 1351 | 0.27 | |
| 11 | Linalyl acetate | 13.30 | 1381 | 1254 | 9.82 |
| 12 | β-Caryophyllene | 13.86 | 1410 | 1418 | 6.73 |
| 13 | Humulene | 15.27 | 1484 | 0.93 | |
| 14 | β-Citral | 15.53 | 1498 | 1316 | 36.06 |
| 15 | α-Citral | 16.48 | 1548 | 1338 | 37.44 |
| 16 | Neryl acetate | 16.80 | 1565 | 1359 | 2.43 |
| 17 | β-Caryophyllene oxide | 20.83 | 1778 | 0.66 |
| Sample | IC50 (µg/mL) | ABTS (Inh%) |
|---|---|---|
| LEO | 92.30 ± 18.00 | 78.20 ± 0.040 |
| AA | 12.67 ± 5.28 | 88.84 ± 0.002 |
| BHA | 6.36 ± 13.56 | 88.79 ± 0.002 |
| State 2 | OXPHOSCI | OXPHOSCI + II | State 4 | ETSCI + II | ETSCI | ||
|---|---|---|---|---|---|---|---|
| A375 | Control | 8.58 | 24.9 | 41.58 | 10.97 | 51.53 | 32.84 |
| LEO | 3.64 ** | 13.97 * | 25.03 *** | 5.443 ** | 34.36 *** | 23.32 ** | |
| RPMI-7951 | Control | 5.21 | 18.75 | 31.12 | 6.505 | 39.18 | 24.04 |
| LEO | 2.84 * | 13.56 *** | 20.57 *** | 3.31 * | 28.79 *** | 15.54 ** | |
| MCF-7 | Control | 9.69 | 30.45 | 50.02 | 17.49 | 62.03 | 44.67 |
| LEO | 5.32 * | 16.07 *** | 32.53 *** | 9.66 ** | 38.88 *** | 27.89 ** | |
| HT-29 | Control | 6.603 | 31.56 | 44.90 | 8.40 | 51.60 | 29.32 |
| LEO | 3.57 * | 24.88 ** | 33.62 *** | 4.35 ** | 40.72 *** | 22.91 ** |
| Compound | Protein Targets | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Egfr1 | Vegfr2 | Mek1 | Akt | PI3Kα | mTOR | Bcl-2 | Bcl-XL | PI3Kγ | PDK1 | |
| Binding Affinity (kcal/mol) | ||||||||||
| NL | −10.9 | −12.1 | −9.4 | −9.4 | −8.8 | −11.2 | −11.3 | −10.8 | −9.3 | −8.7 |
| 1 | −6 | −7 | −6.3 | −6.3 | −5.8 | −6.3 | −5.9 | −5.9 | −5.9 | −6.5 |
| 2 | −5.9 | −6.5 | −6.6 | −6 | −5.7 | −6 | −5.5 | −6 | −6 | −6.2 |
| 3 | −5.9 | −5.9 | −5.4 | −5 | −5.1 | −4 | −5.5 | −6.2 | −5.2 | −5 |
| 4 | −5.7 | −6.6 | −6.6 | −5.9 | −5.7 | −5.9 | −5.8 | −5.9 | −6.1 | −6.2 |
| 5 | −5.1 | −5.7 | −5.2 | −4.9 | −5.1 | −4.9 | −4.8 | −5.2 | −5.2 | −4.8 |
| 6 | −5.5 | −6.1 | −5.4 | −5 | −5.6 | −5.5 | −5.1 | −5.4 | −5.9 | −5.6 |
| 7 | −7.1 | −6.5 | −6.4 | −6.3 | −6.5 | −5.9 | −6.6 | −7.8 | −6.9 | −7.3 |
| 8 | −5.6 | −6.1 | −5.8 | −5.9 | −5.6 | −5.7 | −5.1 | −5.5 | −5.4 | −5.7 |
| 9 | −7 | −6.9 | −6.7 | −5.8 | −6.8 | −5.8 | −6.8 | −6.9 | −6.8 | −6.4 |
| 10 | −5.1 | −5.6 | −5.5 | −5.3 | −4.9 | −5 | −4.9 | −5.2 | −5.1 | −5 |
| 11 | −6.3 | −6.8 | −5.7 | −5.2 | −5.9 | −5.5 | −5.6 | −5.7 | −6.4 | −5.6 |
| 12 | −7.2 | −7.1 | −6.6 | −6.2 | −6.6 | −6.8 | −6.9 | −7 | −6.5 | −7.4 |
| 13 | −6.9 | −6.7 | −6.7 | −5.8 | −6.3 | −5.7 | −6.8 | −7.2 | −6.2 | −7.5 |
| 14 | −5.5 | −6.2 | −5.5 | −5.5 | −5.9 | −5.3 | −5.2 | −5.6 | −6 | −5.6 |
| 15 | −5.5 | −6.3 | −5.4 | −5.3 | −5.7 | −5.8 | −5.5 | −5.5 | −6.2 | −5.4 |
