Peach Leaf Extract (Prunus persica L.) Mitigates Metabolic Syndrome and Oxidative Stress in High-Fructose Diet Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening
2.2. Animal Experimentation
2.2.1. Acute Toxicity Test
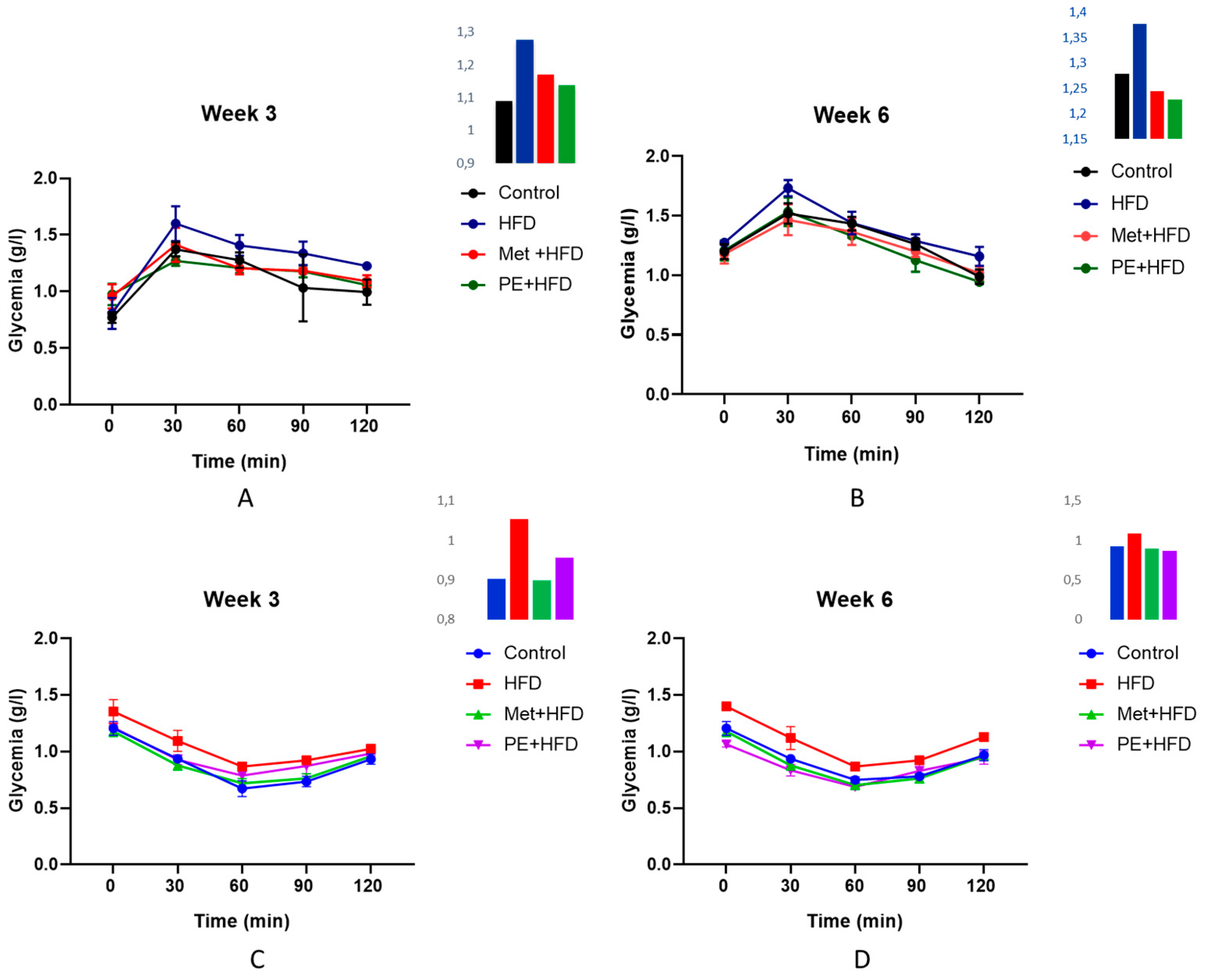
2.2.2. Oral Glucose and Insulin Tolerance Test (OGTT, ITT)
2.2.3. Body Weight
2.2.4. Insulinemia, Total Cholesterol, Triglycerides, Glycemia
2.2.5. Determination of Oxidant/Antioxidant Status Parameters
2.2.6. Organ Weight
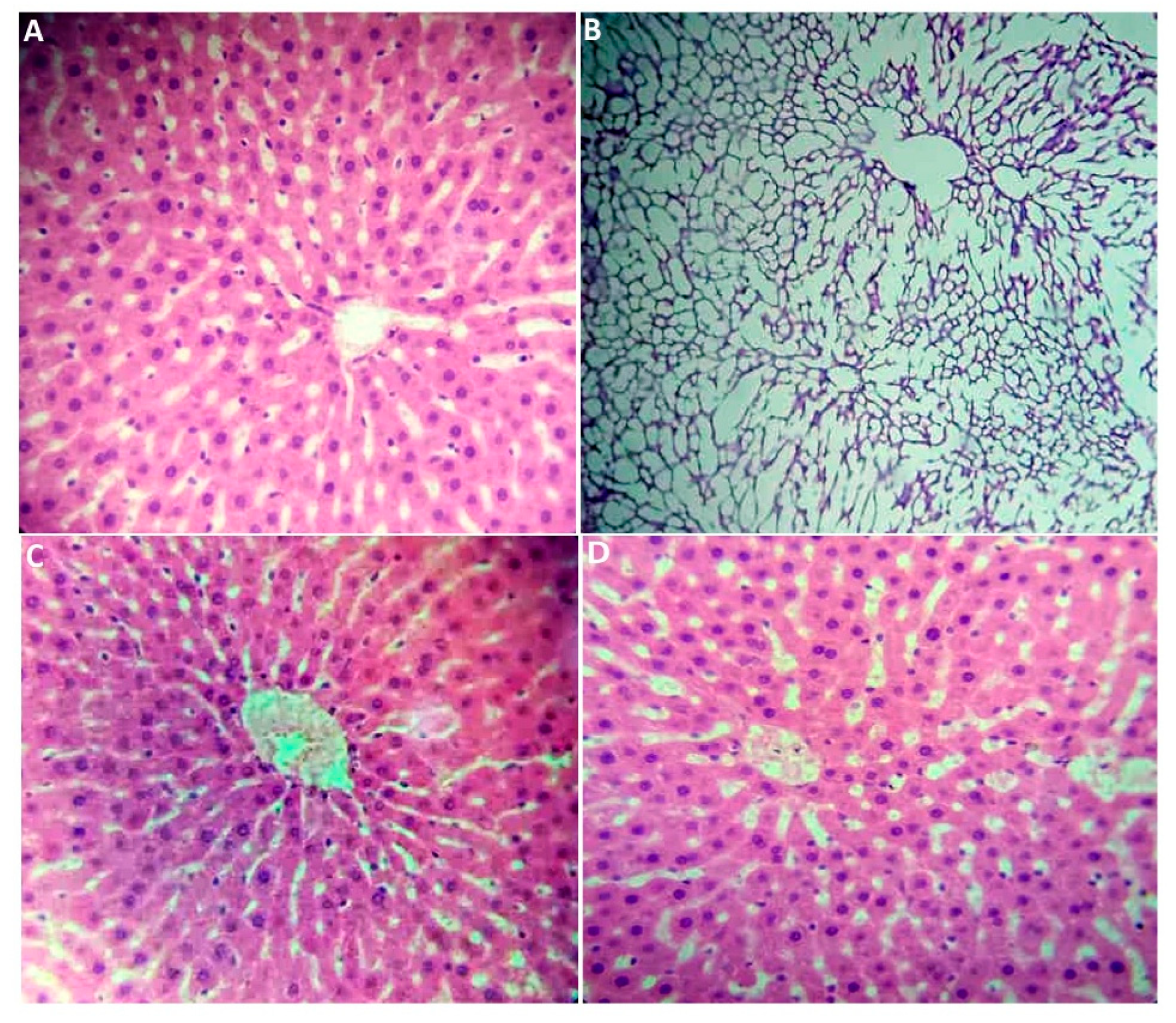
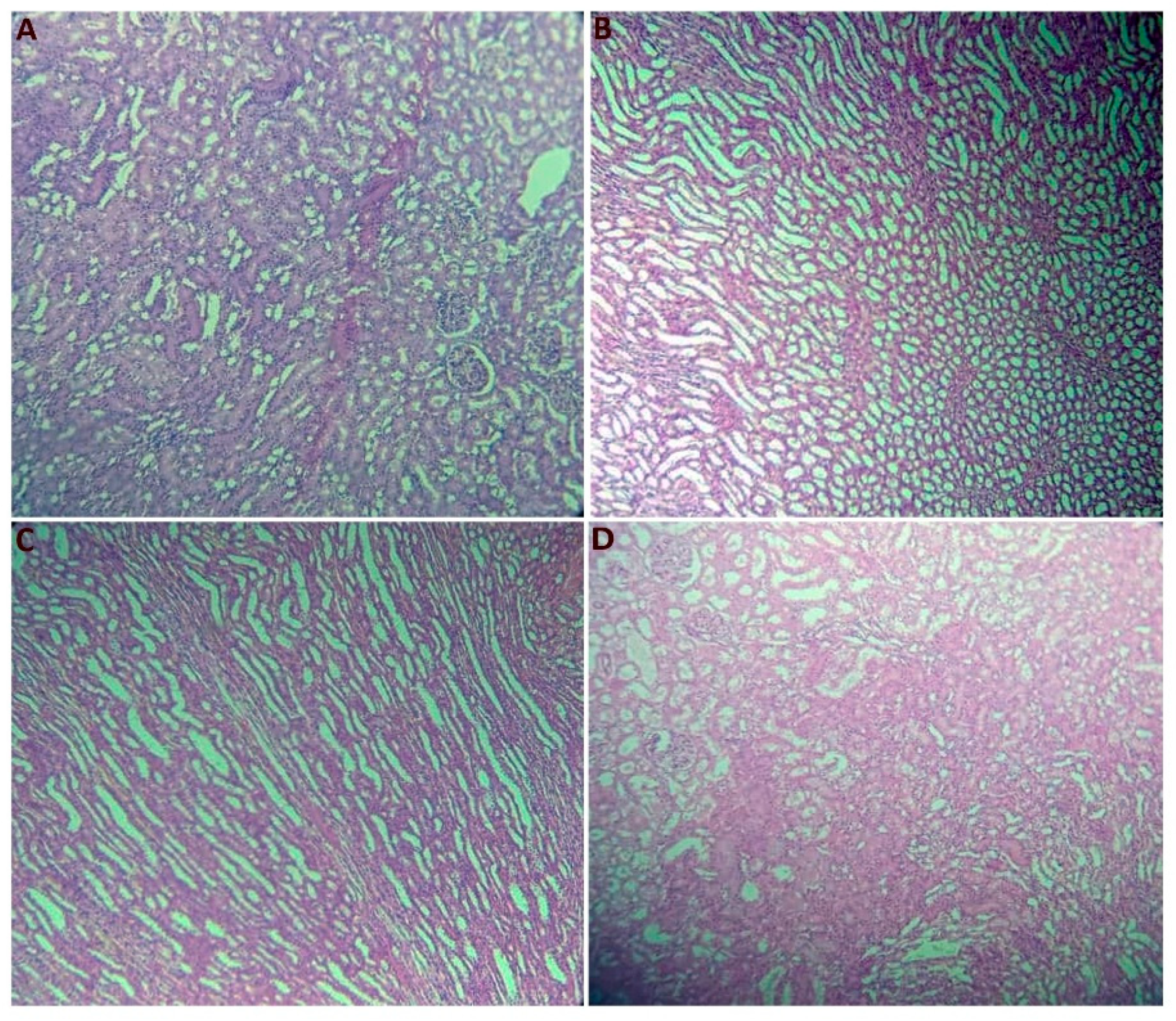
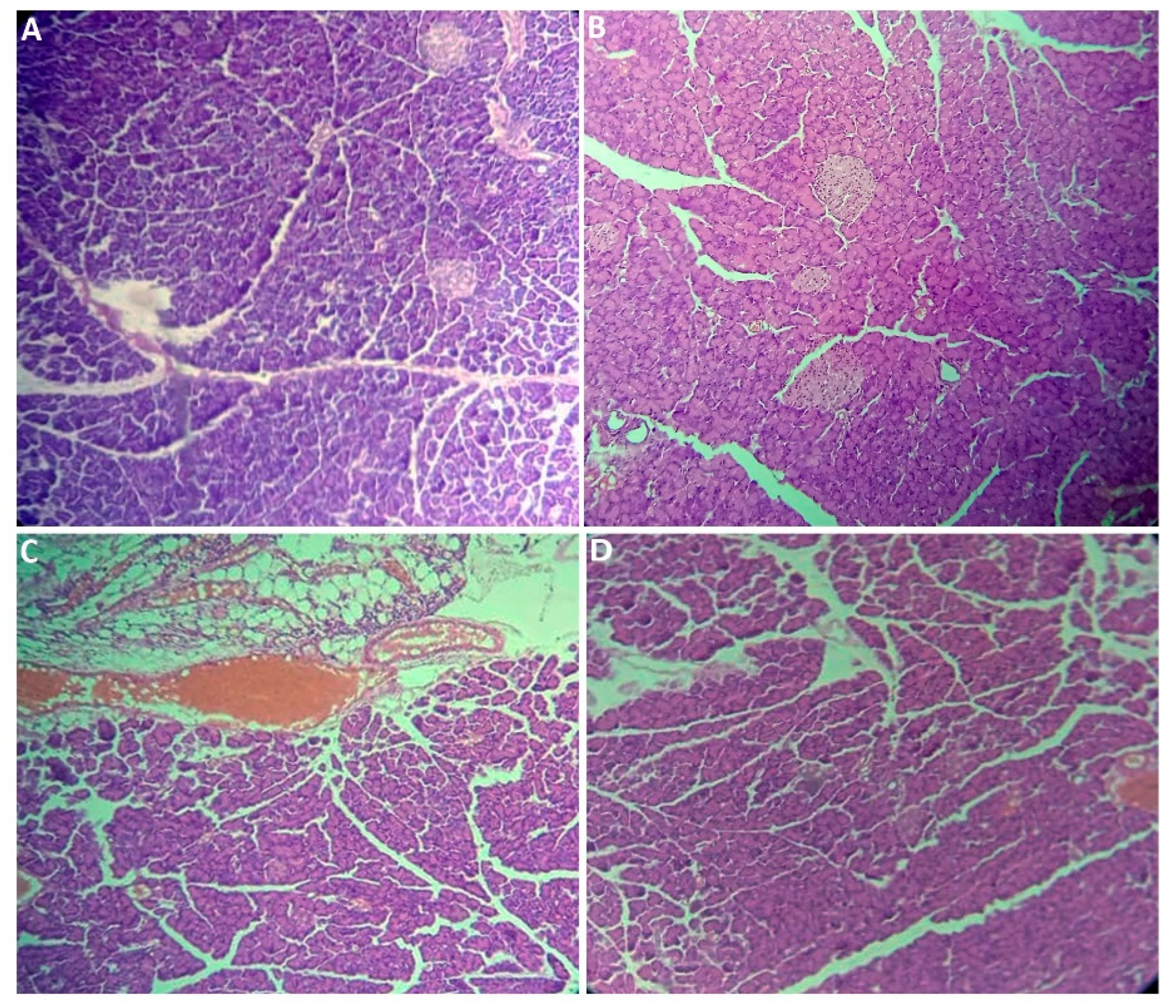
2.2.7. Histological Section Study
3. Materials and Methods
3.1. Plant Material
3.2. Phytochemical Screening
3.3. Preparation of Extracts
3.3.1. Determination of Total Phenolic Content
3.3.2. Determination of Flavonoid Content
3.3.3. Determination of Condensed Tannins
3.3.4. Determination of Flavonols
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging Activity Assay
3.4.2. Reducing Power
3.4.3. Total Antioxidant Capacity
3.5. Experimental Animals
3.5.1. Acute Toxicity Test
3.5.2. Preparation of a High-Fructose Diet
3.5.3. Experimental Design
3.5.4. Oral Glucose Tolerance Test and Insulin Tolerance Test
3.5.5. Animal Euthanasia
3.6. Biochemical Study
Determination of Oxidant/Antioxidant Status Parameters
3.7. Histological Section Study
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.E.; Martínez-Thomas, J.A.; Schürenkämper-Carrillo, K.; de Parrodi, C.A.; López-Mena, E.R.; Mejía-Méndez, J.L.; Lozada-Ramírez, J.D. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants 2024, 13, 1584. [Google Scholar] [CrossRef] [PubMed]
- Sabarathinam, S.; Chandra Satish Kumar, R.; Mahalingam Vijayakumar, T. Necessity of Herbal Medicine in the Management of Metabolic Syndrome; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Zheng, Y.; Crawford, G.W.; Chen, X. Archaeological Evidence for Peach (Prunus persica) Cultivation and Domestication in China. PLoS ONE 2014, 9, e106595. