Characterization of Bunch Compactness in a Diverse Collection of Vitis vinifera L. Genotypes Enriched in Table Grape Cultivars Reveals New Candidate Genes Associated with Berry Number
Abstract
1. Introduction
2. Results
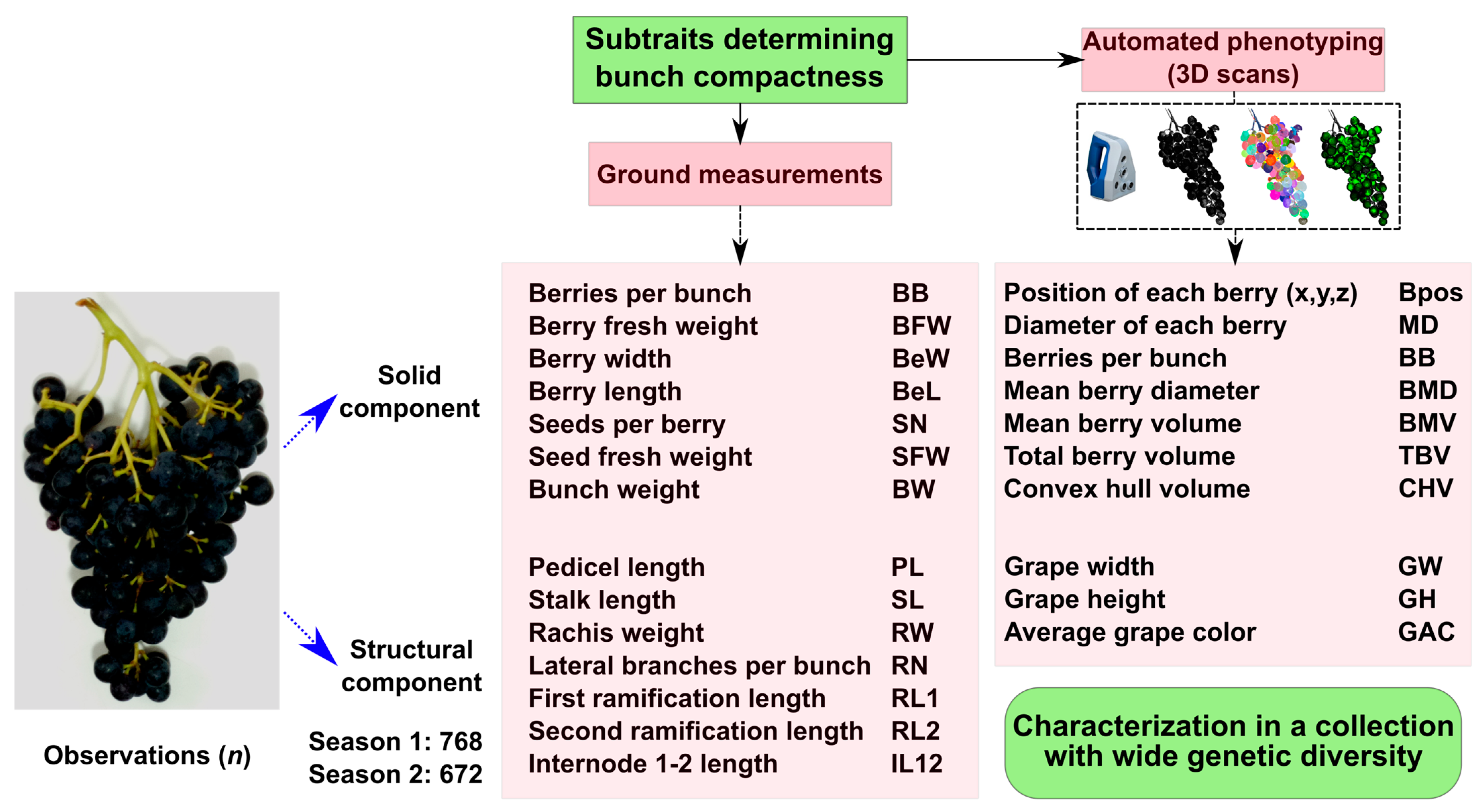
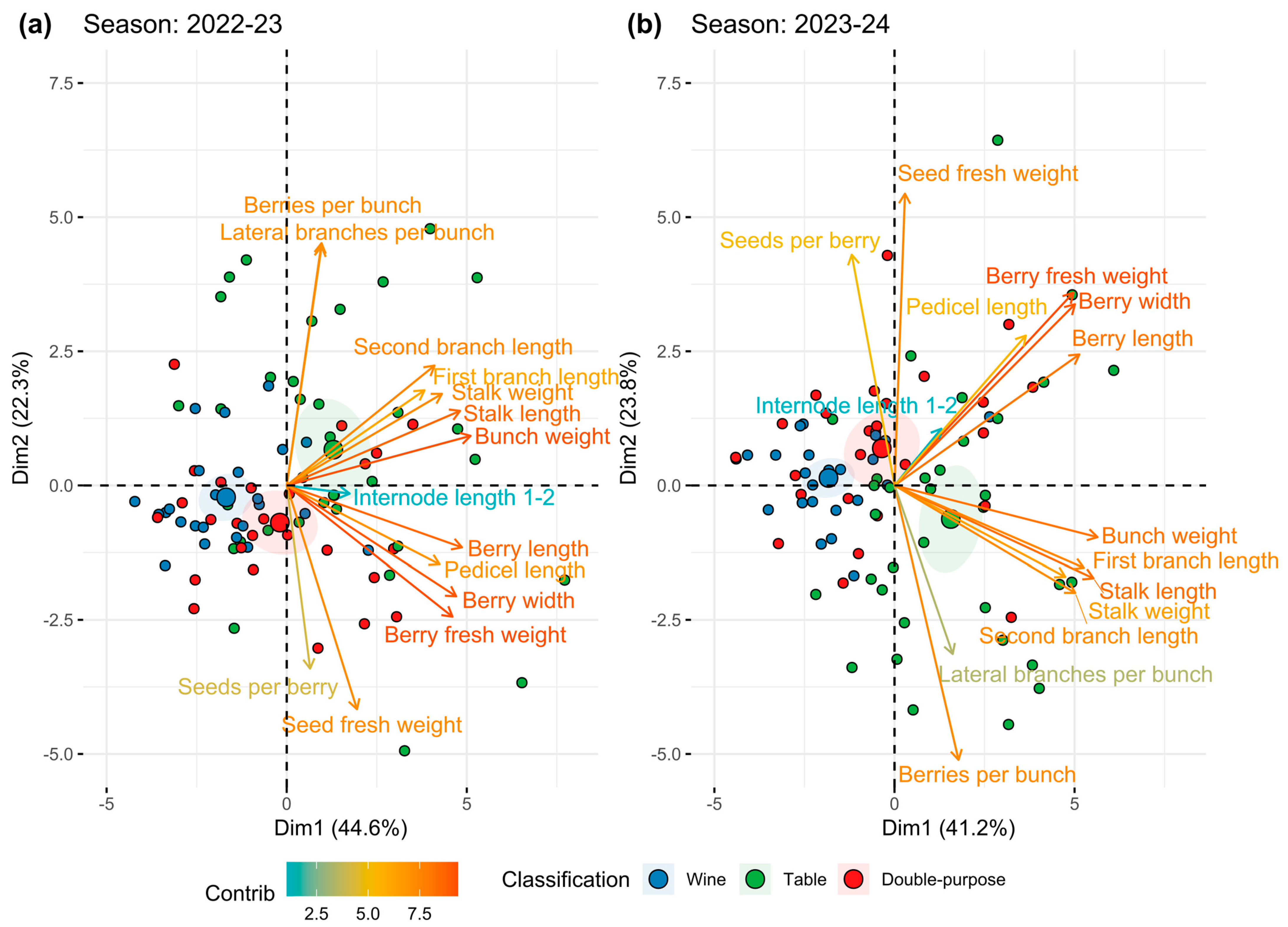
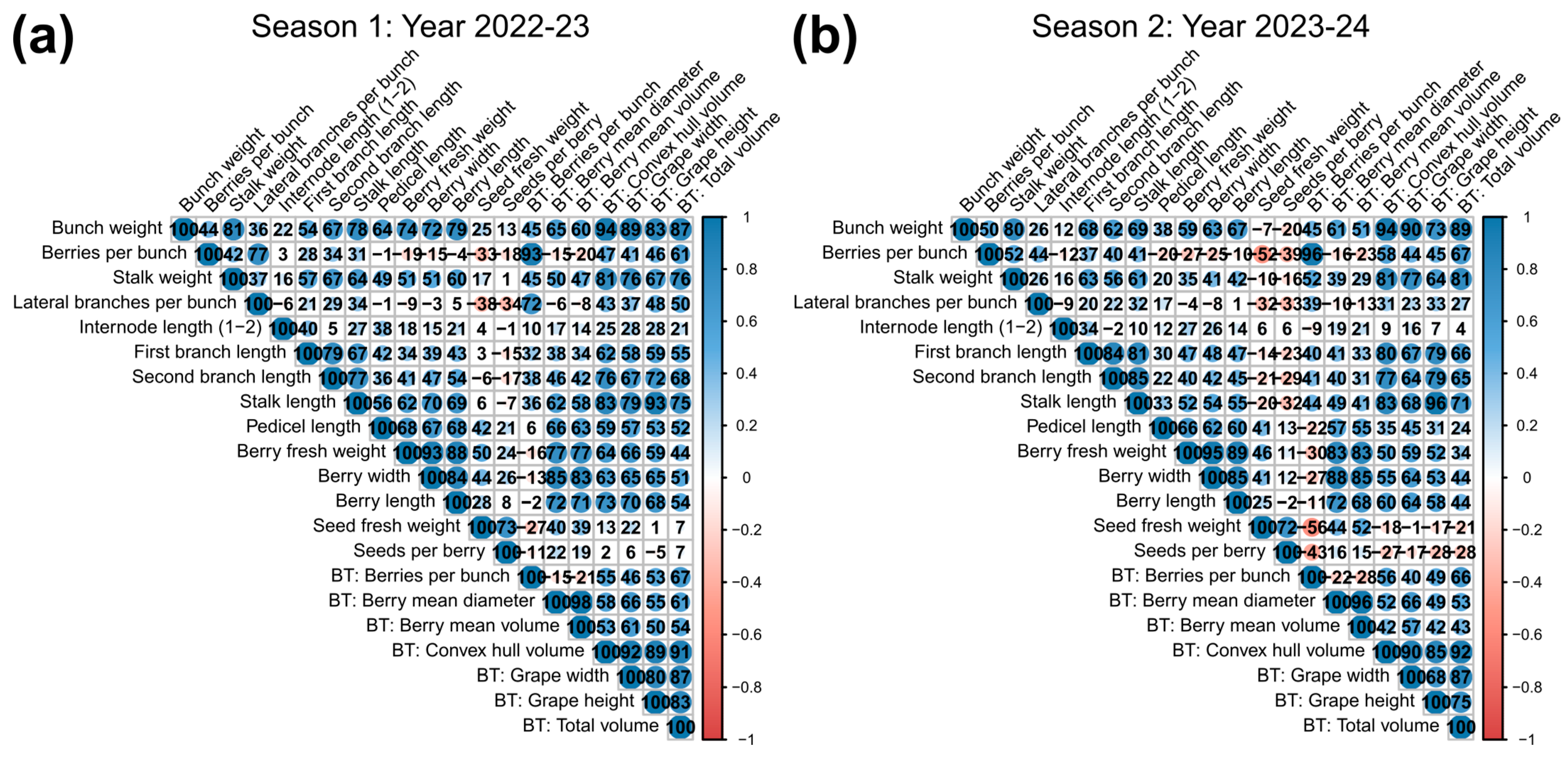
2.1. Relationships Among the Traits Determining Bunch Compactness
- The first group shows close association between berries per bunch and number of lateral branches;
- The second group is related to the structural component of BC. This group includes the traits bunch weight, stalk length, stalk weight, and first and second branch lengths;
- The third group is related to the solid component of BC associated with berry characteristics, including length, width, and fresh weight. Interestingly, it also includes pedicel length, which appears to be more related to the solid component of the bunch rather than the structural one;
- Finally, the fourth group is composed mainly of seed-related traits, showing variable behavior among seasons and inversely correlated with other traits, such as the number of berries per bunch during the second season.
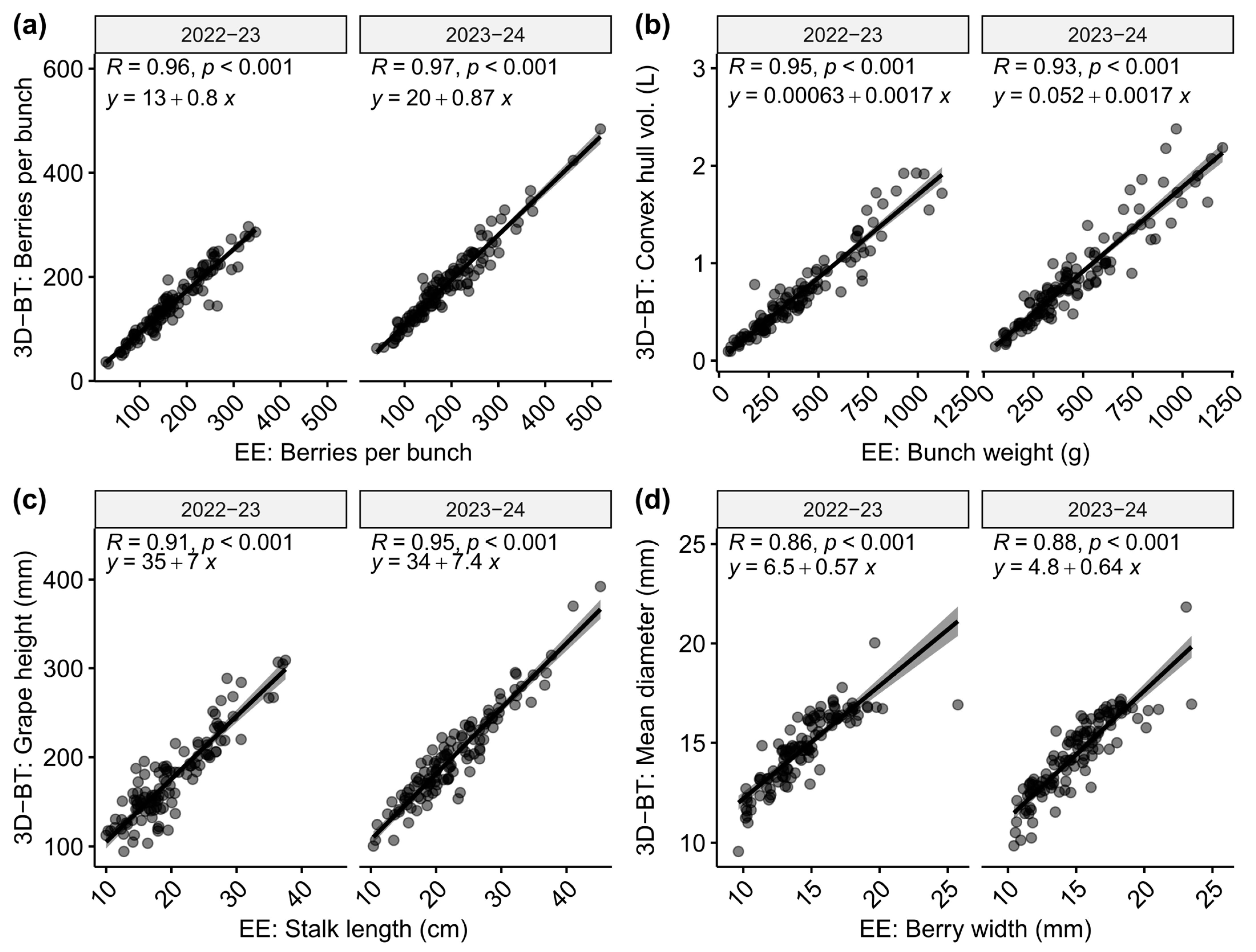