| 16 | −6.8 | −7 | −6.1 | −5.9 | −6 | −6 | −5.3 | −5.9 | −6.5 | −6 |
| 17 | −7.1 | −7.3 | −6.8 | −6.4 | −6.5 | −6.3 | −7.1 | −7 | −6.5 | −7.9 |
| Protein (PDB ID) | Grid Box Center Coordinates | Grid Box Size | References |
|---|---|---|---|
| VEGFR2 (4ASD) | x = −23.4872 | x = 14.6340 | [68] |
| y = −1.3964 | y = 19.2181 | ||
| z = −11.0618 | z = 15.7983 | ||
| EGFR1 (1XKK) | x = 19.4479 | x = 18.8523 | [69] |
| y = 33.9295 | y = 18.8523 | ||
| z = 38.3514 | z = 18.8523 | ||
| MEK1 (3DV3) | x = 38.8352 | x = 13.0549 | [70] |
| y = −14.6371 | y = 19.2181 | ||
| z = 0.0462 | z = 11.5471 | ||
| PDK1 (2PE1) | x = −6.2833 | x = 11.6743 | [71] |
| y = 44.2844 | y = 14.1528 | ||
| z = 44.0516 | z = 11.6743 | ||
| AKT/PKB (4GV1) | x = −19.9894 | x = 14.2711 | [72] |
| y = 3.3152 | y = 13.5948 | ||
| z = 11.0426 | z = 15.4926 | ||
| PI3Kα (6GVF) | x = −17.2065 | x = 14.8956 | [55] |
| y = 147.5732 | y = 14.8956 | ||
| z = 29.1217 | z = 14.8956 | ||
| PI3Kγ (4FA6) | x = 44.2130 | x = 13.5948 | [73] |
| y = 13.1865 | y = 13.5948 | ||
| z = 29.7323 | z = 13.5948 | ||
| mTOR (4JSX) | x = 50.4459 | x = 14.2711 | [74] |
| y = −2.0684 | y = 13.5948 | ||
| z = −48.5963 | z = 15.4926 | ||
| BCL-XL (2YXJ) | x = −9.7398 | x = 17.9927 | [75] |
| y = −16.3876 | y = 26.4869 | ||
| z = 8.8381 | z = 15.9145 | ||
| BCL-2 (4LVT) | x = 7.6196 | x = 16.5597 | [76] |
| y = −3.0737 | y = 26.5085 | ||
| z = −10.3894 | z = 20.6849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimović, T.; Minda, D.; Șoica, C.; Mioc, A.; Mioc, M.; Colibășanu, D.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A. Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells. Plants 2025, 14, 1341. https://doi.org/10.3390/plants14091341
Maksimović T, Minda D, Șoica C, Mioc A, Mioc M, Colibășanu D, Lukinich-Gruia AT, Pricop M-A, Jianu C, Gogulescu A. Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells. Plants. 2025; 14(9):1341. https://doi.org/10.3390/plants14091341
Chicago/Turabian StyleMaksimović, Tamara, Daliana Minda, Codruța Șoica, Alexandra Mioc, Marius Mioc, Daiana Colibășanu, Alexandra Teodora Lukinich-Gruia, Maria-Alexandra Pricop, Calin Jianu, and Armand Gogulescu. 2025. "Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells" Plants 14, no. 9: 1341. https://doi.org/10.3390/plants14091341
APA StyleMaksimović, T., Minda, D., Șoica, C., Mioc, A., Mioc, M., Colibășanu, D., Lukinich-Gruia, A. T., Pricop, M.-A., Jianu, C., & Gogulescu, A. (2025). Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells. Plants, 14(9), 1341. https://doi.org/10.3390/plants14091341