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Cluzet, S.; Madani, K.; Pakina, E.; Gadzhikurbanov, A.; Mesnil, M.; Monvoisin, A.; Richard, T. HPLC-DAD-MS/MS Profiling of Phenolics from Different Varieties of Peach Leaves and Evaluation of Their Antioxidant Activity: A Comparative Study. Int. J. Mass. Spectrom. 2019, 445, 116192. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef]
- Prakash, V.; Sagar, A. Alpha-Amylase and Urease Inhibitory Activity of Leaf Extracts of Prunus persica (L.) Batsch. Bull. Pure Appl. Sci. 2019, 38b, 74–81. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mousa, O.M.; El-Fitiany, R.A.; El Gedaily, R.A. Cytotoxic, Antimicrobial Activities, and Phytochemical Investigation of Three Peach Cultivars and Acerola Leaves. J. Rep. Pharm. Sci. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Preeti Chaudhary, P.C.; Mehra, R.; Ratendra Kumar, R.K.; Shamim Ahamad, S.A. Hepatoprotective Effect of Prunus persica Leaves Extract Against Carbon Tetrachloride Induced Hepatic Injury in Rats. Der Pharm. Lett. 2015, 7, 150–153. [Google Scholar]
- Monika, P.M.; Sharma, K.; Yadav, M. Antioxidant Effect of Some Medicinal Plants: A Review. Inven. Rapid Planta Act. 2016, 1, 1–8. [Google Scholar]
- Bhattacharjee, C.; Gupta, D.; Deb, L.; Debnath, S.; Dutta, A. Effect of Leave Extract of Prunus persica Linn on Acute Inflammation in Rats. Res. J. Pharmacogn. Phytochem. 2011, 3, 38–40. [Google Scholar]
- Haleema, R.; Shenoy, A.; Shabraya, A.R. A review on pharmacological activities of Prunus persica. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 38–40. [Google Scholar]
- Fellah, K.; Amrouche, A.; Benmehdi, H.; Memmou, F. Phenolic Profile, Antioxidants and Kinetic Properties of Flavonoids and Tannins Fractions Isolated from Prunus persica L. Leaves Growing in Southwest Algeria. Res. J. Pharm. Technol. 2019, 12, 4365–4372. [Google Scholar] [CrossRef]
