Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases
Abstract
1. Introduction
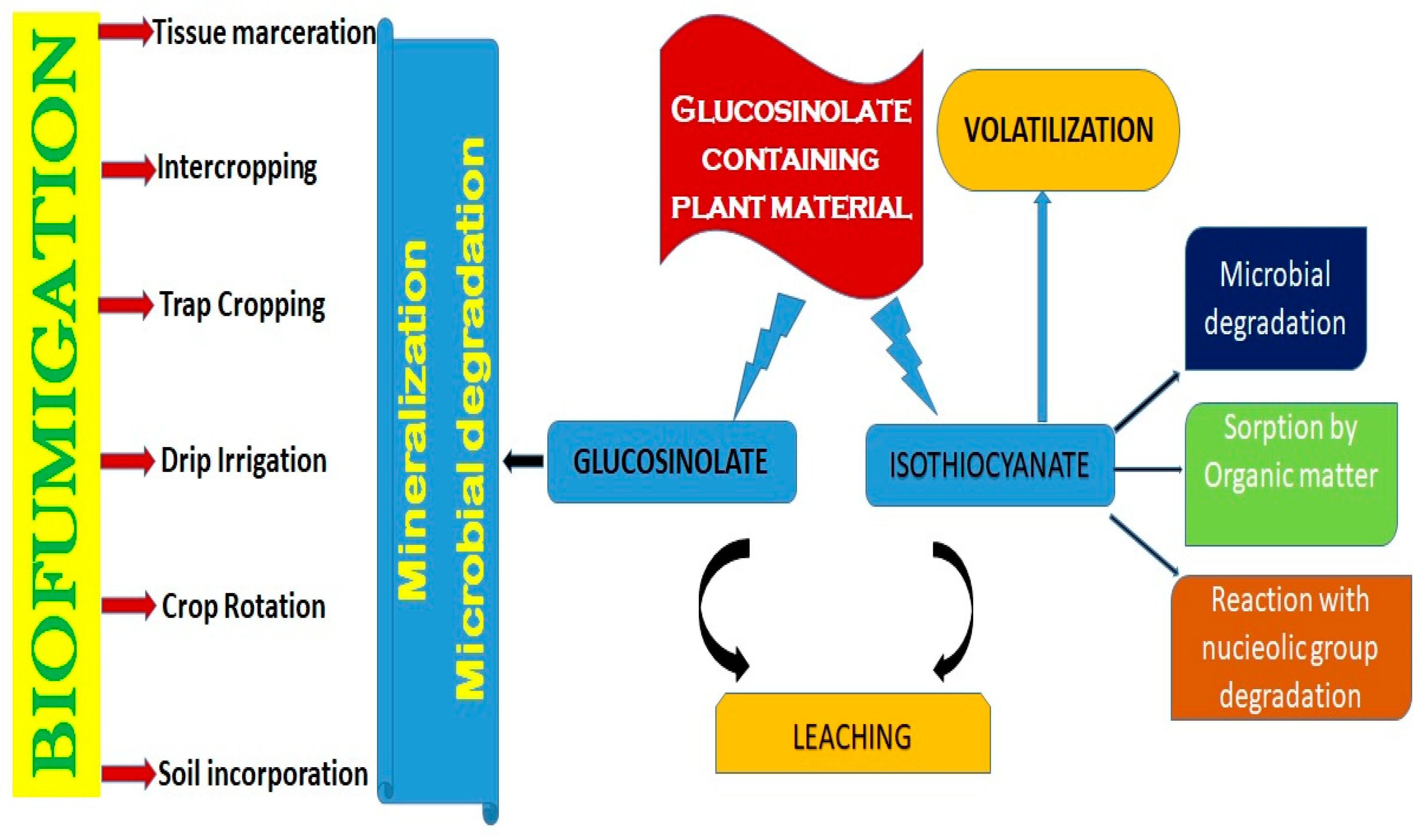
2. Biofumigation Crops
3. Biofumigation Techniques
4. Mechanism of Action
Mode of Action of Biofumigant Crops
5. Role of Glucosinolates and Isothiocyanates in Biofumigation
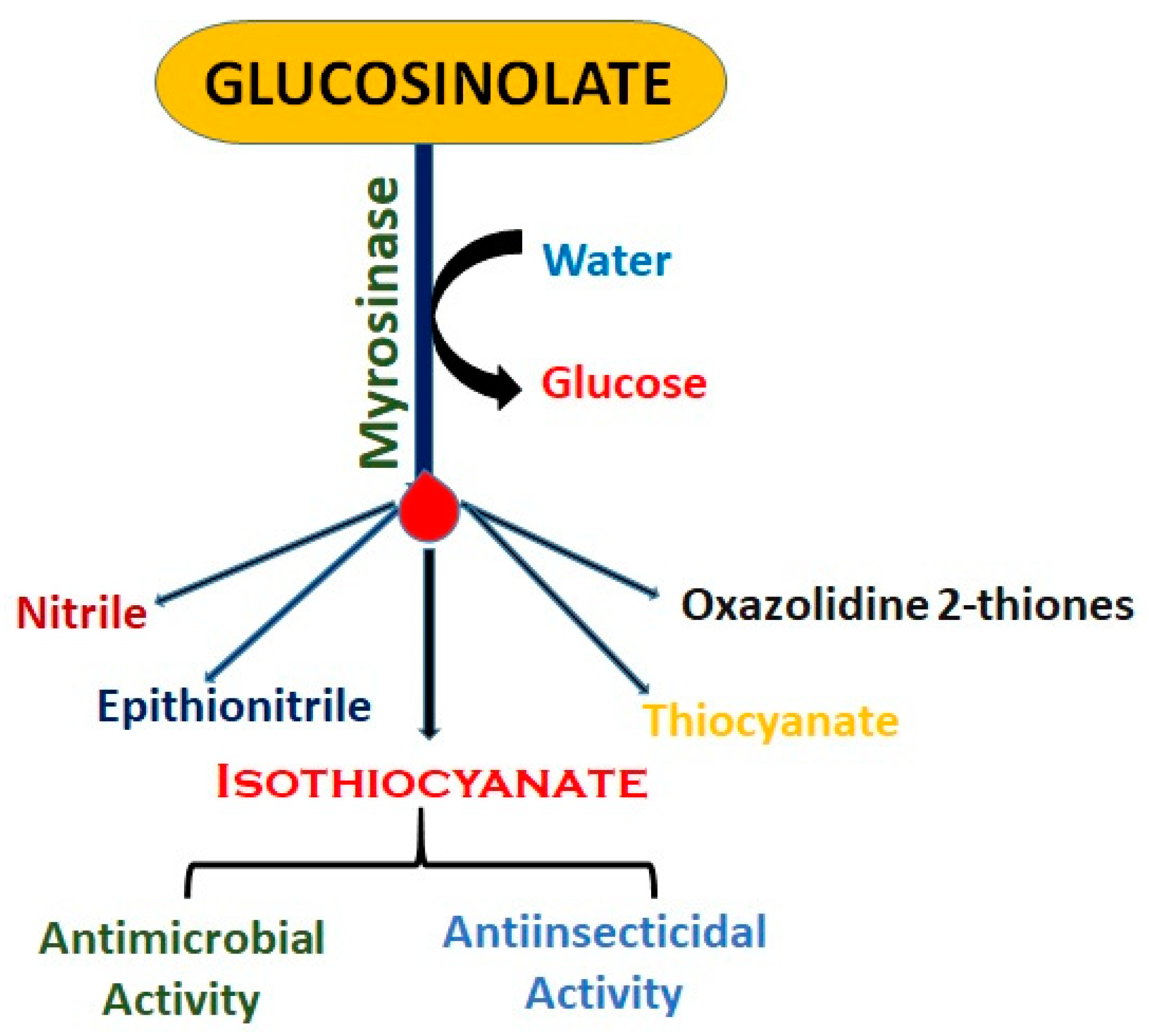
6. Myrosinase Activity Mediating Glucosinolate Breakdown
- Isothiocyanates: These are among the most commonly studied products of glucosinolate breakdown.
- Sulforaphane: A secondary metabolite produced as a result of the hydrolysis of the glucosinolate glucoraphanin by myrosinase. It has been identified as the most effective active substance [35]. It is a potent anticancer phytochemical found in high levels in broccoli and provides cancer protection through the alteration of various epigenetic and non-epigenetic pathways. It is a safe-to-consume and potent anticancer phytochemical [36].