2.2. Automated and Standard Phenotyping Showed High Correlation Values
2.3. Genetic Diversity and Structure of the Collection of Samples
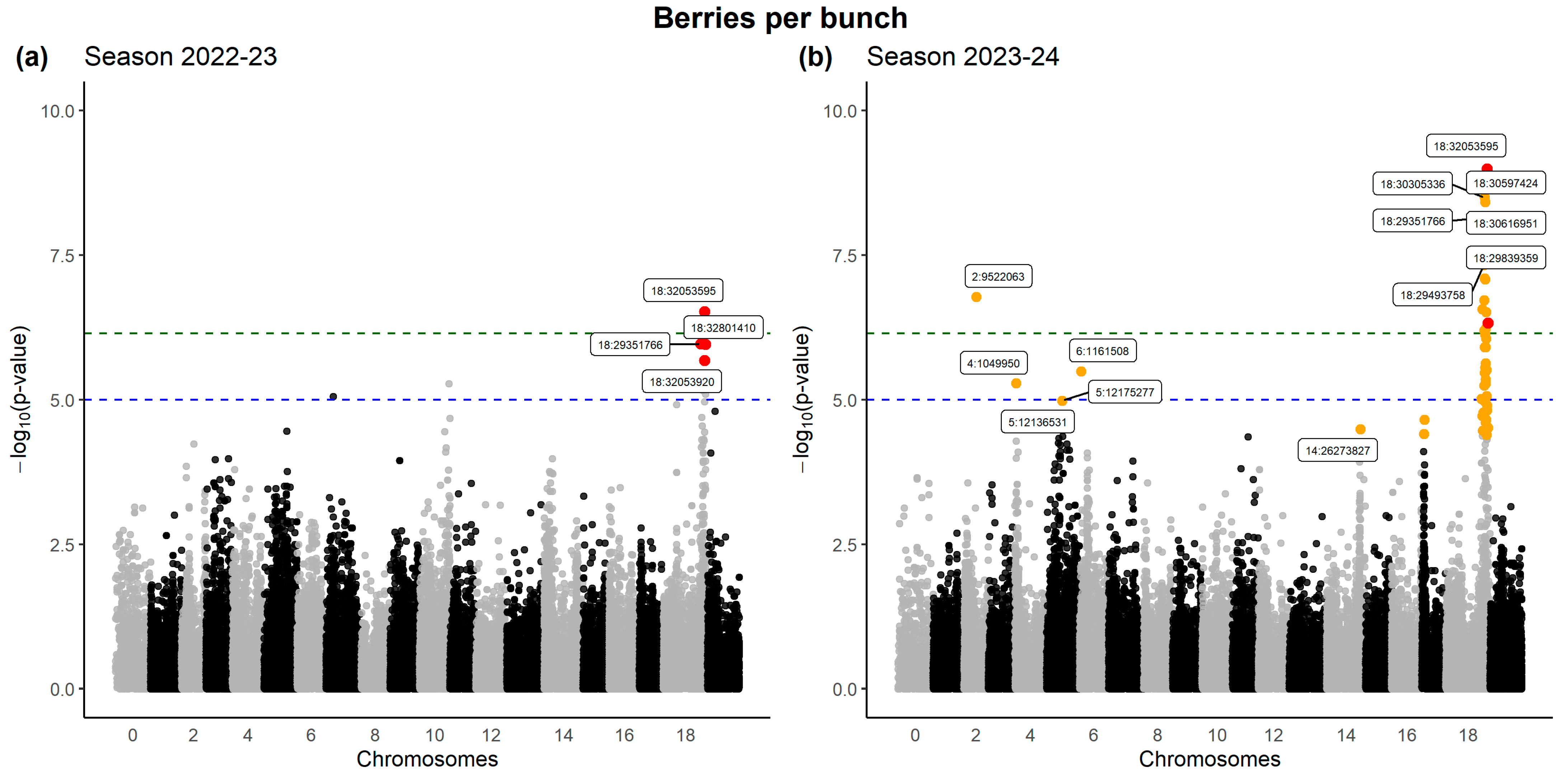
2.4. Genotype–Phenotype Association Studies
2.5. Survey of the Closest Genes Around the Most Frequent SNPs Showing Association with the Traits Describing BC
3. Discussion
3.1. Relevance of Bunch Compactness in Viticulture
3.2. Major Findings
- The identification of sub-traits that are the main determining factors describing BC;
- The confirmation of automated phenotyping as a robust methodology to study BC in table grapes;
- The identification of novel genetic variants (SNPs) associated with the BC-related traits.
3.2.1. Which Traits Are the Main Determinants of Bunch Compactness?
3.2.2. Automated Phenotyping Based on 3D Scans Is a Highly Reliable Methodology to Address the Complexity of Traits Determining BC
3.2.3. How Different Are the Loci and Genes Found in Different Populations?
3.3. Other Aspects Worthy of Consideration
3.3.1. Phenotyping Efficiency Could Be a Key Aspect with Which to Study BC and Achieve Data Reproducibility
3.3.2. Incorporation of Table Grapes in BC Studies
3.3.3. Discussing the Role of the Enriched Terms Found in This Study
3.4. Limitations and Future Directions
4. Materials and Methods
4.1. Plant Material
4.2. Sampling
4.3. Phenotypic Data: 3D Scanning and Manual Measurements
4.4. Genotyping-by-Sequencing and Variant Discovery
4.5. Genotype–Phenotype Association Studies
4.6. In Silico Annotation of Closest Genes Regarding Markers with Significant Association
4.7. Enrichment Analysis of Gene Ontology Categories
4.8. Statistical Analyses
5. Conclusions
- Automated phenotyping based on 3D scanning is suitable for datasets detailing table grapes. The correlation values for a collection of 116 genotypes composed of 24 wine, 66 table, and 26 double-purpose genotype backgrounds (comparing inferred values against ground measurements) revealed high correlation values.
- The analyses of these datasets collected over two consecutive seasons led to the identification of a large set of significant SNPs when using the GWAS approach. The most striking signals were associated with the number of berries per bunch (BB). Other signals for other BC-related traits were distributed on various chromosomes, with high variability among seasons. The list of genes closest to the significant SNPs revealed significant enrichment in discrete functions such as alpha-linolenic acid and glycan degradation, which poses new questions regarding the BC phenotype.