- Benmehdi, H.; Fellah, K.; Amrouche, A.; Memmou, F.; Malainine, H.; Dalile, H.; Siata, W. Phytochemical Study, Antioxidant Activity and Kinetic Behaviour of Flavonoids Fractions Isolated from Prunus persica L. Leaves. Asian J. Chem. 2017, 29, 13–18. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kant, R. Assessment of phytochemical screening by Fourier Transform Infrared spectroscopic analysis of peach (Prunus persica) seed biomass from Uttarakhand region of India. J. Appl. Nat. Sci. 2020, 12, 519–524. [Google Scholar] [CrossRef]
- Asnaashari, M.; Tajik, R.; Khodaparast, M.H.H. Antioxidant Activity of Raspberry (Rubus fruticosus) Leaves Extract and Its Effect on Oxidative Stability of Sunflower Oil. J. Food Sci. Technol. 2015, 52, 5180–5187. [Google Scholar] [CrossRef]
- Dhingra, N.; Sharma, R.; Kar, A. Towards Further Understanding on the Antioxidative Activities of Prunus persica Fruit: A Comparative Study with Four Different Fractions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 582–587. [Google Scholar] [CrossRef]
- Kant, R.; Shukla, R.K.; Shukla, A.; Vishnoi, V.K.; Babu, N. An experimrntal assesment of antimicrobial property of phytochemical derivatives extracted from the peach (Prunus persica L. Batsch) leaves. Rasayan J. Chem. 2022, 15, 1888–1893. [Google Scholar] [CrossRef]
- Kazan, A.; Koyu, H.; Turu, I.C.; Yesil-Celiktas, O. Supercritical Fluid Extraction of Prunus persica Leaves and Utilization Possibilities as a Source of Phenolic Compounds. J. Supercrit. Fluids 2014, 92, 55–59. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica By-Products: A Source of Minerals, Phenols and Volatile Compounds. Sci. Hortic. 2020, 261, 109016. [Google Scholar] [CrossRef]