- Phenethyl isothiocyanate (PEITC): Found in watercress, PEITC has been shown to inhibit the growth of cancer cells [31].
- Nitriles: The formation of nitriles can be favored in certain pH conditions or by the presence of specific proteins. Nitriles are less bioactive than isothiocyanates but can still play a role in the plant’s defense mechanisms [23]
- Thiocyanates: These compounds can affect thyroid function by competing with iodine uptake, potentially impacting thyroid hormone production. However, the effect is generally considered minor in the context of a balanced diet [38].
7. Isothiocyanate Breakdown Mechanism
Breakdown of GSL
8. Maximizing ITC-Mediated Disease Suppression
- Different biofumigant crops must be tested for activity against the target pathogen. This can be achieved through in vitro studies, particularly focusing on the effect on resting structures such as chlamydospores, sclerotia, and microsclerotia, or ideally in soil-based assays under controlled conditions to establish the biofumigant with the best potential for a specific soilborne disease before extensive field experiments are performed. Recently, an optical platform was devised [42] using cation-exchanging Nafion particles to produce transparent soil to study the mechanisms of transmission of pathogens on lettuce plants. This platform may be utilized as a real-time biological screen to examine the effects on target pathogens, as it was shown that transparent soil is ideal for imaging studies of certain plant–microbe interactions in situ, as soil microbes and their interactions with plants enhance the supply of nutrients via nodulation or biological fertilization.
- Brassica species producing aliphatic short-chain ITCs may be more efficient than those producing long-chain aromatic ITCs due to enhanced volatility and lower sorption of these chemicals to organic debris. The biofumigant species may also need to be chosen based on winter hardiness, growth rate, and GSL production during various times of the year, depending on when they are to be integrated. Seed meals and processed biofumigants may be more appropriate (1) for small, intensively cropped areas such as greenhouses and polytunnels and (2) for the suppression of more resistant resting structures, such as Verticillium dahliae’s microsclerotia [43].
- Agronomic aspects, including the seed rate, sowing time, fertilizer treatment, and the best time for inclusion, must all be taken into account in order to maximize the production of biofumigant crops and the GSL level since biofumigation requires large volumes of biomass. For example, fertilization-mediated nitrogen and sulfur delivery has been shown to alter the amount of GSLs in plant tissue [44].
- The breakdown of Brassica plant cells is essential for converting glucosinolates into isothiocyanates (ITCs). The release of ITCs is proportional to the degree of cell damage, with a higher level of disruption leading to increased ITC concentrations [45]. Therefore, pulverizing and crushing plant material is a better use of equipment than chopping. Finely shredding the biofumigant crop before incorporation into the soil is crucial for maximizing ITC release [46].
9. Effectiveness of Processed Biogumifationts Against Pathogens
Effectiveness Against Pathogens
- Factors Affecting Efficacy: The following variables determine the success of biofumigation: soil conditions, climate, and farm management practices, as soil with low organic matter is favorable for biofumigation, and factors like pH, temperature, soil-metal interaction, and water management in farms influence the release of bioactive compounds [11,44,64].
- Knowledge Gaps and Limitations: Research on the impact of biofumigation on free-living nematode species remains limited. A major challenge in commercial adoption is the lack of a consistent experimental framework, leading to variable control outcomes across organisms and studies, as the effects of ITCs on fungal activities include the inhibition of growth and germination of sclerotia or spores [24]. Additional research is required to identify the most effective biofumigants for specific pathogens under diverse conditions.
- Significance of effects on soil health: Biofumigation enhances soil health by promoting greater microbial diversity and activity within the soil ecosystem [65]. The addition of Brassica residues improves the soil structure by enhancing organic matter, thus improving nutrient availability. These benefits contribute to increased soil fertility and resilience to environmental stresses, fostering a sustainable farming system [66,67].
10. Inherent Limitations of Biofumigation and Challenges
- Pathogen-Specific Limitations: The concentration of ITCs needed to suppress pathogens varies depending on the target soil-borne pathogens, nematodes, or weed seeds [16,68]. For example, for Verticillium dahliae, Brassica plants may not produce enough ITCs to control its resilient microsclerotia under field conditions [43].