- Understanding the genetic bases in both genetic groups is highly relevant under climate change scenarios, which are driving the development of new table grapes and wine varieties.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bunch compactness |
| Chr | Chromosome |
| FDR | False discovery ratio |
| GA | Gibberellic acid |
| GBS | Genotyping by sequencing |
| GWAS | Genome-wide association study |
| PGR | Plant growth regulator |
| QTL | Quantitative trait locus |
| SNP | Single nucleotide polymorphism |
References
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New Horizons for Grapevine Breeding. In Fruit, Vegetable and Cereal Science and Biotechnology; Global Science Books: Ikenobe, Kagawa ken, Japan, 2011; pp. 79–100. [Google Scholar]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual Domestications and Origin of Traits in Grapevine Evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Magon, G.; De Rosa, V.; Martina, M.; Falchi, R.; Acquadro, A.; Barcaccia, G.; Portis, E.; Vannozzi, A.; De Paoli, E. Boosting Grapevine Breeding for Climate-Smart Viticulture: From Genetic Resources to Predictive Genomics. Front. Plant Sci. 2023, 14, 1293186. [Google Scholar] [CrossRef] [PubMed]
- Pirrello, C.; Magon, G.; Palumbo, F.; Farinati, S.; Lucchin, M.; Barcaccia, G.; Vannozzi, A. Past, Present, and Future of Genetic Strategies to Control Tolerance to the Main Fungal and Oomycete Pathogens of Grapevine. J. Exp. Bot. 2023, 74, 1309–1330. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Mian, G.; Nassivera, F.; Sillani, S.; Iseppi, L. Grapevine Resistant Cultivars: A Story Review and the Importance on the Related Wine Consumption Inclination. Sustainability 2022, 15, 390. [Google Scholar] [CrossRef]
- Kui, L.; Tang, M.; Duan, S.; Wang, S.; Dong, X. Identification of Selective Sweeps in the Domesticated Table and Wine Grape (Vitis vinifera L.). Front. Plant Sci. 2020, 11, 572. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic Structure and Domestication History of the Grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Di Genova, A.; Almeida, A.M.; Muñoz-Espinoza, C.; Vizoso, P.; Travisany, D.; Moraga, C.; Pinto, M.; Hinrichsen, P.; Orellana, A.; Maass, A. Whole Genome Comparison between Table and Wine Grapes Reveals a Comprehensive Catalog of Structural Variants. BMC Plant Biol. 2014, 14, 7. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Dangl, G.S.; Prins, B.H.; Boursiquot, J.-M.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Genetic Structure and Differentiation in Cultivated Grape, Vitis vinifera L. Genet. Res. 2003, 81, 179–192. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Sawler, J.; Gardner, K.M.; Aradhya, M.K.; Prins, B.H.; Schwaninger, H.R.; Bustamante, C.D.; Buckler, E.S.; Zhong, G.-Y.; Brown, P.J.; et al. Patterns of Genomic and Phenomic Diversity in Wine and Table Grapes. Hortic. Res. 2017, 4, 17035. [Google Scholar] [CrossRef]
- Weisong, M.; Chengcheng, L.; Dong, T.; Jianying, F. Chinese Consumers’ Behavior and Preference to Table Grapes: Based on a Comparative Study of 2009 and 2014. Br. Food J. 2016, 118, 231–246. [Google Scholar] [CrossRef]
- Wolf, M.M. An Analysis of the Impact of Price on Consumer Purchase Interest in Organic Grapes and a Profile of Organic Purchasers. AgEcon. Search 2002, 24. [Google Scholar] [CrossRef]
- Montaigne, E.; Coelho, A.; Zadmehran, S. A Comprehensive Economic Examination and Prospects on Innovation in New Grapevine Varieties Dealing with Global Warming and Fungal Diseases. Sustainability 2021, 13, 13254. [Google Scholar] [CrossRef]
- Piarulli, L.; Pirolo, C.; Roseti, V.; Bellin, D.; Mascio, I.; La Notte, P.; Montemurro, C.; Miazzi, M.M. Breeding New Seedless Table Grapevines for a More Sustainable Viticulture in Mediterranean Climate. Front. Plant Sci. 2024, 15, 1379642. [Google Scholar] [CrossRef]
- Herzog, K.; Wind, R.; Töpfer, R. Impedance of the Grape Berry Cuticle as a Novel Phenotypic Trait to Estimate Resistance to Botrytis Cinerea. Sensors 2015, 15, 12498–12512. [Google Scholar] [CrossRef]
- Rist, F.; Herzog, K.; Mack, J.; Richter, R.; Steinhage, V.; Töpfer, R. High-Precision Phenotyping of Grape Bunch Architecture Using Fast 3D Sensor and Automation. Sensors 2018, 18, 763. [Google Scholar] [CrossRef]
- Tello, J.; Ibáñez, J. Evaluation of Indexes for the Quantitative and Objective Estimation of Grapevine Bunch Compactness. Vitis J. Grapevine Res. 2015, 53, 9–16. [Google Scholar] [CrossRef]
- Tello, J.; Ibáñez, J. What Do We Know about Grapevine Bunch Compactness? A State-of-the-Art Review: Review on Bunch Compactness. Aust. J. Grape Wine Res. 2018, 24, 6–23. [Google Scholar] [CrossRef]
- Hed, B.; Ngugi, H.K.; Travis, J.W. Relationship Between Cluster Compactness and Bunch Rot in Vignoles Grapes. Plant Dis. 2009, 93, 1195–1201. [Google Scholar] [CrossRef]
- Latorre, B.A.; Elfar, K.; Ferrada, E.E. Gray Mold Caused by Botrytis Cinerea Limits Grape Production in Chile. Cienc. Inv. Agrar. 2015, 42, 316–330. [Google Scholar] [CrossRef]
- Vail, M.E.; Wolpert, J.A.; Gubler, W.D.; Rademacher, M.R. Effect of Cluster Tightness on Botrytis Bunch Rot in Six Chardonnay Clones. Plant Dis. 1998, 82, 107–109. [Google Scholar] [CrossRef]
- Valdés-Gómez, H.; Fermaud, M.; Roudet, J.; Calonnec, A.; Gary, C. Grey Mould Incidence Is Reduced on Grapevines with Lower Vegetative and Reproductive Growth. Crop. Prot. 2008, 27, 1174–1186. [Google Scholar] [CrossRef]
- Esterio, M.; Osorio-Navarro, C.; Azócar, M.; Copier, C.; Rubilar, M.; Pizarro, L.; Auger, J. Reduced Fitness Cost and Increased Aggressiveness in Fenhexamid-Resistant Botrytis cinerea Field Isolates from Chile. Phytopathol. Mediterr. 2021, 60, 69–77. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight Exposure and Temperature Effects on Berry Growth and Composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar] [CrossRef]