- Song, W.; Qin, S.T.; Fang, F.X.; Gao, Z.J.; Liang, D.D.; Liu, L.L.; Tian, H.T.; Yang, H.B. Isolation and Purification of Condensed Tannin from the Leaves and Branches of Prunus cerasifera and Its Structure and Bioactivities. Appl. Biochem. Biotechnol. 2018, 185, 464–475. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol Content and Antioxidant Activities of Prunus padus L. and Prunus serotina L. Leaves: Electrochemical and Spectrophotometric Approach and Their Antimicrobial Properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Christabel, P.H.; Nishaa, S.; Vishnupriya, M.; Sasikumar, J.M.; Gopalakrishnan, V.K. In Vitro Antioxidant Studies and the Scavenging Potential of Pulp and Peel of Prunus persica i. Fruit on Different Solvent Systems. World J. Pharm. Res. 2012, 1, 1371–1386. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of High-Fructose Corn Syrup in Beverages May Play a Role in the Epidemic of Obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Teff, K.L.; Elliott, S.S.; Tschöp, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’alessio, D.; Havel, P.J. Dietary Fructose Reduces Circulating Insulin and Leptin, Attenuates Postprandial Suppression of Ghrelin, and Increases Triglycerides in Women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The Fructose-Fed Rat: A Review on the Mechanisms of Fructose-Induced Insulin Resistance and Hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Pektaş, M.B.; Sadi, G.; Akar, F. Long-Term Dietary Fructose Causes Gender-Different Metabolic and Vascular Dysfunction in Rats: Modulatory Effects of Resveratrol. Cell. Physiol. Biochem. 2015, 37, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Won, Y.S.; Cho, H.D.; Hong, S.M.; Moon, K.D.; Seo, K.I. Protective Effect of Prunus mume Fermented with Mixed Lactic Acid Bacteria in Dextran Sodium Sulfate-Induced Colitis. Foods 2020, 10, 58. [Google Scholar] [CrossRef]
- Roomi, A.B.; Al-Salih, R.M.; Kredy, H.M. Study of Polyphenolic Extracts of Prunus domestica L. Wall Nuts as Hypolipidemic Agents. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 154–171. [Google Scholar]