- Soil-Dependent Limitations: The type of soil significantly influences biofumigation outcomes. Lighter soils with sandy textures and a low organic matter content are more favorable for biofumigation [64]. High organic matter in soil reduces ITC availability due to sorption, diminishing their effectiveness against pathogens [69]. In contrast, sandy soils enable better diffusion of toxic gases, enhancing biofumigation efficacy. Variability in glucosinolate levels: The effectiveness of biofumigation relies heavily on the type and concentration of glucosinolates (GSLs) in the Brassica species used. GSL levels can vary significantly between species, cultivars, and even different parts of the plant, making it difficult to standardize its application for consistent results [70].
- Potential Harm to Future Crops: The residual effects of biofumigation, such as an excessive release of isothiocyanates (ITCs), may cause phytotoxicity to subsequent crops if not managed properly. Sensitive crops planted immediately after biofumigation can suffer damage, which necessitates careful planning of crop rotation [9].
- Timing and Application Techniques: The success of biofumigation depends on precise timing and application methods [9]. Factors such as the plant growth stage, pH, incorporation into soil, water management, and moisture levels significantly influence the release and effectiveness of bioactive compounds. Incorrect timing or poor technique may result in reduced efficacy or inconsistent pest and disease control [9,71,72].
- Environmental Influences: Soil conditions, temperature, and microbial activity can alter the hydrolysis of glucosinolates [6] and the release of ITCs. Adverse environmental factors, including local environmental conditions, may limit the biofumigation process or reduce its effectiveness in pest suppression [73]. Several studies have detected both GSLs and ITCs in the rhizosphere, which have been implicated in the suppression of pests and pathogens [74].
- Economic Feasibility: The cost of cultivating biofumigant crops into existing farming systems can be prohibitive for small-scale farmers. This challenge is further compounded by the lack of access to optimized cultivars for specific biofumigation purposes.
- Regulatory and Adoption Barriers: The adoption of biofumigation practices may be hindered by limited awareness, a lack of extension services, and the need for regulatory frameworks to ensure the safety and sustainability of the practice.
11. Conclusions and Future Scope
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, J.N.; Ghatak, A. Biofumigation: A control method for the soil-borne diseases. Internat. J. Plant Protec. 2017, 10, 453–460. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Plant isothiocyanates as an alternative for sustainable disease control of horticultural crops. In Sustainable Crop Disease Management Using Natural Products; Ganesan, S., Vedivel, K.V.K., Jayaraman, J., Eds.; CABI: Wallingford, UK, 2015; pp. 74–94. [Google Scholar] [CrossRef]
- Petersen, A.; Wang, C.; Crocoll, C.; Halkier, B.A. Biotechnological approaches in glucosinolate production. J. Integr. Plant Biol. 2018, 60, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Gantait, A.; Masih, S.A.; Addesso, R.; Maxton, A.; Sofo, A. Glucosinolates Mediated Regulation of Enzymatic Activity in Response to Oxidative Stress in Brassica spp. Plants 2024, 13, 3422. [Google Scholar] [CrossRef]
- Lu, P.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Biofumigation with Brassica plants and its effect on the inoculum potential of Fusarium yellows of Brassica crops. Eur. J. Plant Pathol. 2010, 126, 387–402. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Yim, B.; Winkelmann, T.; Smalla, K.; Schreiner, M. Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS ONE 2015, 10, e0132931. [Google Scholar] [CrossRef]
- Antônio dos Santos, C.; Carlos de Souza Abboud, A.; Goréte Ferreira do Carmo, M. Biofumigation with species of the Brassicaceae family: A review. Ciênc. Rural. 2020, 51, e20200440. [Google Scholar] [CrossRef]