- Pieri, P.; Zott, K.; Gomès, E.; Hilbert, G. Nested Effects of Berry Half, Berry and Bunch Microclimate on Biochemical Composition in Grape. OENO One 2016, 50, 23. [Google Scholar] [CrossRef]
- Dokoozlian, N.; Peacock, B.; Luvisi, D.; Vasquez, S. Cultural Practices for Crimson Seedless Table Grapes. In Cooperative Extension; University of California: Los Angeles, CA, USA, 1993. [Google Scholar]
- Dokoozlian, N.K.; Peacock, W.L. Gibberellic Acid Applied at Bloom Reduces Fruit Set and Improves Size of “Crimson Seedless” Table Grapes. HortSci. 2001, 36, 706–709. [Google Scholar] [CrossRef]
- Molitor, D.; Behr, M.; Hoffmann, L.; Evers, D. Impact of Grape Cluster Division on Cluster Morphology and Bunch Rot Epidemic. Am. J. Enol. Vitic. 2012, 63, 508–514. [Google Scholar] [CrossRef]
- Correa, J.; Mamani, M.; Muñoz-Espinoza, C.; Laborie, D.; Muñoz, C.; Pinto, M.; Hinrichsen, P. Heritability and Identification of QTLs and Underlying Candidate Genes Associated with the Architecture of the Grapevine Cluster (Vitis Vinifera L.). Theor. Appl. Genet. 2014, 127, 1143–1162. [Google Scholar] [CrossRef]
- Houel, C.; Martin-Magniette, M.-L.; Nicolas, S.D.; Lacombe, T.; Le Cunff, L.; Franck, D.; Torregrosa, L.; Conéjéro, G.; Lalet, S.; This, P.; et al. Genetic Variability of Berry Size in the Grapevine (Vitis Vinifera L.): Berry Size. Aust. J. Grape Wine Res. 2013, 19, 208–220. [Google Scholar] [CrossRef]
- Muñoz-Espinoza, C.; Di Genova, A.; Sánchez, A.; Correa, J.; Espinoza, A.; Meneses, C.; Maass, A.; Orellana, A.; Hinrichsen, P. Identification of SNPs and InDels Associated with Berry Size in Table Grapes Integrating Genetic and Transcriptomic Approaches. BMC Plant Biol. 2020, 20, 365. [Google Scholar] [CrossRef]
- Muñoz-Espinoza, C.; Di Genova, A.; Correa, J.; Silva, R.; Maass, A.; González-Agüero, M.; Orellana, A.; Hinrichsen, P. Transcriptome Profiling of Grapevine Seedless Segregants during Berry Development Reveals Candidate Genes Associated with Berry Weight. BMC Plant Biol. 2016, 16, 104. [Google Scholar] [CrossRef]
- Shavrukov, Y.N.; Dry, I.B.; Thomas, M.R. Inflorescence and Bunch Architecture Development in Vitis Vinifera L. Aust. J. Grape Wine Res. 2004, 10, 116–124. [Google Scholar] [CrossRef]
- Schöler, F.; Steinhage, V. Automated 3D Reconstruction of Grape Cluster Architecture from Sensor Data for Efficient Phenotyping. Comput. Electron. Agric. 2015, 114, 163–177. [Google Scholar] [CrossRef]
- Mack, J.; Rist, F.; Herzog, K.; Töpfer, R.; Steinhage, V. Constraint-Based Automated Reconstruction of Grape Bunches from 3D Range Data for High-Throughput Phenotyping. Biosyst. Eng. 2020, 197, 285–305. [Google Scholar] [CrossRef]
- Torres-Lomas, E.; Lado-Bega, J.; Garcia-Zamora, G.; Diaz-Garcia, L. Segment Anything for Comprehensive Analysis of Grapevine Cluster Architecture and Berry Properties. Plant Phenomics 2024, 6, 0202. [Google Scholar] [CrossRef]
- Tello, J.; Torres-Pérez, R.; Flutre, T.; Grimplet, J.; Ibáñez, J. VviUCC1 Nucleotide Diversity, Linkage Disequilibrium and Association with Rachis Architecture Traits in Grapevine. Genes 2020, 11, 598. [Google Scholar] [CrossRef]
- Rossmann, S.; Richter, R.; Sun, H.; Schneeberger, K.; Töpfer, R.; Zyprian, E.; Theres, K. Mutations in the miR396 Binding Site of the Growth-regulating Factor Gene VvGRF4 Modulate Inflorescence Architecture in Grapevine. Plant J. 2020, 101, 1234–1248. [Google Scholar] [CrossRef]
- Marguerit, E.; Boury, C.; Manicki, A.; Donnart, M.; Butterlin, G.; Némorin, A.; Wiedemann-Merdinoglu, S.; Merdinoglu, D.; Ollat, N.; Decroocq, S. Genetic Dissection of Sex Determinism, Inflorescence Morphology and Downy Mildew Resistance in Grapevine. Theor. Appl. Genet. 2009, 118, 1261–1278. [Google Scholar] [CrossRef]
- Richter, R.; Gabriel, D.; Rist, F.; Töpfer, R.; Zyprian, E. Identification of Co-Located QTLs and Genomic Regions Affecting Grapevine Cluster Architecture. Theor. Appl. Genet. 2019, 132, 1159–1177. [Google Scholar] [CrossRef]
- Underhill, A.; Hirsch, C.; Clark, M. Image-Based Phenotyping Identifies Quantitative Trait Loci for Cluster Compactness in Grape. J. Amer. Soc. Hort. Sci. 2020, 145, 363–373. [Google Scholar] [CrossRef]
- Tello, J.; Torres-Pérez, R.; Grimplet, J.; Ibáñez, J. Association Analysis of Grapevine Bunch Traits Using a Comprehensive Approach. Theor. Appl. Genet. 2016, 129, 227–242. [Google Scholar] [CrossRef]
- Canaguier, A.; Grimplet, J.; Scalabrin, S.; Di Gaspero, G.; Mohellibi, N.; Choisne, N.; Rombault, S.; Rustenholtz, C.; Morgante, M.; Quesneville, H.; et al. A New Version of the Grapevine Reference Genome Assembly (12X.v2) and of Its Annotation (VCost.V3). Genome Data 2017, 14, 56–62. [Google Scholar] [CrossRef]
- García-Abadillo, J.; Barba, P.; Carvalho, T.; Sosa-Zuñiga, V.; Lozano, R.; Carvalho, H.F.; Garcia-Rojas, M.; Salazar, E.; Y Sánchez, J.I. Dissecting the Complex Genetic Basis of Pre- and Post-Harvest Traits in Vitis Vinifera L . Using Genome-Wide Association Studies. Hortic. Res. 2024, 11, uhad283. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-Wide Efficient Mixed-Model Analysis for Association Studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mejía, N.; Gebauer, M.; Muñoz, L.; Hewstone, N.; Muñoz, C.; Hinrichsen, P. Identification of QTLs for Seedlessness, Berry Size, and Ripening Date in a Seedless × Seedless Table Grape Progeny. Am. J. Enol. Vitic. 2007, 58, 499–507. [Google Scholar] [CrossRef]
- Mejía, N.; Soto, B.; Guerrero, M.; Casanueva, X.; Houel, C.; De Los Ángeles Miccono, M.; Ramos, R.; Le Cunff, L.; Boursiquot, J.-M.; Hinrichsen, P.; et al. Molecular, Genetic and Transcriptional Evidence for a Role of VvAGL11 in Stenospermocarpic Seedlessness in Grapevine. BMC Plant Biol. 2011, 11, 57. [Google Scholar] [CrossRef]
- Rist, F.; Schwander, F.; Richter, R.; Mack, J.; Schwandner, A.; Hausmann, L.; Steinhage, V.; Töpfer, R.; Herzog, K. Relieving the Phenotyping Bottleneck for Grape Bunch Architecture in Grapevine Breeding Research: Implementation of a 3D-Based Phenotyping Approach for Quantitative Trait Locus Mapping. Horticulturae 2022, 8, 907. [Google Scholar] [CrossRef]