- Giannarelli, R.; Aragona, M.; Coppelli, A.; Del Prato, S. Reducing Insulin Resistance with Metformin: The Evidence Today. Diabetes Metab. 2003, 29, 6S28–6S35. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Bidi, A.; El Bouhali, B.; Hajji, L.; Zeggwagh, N.A. Antidiabetic Plants Improving Insulin Sensitivity. J. Pharm. Pharmacol. 2014, 66, 1197–1214. [Google Scholar] [CrossRef]
- Prada, P.O.; Zecchin, H.G.; Gasparetti, A.L.; Torsoni, M.A.; Ueno, M.; Hirata, A.E.; Corezola do Amaral, M.E.; Höer, N.F.; Boschero, A.C.; Saad, M.J.A. Western Diet Modulates Insulin Signaling, c-Jun N-Terminal Kinase Activity, and Insulin Receptor Substrate-1ser307 Phosphorylation in a Tissue-Specific Fashion. Endocrinology 2005, 146, 1576–1587. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant Properties of Sour Cherries (Prunus cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective Effects of Digested Polyphenols from Wild Blackberry Species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Benkhaled, A.; Réggami, Y.; Boudjelal, A.; Senator, A.; Bouriche, H.; Demirtaş, I.; Kheniche, A.; Benyettou, H.; Larabi, N.; Ruberto, G. Chemical Characterisation, Hypoglycaemic and Renoprotective Effects of Aqueous Leaf Extract of Limoniastrum guyonianum on Fructose-Induced Metabolic Syndrome in Rats. Arch. Physiol. Biochem. 2022, 128, 914–923. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Plum (Prunus domestica): Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas: Nutritional and Health Benefits; Springer: Cham, Switzerland, 2021; pp. 169–179. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Sun, M.; Cai, Z.; Guo, S.; Li, J.; Zhao, B.; Shen, Z.; Ma, R.; Yan, J.; Yu, M. Identification and Expression Analysis of Chlorogenic Acid Biosynthesis Key Gene PpHCT in Peach. Hortic. Plant J. 2023, 9, 670–680. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Theory and Practice of Histological Techniques Eighth; Elsevier Health Science: London, UK, 2019. [Google Scholar]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Benmahieddine, A.; El Zerey-Belaskri, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Gismondi, A. Nutraceutical Content and Biological Properties of Lipophilic and Hydrophilic Fractions of the Phytocomplex from Pistacia atlantica Desf. Buds, Roots, and Fruits. Plants 2024, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Benmeliani-Yousfi, F.; Guermouche, B.; Belyagoubi-Benhammou, N.; Kherraf, Y.; Benzazoua, N.; Merzouk, H.; Mokhtari-Soulimane, N. Antioxidant and Antidiabetic Properties of Olive Pomace’s Hydro-Ethanolic Extract and Aqueous Fraction Using an Animal Model of Diabetes Produced by Streptozotocin (STZ). Waste Biomass Valorization 2024, 1–16. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Vijayalakshmi, M.; Ruckmani, K. Ferric Reducing Antioxidant Power Assay in Plant Extract. Bangladesh J. Pharmacol. 2016, 11, 570–572. [Google Scholar] [CrossRef]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Amari, N.O.; Zerey-Belaskri, A.E.; Gismondi, A.; Djebli, N. Leaf-Buds of Pistacia atlantica: A Novel Source of Bioactive Molecules with High Anti-Inflammatory, Antioxidant, Anti-Tyrosinase and Antimicrobial Properties. Physiol. Mol. Biol. Plants 2023, 29, 209–219. [Google Scholar] [CrossRef]
- Shirly, G.; Ahmad, A.; Vivian, S. Development of Rat Metabolic Syndrome Models: A Review. Vet. World 2021, 7, 1774–1783. [Google Scholar]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological Biomarkers of Chronic Stress: A Systematic Review. Int. J. Health Sci. 2021, 15, 46–59. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]


| Compounds | Reactive | P. persica Extract |
|---|---|---|
| Alkaloids | Mayer | +++ |
| Wagner | +++ | |
| Tannins | FeCl3 | ++ |
| Flavonoids | Shinoda test (Mg) | ++ |
| Coumarins | UV Fluorescence | - |
| Terpenoids | Slakowski Test | + |
| Saponins | Foam Test | - |
| Contents | IC50 (mg/mL) | ||
|---|---|---|---|
| DPPH Test | Reducing Power | ||
| Yield (%) | 53.8 | ||
| Total Phenolics (mg GAE/g DW) | 273.36 ± 1.929 | ||