- Szczygłowska, M.; Piekarska, A.; Konieczka, P.; Namieśnik, J. Use of Brassica plants in the phytoremediation and biofumigation processes. Int. J. Mol. Sci. 2011, 12, 7760–7771. [Google Scholar] [CrossRef]
- Ngala, B.M.; Haydock, P.P.J.; Woods, S.; Back, M.A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest. Manag. Sci. 2015, 71, 759–769. [Google Scholar] [CrossRef]
- Shwetha, H.M.; Kumar, M.K.P.; Teli, K.; Puneeth, M.E. Soil Incorporation of Different Brassica spp. Reduces Incidence of Bacterial Wilt Caused by Ralstonia solanacearum. Int. J. Pure Appl. Biosci. 2018, 6, 904–910. [Google Scholar] [CrossRef]
- Abdallah, I.; Yehia, R.; Kandil, M.A. Biofumigation potential of Indian mustard (Brassica juncea) to manage Rhizoctonia solani. Egypt J. Biol. Pest. Cont. 2020, 30, 99. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Liyanapathiranage, P.; Addesso, K.M. Effect of Brassica crop-based biofumigation on soilborne disease suppression in woody ornamentals. Can. J. Plant Pathol. 2020, 42, 94–106. [Google Scholar] [CrossRef]
- Swetha, B.; Preethika, S.; Babu, S. Biofumigation in Crop Protection. Agri. Mirror Future India 2020, 1, 29–32. [Google Scholar]
- Rice, A.R.; Johnson-Maynard, J.L.; Thill, D.C.; Morra, M.J. Vegetable crop emergence and weed control following amendment with different Brassicaceae seed meals. Renew. Agric. Food Syst. 2007, 22, 204–212. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Libbey, C.; Boydston, R. Weed Suppression with brassica Green Manure Crops in Green Pea. Weed Sci. 1997, 45, 439–445. Available online: http://www.jstor.org/stable/4046046 (accessed on 2 March 2025). [CrossRef]
- Sencenbaugh, L.; Mangold, J.M.; Ulrich, D.E.M.; Rew, L.J. Efficacy of multiple Brassica biofumigation techniques in the suppression of non-native and native grass seedling emergence and productivity. Weed Res. 2025, 65e, 12670. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Matthiessen, J.N. Developing and refining the biofumigation concept. Agroindustria 2004, 3, 233–239. [Google Scholar]
- Gamliel, A.; Austerweil, M.; Kritzman, G. Non-chemical approach to soilborne pest management—Organic amendments. Crop Prot. 2000, 19, 847–853. [Google Scholar] [CrossRef]
- Badenes-Pérez, F. Trap Crops and Insectary Plants in the Order Brassicales. Ann. Entomol. Soc. Am. 2019, 112, 318–329. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Trout, T.; Mueller, J.; Wilhelm, S.; Nelson, S.D.; Soppe, R.; Shatley, D. Application of Alternative Fumigants Through Drip Irrigation Systems. Phytopathology 2003, 92, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Dhankhar, J.; Kundu, P. Isothiocyanates—A Review of their Health Benefits and Potential Food Applications. Curr. Res. Nutr. Food Sci. 2022, 10, 476–502. [Google Scholar] [CrossRef]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates-A review. LWT–Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Hallam, J. Soil hydraulic function: Earthworm-plant root interactions. Ph.D. Thesis, University of York, York, UK, December 2018. [Google Scholar]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Potter, M.; Vanstone, V.; Davies, K.; Rathjen, A. Breeding to increase the concentration of 2-phenylethyl glucosinolate in the roots of Brassica napus. J. Chem. Ecol. 2000, 26, 1811–1820. [Google Scholar] [CrossRef]
- Manici, L.M.; Lazzeri, L.; Palmieri, S. In vitro fungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J. Agri. Food Chem. 1997, 45, 2768–2773. [Google Scholar] [CrossRef]
- Chew, F.S. Biologically active natural products—Potential use in agriculture. In ACS Symposium Series. American Chemical; Comstock, M.J., Ed.; Society: Washington, DC, USA, 1987. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Tomkins, B.; Nicolas, M.E.; Premier, R.R.; Bennett, R.N.; Eagling, D.R.; Taylor, P.W. The effect of post-harvest and packaging treatments on glucoraphanin concentration in broccoli (Brassica oleracea var. italica). J. Agric. Food Chem. 2002, 50, 7386–7391. [Google Scholar] [CrossRef]