- Fanizza, G.; Lamaj, F.; Costantini, L.; Chaabane, R.; Grando, M.S. QTL Analysis for Fruit Yield Components in Table Grapes (Vitis vinifera). Theor. Appl. Genet. 2005, 111, 658–664. [Google Scholar] [CrossRef]
- Costantini, L.; Moreno-Sanz, P.; Nwafor, C.C.; Lorenzi, S.; Marrano, A.; Cristofolini, F.; Gottardini, E.; Raimondi, S.; Ruffa, P.; Gribaudo, I.; et al. Somatic Variants for Seed and Fruit Set in Grapevine. BMC Plant Biol. 2021, 21, 135. [Google Scholar] [CrossRef]
- Flutre, T.; Le Cunff, L.; Fodor, A.; Launay, A.; Romieu, C.; Berger, G.; Bertrand, Y.; Terrier, N.; Beccavin, I.; Bouckenooghe, V.; et al. A Genome-Wide Association and Prediction Study in Grapevine Deciphers the Genetic Architecture of Multiple Traits and Identifies Genes under Many New QTLs. G3 Genes Genomes Genet. 2022, 12, jkac103. [Google Scholar] [CrossRef]
- Tello, J.; Aguirrezábal, R.; Hernáiz, S.; Larreina, B.; Montemayor, M.I.; Vaquero, E.; Ibáñez, J. Multicultivar and Multivariate Study of the Natural Variation for Grapevine Bunch Compactness: Multicultivar Study of Grapevine Bunch Compactness. Aust. J. Grape Wine Res. 2015, 21, 277–289. [Google Scholar] [CrossRef]
- Le Cunff, L.; Fournier-Level, A.; Laucou, V.; Vezzulli, S.; Lacombe, T.; Adam-Blondon, A.-F.; Boursiquot, J.-M.; This, P. Construction of Nested Genetic Core Collections to Optimize the Exploitation of Natural Diversity in Vitis vinifera L. Subsp Sativa. BMC Plant Biol. 2008, 8, 31. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Eibach, R. Molecular Breeding. In Genetics, Genomics and Breeding of Grapes; Adam-Blondon, A.-F., Martínez-Zapater, J.M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-429-18941-8. [Google Scholar]
- Akkurt, M.; Tahmaz, H.; Veziroğlu, S. Recent Developments in Seedless Grapevine Breeding. SAJEV 2019, 40, 260–265. [Google Scholar] [CrossRef]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in Jasmonate Signaling for Multistress Resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef]
- Gabay, G.; Faigenboim, A.; Dahan, Y.; Izhaki, Y.; Itkin, M.; Malitsky, S.; Elkind, Y.; Flaishman, M.A. Transcriptome Analysis and Metabolic Profiling Reveal the Key Role of α-Linolenic Acid in Dormancy Regulation of European Pear. J. Exp. Bot. 2019, 70, 1017–1031. [Google Scholar] [CrossRef]
- Ma, L.; Wang, P.; Zhu, Q.-H.; Cheng, X.; Zhang, T.; Zhang, X.; Zhu, H.; Yang, Z.; Sun, J.; Liu, F. Unbalanced Lipid Metabolism in Anther, Especially the Disorder of the Alpha-Linolenic Acid Metabolism Pathway, Leads to Cotton Male Sterility. J. Integr. Agric. 2024, S209531192400279X. [Google Scholar] [CrossRef]
- Lavell, A.A.; Benning, C. Cellular Organization and Regulation of Plant Glycerolipid Metabolism. Plant Cell Physiol. 2019, 60, 1176–1183. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, S.; Mao, J.; Li, W.; Li, W.; Zuo, C.; Chu, M.; Zhao, X.; Zhou, Q.; Chen, B. Effects of Shading on the Synthesis of Volatile Organic Compounds in ‘Marselan’ Grape Berries (Vitis vinifera L.). J. Plant Growth Regul. 2021, 40, 679–693. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.; Zhang, W.; Cao, D.; Ye, G.; Chen, J.; Lei, Y.; Wei, X. Transcriptomic and Metabolomic Analyses Reveal Mechanisms Underpinning Resistance of Chinese Wild Grape to Colletotrichum viniferum. Plant Physiol. Biochem. 2024, 215, 108851. [Google Scholar] [CrossRef]
- Mielke, S.; Zimmer, M.; Meena, M.K.; Dreos, R.; Stellmach, H.; Hause, B.; Voiniciuc, C.; Gasperini, D. Jasmonate Biosynthesis Arising from Altered Cell Walls Is Prompted by Turgor-Driven Mechanical Compression. Sci. Adv. 2021, 7, eabf0356. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Chen, W.; Xu, Z.; Chen, M.; Wang, H.; Yu, D. The Emerging Role of Jasmonate in the Control of Flowering Time. J. Exp. Bot. 2022, 73, 11–21. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Powell, J.J.; Aitken, E.A.B.; Kazan, K.; Manners, J.M. The Lateral Organ Boundaries Domain Transcription Factor LBD20 Functions in Fusarium Wilt Susceptibility and Jasmonate Signaling in Arabidopsis. Plant Physiol. 2012, 160, 407–418. [Google Scholar] [CrossRef]
- Shuai, B.; Reynaga-Peña, C.G.; Springer, P.S. The Lateral Organ Boundaries Gene Defines a Novel, Plant-Specific Gene Family. Plant Physiol. 2002, 129, 747–761. [Google Scholar] [CrossRef]
- Grimplet, J.; Tello, J.; Laguna, N.; Ibáñez, J. Differences in Flower Transcriptome between Grapevine Clones Are Related to Their Cluster Compactness, Fruitfulness, and Berry Size. Front. Plant Sci. 2017, 8, 632. [Google Scholar] [CrossRef]
- Guo, D.-L.; Zhao, H.-L.; Li, Q.; Zhang, G.-H.; Jiang, J.-F.; Liu, C.-H.; Yu, Y.-H. Genome-Wide Association Study of Berry-Related Traits in Grape [Vitis vinifera L.] Based on Genotyping-by-Sequencing Markers. Hortic. Res. 2019, 6, 11. [Google Scholar] [CrossRef]
- Correa, J.; Ravest, G.; Laborie, D.; Mamani, M.; Torres, E.; Muñoz, C.; Pinto, M.; Hinrichsen, P. Quantitative Trait Loci for the Response to Gibberellic Acid of Berry Size and Seed Mass in Tablegrape (V Itis vinifera L.): Genetics of the Gibberellic Acid Effect on Tablegrapes. Aust. J. Grape Wine Res. 2015, 21, 496–507. [Google Scholar] [CrossRef]
- Meneses, M.; García-Rojas, M.; Muñoz-Espinoza, C.; Carrasco-Valenzuela, T.; Defilippi, B.; González-Agüero, M.; Meneses, C.; Infante, R.; Hinrichsen, P. Transcriptomic Study of Pedicels from GA3-Treated Table Grape Genotypes with Different Susceptibility to Berry Drop Reveals Responses Elicited in Cell Wall Yield, Primary Growth and Phenylpropanoids Synthesis. BMC Plant Biol. 2020, 20, 66. [Google Scholar] [CrossRef]
- Peppi, M.C.; Álvarez, E. Gibberellic Acid for Table Grape Inflorescence Elongation: Is It Worth It? Am. J. Enol. Vitic. 2023, 74, 0740012. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar]