| Flavonoids (mg QE/ g DW) | 149.02 ± 57.478 | ||
| Condensed Tannins (mg CE/g DW) | 2.34 ± 0.171 | ||
| Flavonols (mg QE/g DW) | 81.67 ± 0.497 | ||
| TAC (AAE/g DW) | 44.11 ± 6.328 | ||
| Extract | 4.89 ± 0.010 | 0.525 ± 0.059 | |
| BHT | 0.53 ± 0.040 | 0.091 ± 0.48 | |
| Control | HFD | Met + HFD | PE + HFD | DF | f | p-Value | |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 207 ± 4.69 b | 214.25 ± 6.45 b | 231 ± 4.69 a | 234.50 ± 7.14 a | 3 | 15.29 | 0.000 |
| Final body weight (g) | 280.50 ± 11.82 b | 345.50 ± 12.15 a | 326 ± 22.50 a | 321.50 ± 6.45 a | 3 | 10.72 | 0.000 |
| Body weight gain (g) | 73.50 ± 15.29 b | 131.25 ± 15.24 a | 95.00 ± 19.06 b | 87.00 ± 9.42 b | 3 | 7.97 | 0.000 |
| Control | HFD | Met + HFD | PE + HFD | DF | F | p-Value | |
|---|---|---|---|---|---|---|---|
| Total Cholesterol (g/L) | 0.525 ± 0.092 a | 0.745 ± 0.106 a | 0.595 ± 0.0636 a | 0.555 ± 0.177 a | 3 | 1.29 | 0.392 |
| Triglycerides (g/L) | 0.630 ± 0.042 b | 1.395 ± 0.035 a | 0.780 ± 0.156 b | 0.720 ± 0.141 b | 3 | 0.196 | 0.011 |
| Insulinemia (U(IU)/mL) | 1.32 ± 1.44 a | 1.92 ± 1.65 a | 0.360 ± 0.085 a | 0.30 ± 0.00 a | 3 | 1.02 | 0.471 |
| Glycemia (g/L) | 0.847 ± 0.091 b | 1.462 ± 0.062 a | 0.940 ± 0.182 b | 0.955 ± 0.068 b | 3 | 36.32 | 0.000 |
| Control | HFD | Met + HFD | PE + HFD | p-Value | |
|---|---|---|---|---|---|
| Vit C (mg AAE/L plasma) | 22.980 ± 1.495 a | 22.68 ± 7.10 a | 23.22 ± 4.10 a | 25.84 ± 3.00 a | 0.478 |
| Catalase (μmol/L) | 0.0051 ± 0.0002 a | 0.0037 ± 0.0001 b | 0.0052 ± 0.00007 a | 0.0052 ± 0.00008 a | 0.000 |
| ORAC (UI) | 5.064 ± 0.134 a | 1.985 ± 0.952 b | 5.246 ± 0.646 a | 6.043 ± 0.345 a | 0.000 |
| MDA (μmol/L) | 3199 ± 509 b | 9471 ± 2125 a | 3574 ± 910 b | 4567 ± 1219 b | 0.000 |
| Hydroperoxides (μmol/L) | 1389 ± 460 b | 2207 ± 413 a | 1533 ± 420 b | 1304 ± 288 b | 0.044 |
| Carboniled-proteins (μmol/L) | 0.031 ± 0.020 b | 0.067 ± 0.032 a | 0.038 ± 0.016 a | 0.029 ± 0.020 a | 0.168 |