- Lazzeri, L.; Manici, L.M. The Glucosinolate-Myrosinase System: A Natural and Practical tool for Biofumigation. Acta Hortic. 2000, 532, 89–96. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Khan, N.; Kaleem, N.; Ahmad, W.; Alharethi, S.H.; Alharbi, B.; Alhassan, H.H.; Al-Enazi, M.M.; Razis, A.F.A.; Modu, B.; et al. Anticancer properties of sulforaphane: Current insights at the molecular level. Front. Oncol. 2023, 13, 1168321. [Google Scholar] [CrossRef]
- Coll, D.A.; Rosen, C.A.; Auborn, K.; Potsic, W.P.; Bradlow, H.L. Treatment of recurrent respiratory papillomatosis with indole-3-carbinol. Am. J. Otolaryngol. 1997, 18, 283–285. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef]
- Lin, C.M.; Preston, J.F., 3rd; Wei, C.I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef]
- Dahlin, P.; Hallmann, J. New Insights on the Role of Allyl Isothiocyanate in Controlling the Root Knot Nematode Meloidogyne hapla. Plants 2020, 9, 603. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Fang, W.; Yan, D.; Han, D.; Huang, B.; Zhang, Y.; Li, Y.; Ouyang, C.; Cao, A.; et al. Soil properties, presence of microorganisms, application dose, soil moisture and temperature influence the degradation rate of Allyl isothiocyanate in soil. Chemosphere 2020, 244, 125540. [Google Scholar] [CrossRef]
- Downie, H.; Holden, N.; Otten, W.; Spiers, A.J.; Velntine, T.A.; Dupuy, L.X. Transparent soil for imaging the rhizosphere. PLoS ONE 2012, 7, e44276. [Google Scholar] [CrossRef]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Li, S.; Schonhof, I.; Krumbein, A.; Li, L.; Stützel, H.; Schreiner, M. Glucosinolate concentration in turnip (Brassica rapa ssp. rapifera L.) roots as affected by nitrogen and sulfur supply. J. Agri. Food Chem. 2007, 55, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.J.; Kirkegaard, J.A. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690. [Google Scholar] [CrossRef]
- Dutta, T.K.; Khan, M.R.; Phani, V. Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: Current status and future prospects. Curr. Plant Biol. 2019, 17, 17–32. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Liyanapathiranage, P. Pathogenicity of Rhizoctonia solani and Phytophthora nicotianae to Brassicaceae cover crops. Arc. Phytopathol. Plant Protec. 2019, 52, 288–302. [Google Scholar] [CrossRef]
- Gouws-Meyer, R.; Mcleod, A.; Mazzola, M. Potato scab management with Brassica biofumigation and effect of volatiles on Streptomyces growth. Acta Hortic. 2020, 1269, 25–32. [Google Scholar] [CrossRef]
- Hossain, S.; Bergkvist, G.; Glinwood, R.; Berglund, K.; Mårtensson, A.; Hallin, S.; Persson, P. Brassicaceae cover crops reduce Aphanomyces pea root rot without suppressing genetic potential of microbial nitrogen cycling. Plant Soil 2015, 39, 227–238. [Google Scholar] [CrossRef]
- Lord, J.S.; Lazzeri, L.; Atkinson, H.J.; Urwin, P.E. Biofumigation for control of pale potato cyst nematodes: Activity of Brassica leaf extracts and green manures on Globodera pallida in vitro and in soil. J. Agri. Food Chem. 2011, 59, 7882–7890. [Google Scholar] [CrossRef]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 2001, 91, 673–679. [Google Scholar] [CrossRef]
- Bensen, T.A.; Smith, R.F.; Subbarao, K.V.; Koike, S.T.; Fennimore, S.A.; Shem-Tov, S. Mustard and other cover crop effects vary on lettuce drop caused by Sclerotinia minor and on weeds. Plant Dis. 2009, 93, 1019–1027. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M.; Wong, P.T.W.; Mead, A.; Howe, G.; Newell, M. Field studies on the biofumigation of take-all by Brassica break crops. Aus. J. Agri. Res. 2000, 51, 445–456. [Google Scholar] [CrossRef]
- Deadman, M.; Hasani, H.A.; Sa’di, A.A. Solarization and biofumigation reduce Pythium aphanidermatum induced damping off and enhance vegetative growth of greenhouse cucumber in Oman. J. Plant Pathol. 2006, 88, 335–337. [Google Scholar] [CrossRef]