- Browning, B.L.; Tian, X.; Zhou, Y.; Browning, S.R. Fast Two-Stage Phasing of Large-Scale Sequence Data. Am. J. Hum. Genet. 2021, 108, 1880–1890. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaSci. 2015, 4, 7. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila melanogaster Strain w1118 ; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Knaus, B.J.; Grünwald, N.J. vcfr: A Package to Manipulate and Visualize Variant Call Format Data in R. Mol. Ecol. Resour. 2017, 17, 44–53. [Google Scholar] [CrossRef]
- Yu, G. Using Ggtree to Visualize Data on Tree-Like Structures. CP Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin, Germany, 2016; ISBN 978-3-319-24277-4. [Google Scholar]

| Trait | Season | Chr | Position | p-Value (Raw) | Ref | Alt | Annotated Effect | Candidate Gene | Description |
|---|---|---|---|---|---|---|---|---|---|
| BB | 2022–2023 | 18 | 29,351,766 | 1.10 × 10−6 | G | A | 3_prime_UTR_variant | Vitvi18g02073 | Adenylate kinase, chloroplast |
| BB | 2022–2023 | 18 | 32,053,595 | 3.00 × 10−7 | T | C | upstream_gene_variant | Vitvi18g02260 | |
| BB | 2022–2023 | 18 | 32,053,920 | 2.10 × 10−6 | G | T | missense_variant | Vitvi18g03207 | |
| BB | 2022–2023 | 18 | 32,801,410 | 1.10 × 10−6 | C | T | synonymous_variant | Vitvi18g02321 | Aspartokinase–homoserine dehydrogenase |
| BB | 2023–2024 | 2 | 9,522,063 | 1.70 × 10−7 | C | T | intron_variant | Vitvi02g01526 | NADP-malic enzyme |
| BB | 2023–2024 | 4 | 1,049,950 | 5.20 × 10−6 | G | T | upstream_gene_variant | Vitvi04g01789 | |
| BB | 2023–2024 | 5 | 12,136,531 | 1.00 × 10−5 | G | C | intron_variant | Vitvi05g02021 | Zinc finger (C3HC4-type ring finger) |
| BB | 2023–2024 | 5 | 12,175,277 | 1.00 × 10−5 | A | G | downstream_gene_variant | Vitvi05g00968 | Zinc finger (C3HC4-type ring finger) |
| BB | 2023–2024 | 6 | 1,161,508 | 3.20 × 10−6 | C | G | upstream_gene_variant | Vitvi06g00101 | |
| BB | 2023–2024 | 14 | 26,273,827 | 3.20 × 10−5 | G | A | missense_variant | Vitvi14g01604 | F-box domain containing protein |
| BB | 2023–2024 | 17 | 1,640,664 | 3.90 × 10−5 | G | T | upstream_gene_variant | Vitvi17g01358 | Homeobox protein knotted-1-like 1 (KNAT1) |
| BB | 2023–2024 | 17 | 2,126,568 | 2.20 × 10−5 | A | G | upstream_gene_variant | Vitvi17g00197 | POT1 (protection of telomeres 1) |
| BB | 2023–2024 | 18 | 29,351,766 | 7.10 × 10−9 | G | A | 3_prime_UTR_variant | Vitvi18g02073 | Adenylate kinase, chloroplast |
| BB | 2023–2024 | 18 | 32,053,595 | 1.00 × 10−9 | T | C | upstream_gene_variant | Vitvi18g02260 | |
| BB | 2023–2024 | 18 | 32,053,920 | 9.00 × 10−11 | G | T | missense_variant | Vitvi18g03207 | |
| BB | 2023–2024 | 18 | 32,801,410 | 4.70 × 10−7 | C | T | synonymous_variant | Vitvi18g02321 | Aspartokinase–homoserine dehydrogenase |
| CI-03 | 2023–2024 | 2 | 9,522,063 | 1.90 × 10−6 | C | T | intron_variant | Vitvi02g01526 | NADP-malic enzyme |
| CI-03 | 2023–2024 | 14 | 25,584,904 | 1.70 × 10−6 | T | C | synonymous_variant | Vitvi14g01530 | Nodulin |
| CI-03 | 2023–2024 | 14 | 26,067,440 | 9.10 × 10−6 | T | C | upstream_gene_variant | Vitvi14g02428 | Transcription termination factor mitochondrial mTERF |
| CI-03 | 2023–2024 | 14 | 26,067,815 | 8.70 × 10−6 | A | T | upstream_gene_variant | Vitvi14g02428 | Transcription termination factor mitochondrial mTERF |
| CI-03 | 2023–2024 | 14 | 26,097,728 | 1.10 × 10−6 | T | C | 3_prime_UTR_variant | Vitvi14g01585 | Phosducin |
| CI-03 | 2023–2024 | 14 | 26,097,791 | 2.30 × 10−6 | A | T | 3_prime_UTR_variant | Vitvi14g01585 | Phosducin |
| CI-03 | 2023–2024 | 18 | 29,351,766 | 1.60 × 10−6 | G | A | 3_prime_UTR_variant | Vitvi18g02073 | Adenylate kinase, chloroplast |
| CI-03 | 2023–2024 | 18 | 32,053,595 | 1.40 × 10−6 | T | C | upstream_gene_variant | Vitvi18g02260 | |
| CI-03 | 2023–2024 | 18 | 32,053,920 | 3.40 × 10−7 | G | T | missense_variant | Vitvi18g03207 |
| Gene ID | Description | No. of Hits | Traits on Which Associated SNP Was Detected |
|---|---|---|---|
| Vitvi11g00545 | Chloride channel protein CLC | 6 | S1: CI_18 (3x); S2: CI_18 (3x) |
| Vitvi18g02073 | Adenylate kinase, chloroplast | 5 | S1: BT_BB, RN; S2: BB, BT_BB, CI_03 |
| Vitvi18g02242 | 2 | S1: CI_17; S2: BT_BB | |
| Vitvi18g02260 | 4 | S1: BT_BB; S2: BB, BT_BB, CI_03 | |
| Vitvi18g03207 | 4 | S1: BT_BB; S2: BB, BT_BB, CI_03 | |
| Vitvi18g02321 | Aspartokinase–homoserine dehydrogenase | 4 | S1: BT_BB; S2: BB, BT_BB (2x) |
| Vitvi02g00318 | Tyrosyl-tRNA synthetase | 2 | S1: CI_19 (2x) |
| Vitvi02g00387 | Heat shock transcription factor B2B | 2 | S1: CI_17, CI_19 |
| Vitvi02g00399 | SEU1 protein | 4 | S1: CI_17, CI_19 (3x) |
| Vitvi02g00417 | Copper amine oxidase | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi02g00607 | Unknown protein | 2 | S1: CI_17, CI_18 |
| Vitvi02g01526 | NADP-malic enzyme | 3 | S2: BB, BT_BB, CI_03 |
| Vitvi03g00829 | 2 | S1: CI_08, RW | |
| Vitvi04g01789 | 2 | S2: BB, BT_BB | |
| Vitvi04g00282 | Lachrymatory factor synthase | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi04g00374 | DnaJ homolog, subfamily B, member 4 | 2 | S1: CI_17 (2x) |
| Vitvi04g00375 | Fertility restorer homologue A | 2 | S1: CI_17 (2x) |
| Vitvi04g00732 | Ubiquitin-specific protease 15 | 2 | S2: BT_BMV (2x) |
| Vitvi05g02021 | Zinc finger (C3HC4-type ring finger) | 2 | S2: BB, BT_BB |
| Vitvi05g00968 | Zinc finger (C3HC4-type ring finger) | 2 | S2: BB, BT_BB |
| Vitvi05g00990 | DNA primase large subunit | 3 | S2: BB, BB, BB |
| Vitvi06g00101 | 2 | S2: BB, BT_BB | |
| Vitvi06g00538 | Chlororespiratory reduction 2 (CRR2) | 2 | S1: CI_17, CI_18 |
| Vitvi07g00654 | Ankyrin | 2 | S1: BFW, PL |
| Vitvi07g01536 | Major facilitator superfamily protein (MFS) Spinster | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi07g01999 | U3 small nucleolar ribonucleoprotein protein IMP4 | 2 | S2: CI_12, CI_16 |
| Vitvi08g02237 | 2 | S1: CI_17, CI_19 | |
| Vitvi09g00430 | EMB2753 | 3 | S1: CI_17 (2x), CI_18 |
| Vitvi10g00060 | 4 | S2: CI_17 (2x), CI_18 (2x) | |
| Vitvi10g01508 | F-box/LRR-repeat protein 2 | 3 | S2: RL2 (3x) |