| Control | HFD | Met + HFD | PE + HFD | p-Value | |
|---|---|---|---|---|---|
| Liver | 8.532 ± 1.193 ab | 10.043 ± 0.1895 a | 9.627 ± 1.245 ab | 7.716 ± 0.523 b | 0.016 |
| Kidneys | 1.043 ± 0.351 b | 2.1060 ± 0.0636 a | 1.988 ± 0.229 a | 1.7993 ± 0.0511 a | 0.000 |
| Pancreas | 0.943 ± 0.270 a | 0.984 ± 0.208 a | 0.890 ± 0.203 a | 0.927 ± 0.224 a | 0.949 |
| Spleen | 0.8540 ± 0.0998 a | 1.147 ± 0.359 a | 0.9748 ± 0.1658 a | 0.8453 ± 0.1804 a | 0.242 |
| Peri-renal tissue | 2.002 ± 1.124 ab | 3.638 ± 0.331 a | 2.071 ± 0.926 ab | 1.837 ± 0.893 b | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bali, D.; Mami-Soualem, Z.; Belyagoubi-Benhammou, N.; Benzazoua, N.; Belarbi, C.; Kachekouche, Y.; Aldahmash, W.; Rahman, M.A.; Harrath, A.H. Peach Leaf Extract (Prunus persica L.) Mitigates Metabolic Syndrome and Oxidative Stress in High-Fructose Diet Rats. Plants 2025, 14, 1332. https://doi.org/10.3390/plants14091332
Bali D, Mami-Soualem Z, Belyagoubi-Benhammou N, Benzazoua N, Belarbi C, Kachekouche Y, Aldahmash W, Rahman MA, Harrath AH. Peach Leaf Extract (Prunus persica L.) Mitigates Metabolic Syndrome and Oxidative Stress in High-Fructose Diet Rats. Plants. 2025; 14(9):1332. https://doi.org/10.3390/plants14091332
Chicago/Turabian StyleBali, Djihane, Zoubida Mami-Soualem, Nabila Belyagoubi-Benhammou, Nassima Benzazoua, Chahrazed Belarbi, Youssouf Kachekouche, Waleed Aldahmash, Md Ataur Rahman, and Abdel Halim Harrath. 2025. "Peach Leaf Extract (Prunus persica L.) Mitigates Metabolic Syndrome and Oxidative Stress in High-Fructose Diet Rats" Plants 14, no. 9: 1332. https://doi.org/10.3390/plants14091332
APA StyleBali, D., Mami-Soualem, Z., Belyagoubi-Benhammou, N., Benzazoua, N., Belarbi, C., Kachekouche, Y., Aldahmash, W., Rahman, M. A., & Harrath, A. H. (2025). Peach Leaf Extract (Prunus persica L.) Mitigates Metabolic Syndrome and Oxidative Stress in High-Fructose Diet Rats. Plants, 14(9), 1332. https://doi.org/10.3390/plants14091332