| Vitvi11g00440 | Zinc finger (CCCH-type) family protein | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi11g00475 | EIN3-binding F-box protein 1 | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi11g01443 | 4 | S1: CI_17 (2x), CI_18, CI_19 | |
| Vitvi11g00487 | Glucan endo-1,3-beta-glucosidase 7 precursor | 2 | S1: CI_17, CI_18 |
| Vitvi11g00512 | Unknown protein | 2 | S1: CI_17, CI_18 |
| Vitvi11g00517 | CCR4-NOT transcription complex subunit 10 | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi11g00523 | Clathrin assembly protein 10 | 2 | S1: CI_17, CI_18 |
| Vitvi11g00539 | HHP1 (heptahelical protein 1) | 2 | S1: CI_17, CI_18 |
| Vitvi11g00542 | Sucrose-phosphate synthase | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi11g00543 | Nodulation receptor kinase | 7 | S1: CI_17 (3x), CI_18 (4x) |
| Vitvi11g00549 | IMP dehydrogenase/GMP reductase | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi12g00056 | 1,3-beta-glucan synthase | 2 | S2: BB, BB |
| Vitvi12g00124 | S-receptor kinase | 2 | S2: CI_12, CI_16 |
| Vitvi12g02121 | U2(RNU2) small nuclear RNA auxiliary factor 1-like 2 | 2 | S2: CI_18, CI_19 |
| Vitvi13g01903 | 3 | S1: CI_17, CI_18, CI_19 | |
| Vitvi13g00145 | Myosin heavy chain | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi13g01924 | Cytochrome c oxidase subunit VIb | 2 | S1: CI_18, CI_19 |
| Vitvi13g00779 | ABC transporter G member 22 | 2 | S1: CI_17, CI_19 |
| Vitvi14g01171 | 2 | S1: CI_17 (2x) | |
| Vitvi14g01232 | Nuclear matrix constituent protein 1 | 4 | S1: CI_17 (2x), CI_18, CI_19 |
| Vitvi14g01448 | Chalcone synthase (VviCHS1) | 2 | S1: CI_17, CI_19 |
| Vitvi14g02428 | Transcription termination factor mitochondrial mTERF | 2 | S2: CI_03 (2x) |
| Vitvi14g01585 | Phosducin | 4 | S2: CI_03 (4x) |
| Vitvi14g01604 | F-box domain containing protein | 2 | S2: BB, BT_BB |
| Vitvi15g00571 | Palmitoyl-protein thioesterase 1 precursor | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi15g00585 | 2 | S1: CI_18, CI_19 | |
| Vitvi16g00899 | Subtilisin protease C1 | 2 | S1: CI_17 (2x) |
| Vitvi16g01191 | 3 | S2: BT_BMV (3x) | |
| Vitvi17g00284 | Kinesin motor HIK (HINKEL) | 2 | S1: CI_17, CI_19 |
| Vitvi17g00288 | Receptor serine/threonine kinase PR5K | 2 | S1: CI_17, CI_19 |
| Vitvi17g00289 | Wall-associated kinase 4 | 5 | S1: CI_17 (2x), CI_18, CI_19 (2x) |
| Vitvi17g00299 | EMB2454 (embryo defective 2454) | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi17g00321 | Kinesin motor protein-related | 3 | S1: CI_17, CI_18, CI_19 |
| Vitvi18g00533 | CTR1-like protein kinase | 2 | S2: CI_18 (2x) |
| Vitvi18g01662 | Sensitive to proton rhizotoxicity 1 | 4 | S1: CI_17 (2x), CI_18 (2x) |
| Vitvi18g01948 | 2 | S2: BT_BB (2x) | |
| Vitvi18g01981 | Proton-dependent oligopeptide transport (POT) family protein | 2 | S2: BB, BT_BB |
| Vitvi18g02081 | Protein translocon component Tic40, chloroplast | 3 | S2: BB, BT_BB, CI_03 |
| Vitvi18g02103 | Taxane 10-beta-hydroxylase | 5 | S2: BB (2x), BT_BB (2x), CI_03 |
| Vitvi18g03151 | 2 | S2: BT_BB (2x), | |
| Vitvi18g02121 | Nematode resistance protein | 3 | S2: BB, BT_BB, CI_03 |
| Vitvi18g02122 | 2 | S2: BB, BT_BB | |
| Vitvi18g02128 | Glyoxylate reductase | 4 | S2: BB (2x), BT_BB (2x) |
| Vitvi18g02133 | MADS-box protein SEEDSTICK (VviAG3) | 5 | S2: BB, BT_BB (2x), CI_03, ED |
| Vitvi18g03155 | GORK (gated outwardly rectifying K+ channel) | 2 | S2: BB, BT_BB |
| Vitvi18g02139 | 12-oxophytodienoate reductase 2 | 2 | S2: BB, BT_BB |
| Vitvi18g03160 | 12-oxophytodienoate reductase 1 | 7 | S2: BB (3x), BT_BB (3x), CI_03 |
| Vitvi18g02142 | 12-oxophytodienoate reductase 1 | 3 | S2: BB, BT_BB, BT_CHV |
| Vitvi18g02159 | R protein disease resistance protein | 2 | S2: BB, BT_BB |
| Vitvi18g02171 | Aldehyde oxidase 1 | 2 | S2: BB, BT_BB |
| Vitvi18g02180 | Disease resistance protein (TIR-NBS-LRR class) | 4 | S2: BB, BT_BB (3x) |
| Vitvi18g02184 | 4 | S2: BB (2x), BT_BB (2x) | |
| Vitvi18g02185 | Aminoacyl-tRNA synthetase, class Ia | 2 | S2: BB, BT_BB |
| Vitvi18g02186 | 4 | S2: BB (2x), BT_BB (2x) | |
| Vitvi18g02215 | 2 | S2: BB, BT_BB | |
| Vitvi18g02225 | 2 | S2: BB, BT_BB | |
| Vitvi18g02248 | 2 | S2: BB, BT_BB | |
| Vitvi18g02267 | VviTPS89 | 2 | S2: BB, BT_BB |
| Vitvi18g02462 | VviTPS81 | 2 | S2: BB, BT_BB |
| Vitvi18g02337 | R protein L6 | 2 | S1: CI_17, CI_18 |
| Vitvi19g01412 | Agenet domain-containing protein | 4 | S1: CI_14, CI_17, CI_18, CI_19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses, M.; Muñoz-Espinoza, C.; Reyes-Impellizzeri, S.; Salazar, E.; Meneses, C.; Herzog, K.; Hinrichsen, P. Characterization of Bunch Compactness in a Diverse Collection of Vitis vinifera L. Genotypes Enriched in Table Grape Cultivars Reveals New Candidate Genes Associated with Berry Number. Plants 2025, 14, 1308. https://doi.org/10.3390/plants14091308
Meneses M, Muñoz-Espinoza C, Reyes-Impellizzeri S, Salazar E, Meneses C, Herzog K, Hinrichsen P. Characterization of Bunch Compactness in a Diverse Collection of Vitis vinifera L. Genotypes Enriched in Table Grape Cultivars Reveals New Candidate Genes Associated with Berry Number. Plants. 2025; 14(9):1308. https://doi.org/10.3390/plants14091308
Chicago/Turabian StyleMeneses, Marco, Claudia Muñoz-Espinoza, Sofía Reyes-Impellizzeri, Erika Salazar, Claudio Meneses, Katja Herzog, and Patricio Hinrichsen. 2025. "Characterization of Bunch Compactness in a Diverse Collection of Vitis vinifera L. Genotypes Enriched in Table Grape Cultivars Reveals New Candidate Genes Associated with Berry Number" Plants 14, no. 9: 1308. https://doi.org/10.3390/plants14091308
APA StyleMeneses, M., Muñoz-Espinoza, C., Reyes-Impellizzeri, S., Salazar, E., Meneses, C., Herzog, K., & Hinrichsen, P. (2025). Characterization of Bunch Compactness in a Diverse Collection of Vitis vinifera L. Genotypes Enriched in Table Grape Cultivars Reveals New Candidate Genes Associated with Berry Number. Plants, 14(9), 1308. https://doi.org/10.3390/plants14091308