- Fayzalla, E.A.; El-Barougy, E.; El-Rayes, M.M. Control of soil-borne pathogenic fungi of soybean by biofumigation with mustard seed meal. J. Appl. Sci. 2009, 9, 2272–2279. [Google Scholar] [CrossRef]
- Galletti, S.; Sala, E.; Leoni, O.; Burzi, P.L.; Cerato, C. Trichoderma spp. tolerance to Brassica carinata seed meal for a combined use in biofumigation. Biol. Cont. 2008, 45, 319–327. [Google Scholar] [CrossRef]
- Ramirez-Villapudua, J.; Munnecke, D.E. Effect of solar heating and soil amendments of cruciferous residues on Fusarium oxysporum f. sp. conglutinans and other organisms. Phytopathology 1988, 78, 289–295. [Google Scholar] [CrossRef]
- Koike, S.; Subbarao, K. Broccoli residues can control Verticillium wilt of 147 cauliflower. Calif. Agric. 2000, 54, 30–33. [Google Scholar] [CrossRef][Green Version]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Santo, G.S.; Wilson, J.H.; Hang, A.N. Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. Plant Dis. 1993, 77, 42–46. [Google Scholar] [CrossRef]
- Mazzola, M.; Brown, J.; Izzo, A.D.; Cohen, M.F. Mechanism of action and efficacy of seed meal-induced pathogen suppression differ in a Brassicaceae species and time dependent manner. Phytopathology 2007, 97, 454–460. [Google Scholar] [CrossRef]
- Weerakoon, D.M.N.; Reardon, C.L.; Paulitz, T.C.; Izzo, A.D.; Mazzola, M. Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biol. Biochem. 2012, 51, 44–52. [Google Scholar] [CrossRef]
- Chung, W.C.; Huang, J.W.; Huang, H.C.; Jen, J.F. Effect of ground Brassica seed meal on control of Rhizoctonia damping-off of cabbage. Can. J. Plant Pathol. 2002, 24, 211–218. [Google Scholar] [CrossRef]
- Kirkegaard, J. Biofumigation for plant disease control—From the fundamentals to the farming system. In Disease control in crops: Biological and environmentally friendly approaches; Walter, D., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 172–195. [Google Scholar]
- Waisen, P.; Cheng, Z.; Sipes, B.S.; Koon-Hui, W. Biofumigation effects of Brassicaceous cover crops on soil health in cucurbit agroecosystems in Hawaii, USA. Pedosphere 2022, 32, 521–531. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, R.H.; Shin, J.H. Interactions between Brassica Biofumigants and Soil Microbiota: Causes and Impacts. J. Agric. Food Chem. 2021, 69, 11538–11553. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.R.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Ten years of green manuring and biofumigation alters soil characteristics and microbiota. Appl. Soil. Ecol. 2023, 187, 104836. [Google Scholar] [CrossRef]
- Vandicke, J.; Visschere, K.D.; Deconinck, S.; Leenknecht, D.; Vermeir, P.; Audenaert, K.; Haesaert, G. Uncovering the biofumigant capacity of allyl isothiocyanate from several Brassicaceae crops against Fusarium pathogens in maize. J. Sci. Food Agric. 2020, 100, 5476–5486. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Shackleton, M.A. Biofumigation: Environmental impacts on the biological activity of diverse pure and plant-derived isothiocyanates. Pest. Manag. Sci. 2005, 61, 1043–1051. [Google Scholar] [CrossRef]
- Kim, S.H.; Ochar, K.; Hwang, A.; Lee, Y.J.; Kang, H.J. Variability of Glucosinolates in Pak Choy (Brassica rapa subsp. chinensis) Germplasm. Plants 2023, 13, 9. [Google Scholar] [CrossRef]
- Wang, L.; Mazzola, M. Effect of soil physical conditions on emission of allyl isothiocyanate and subsequent microbial inhibition in response to Brassicaceae seed meal amendment. Plant Dis. 2019, 103, 846–852. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Fang, W.; Li, Y.; Yan, D.; Cao, A.; Wang, Q. A review of biofumigation effects with plant materials. New Plant Prot. 2024, 1, e21. [Google Scholar] [CrossRef]
- Van Dam, N.; Tytgat, T.G.; Kirkegaard, J. Root and shoot glucosinolates: A comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem. Rev. 2009, 8, 171–186. [Google Scholar] [CrossRef]
| Compound/Structure | Description |
|---|---|
Glucosinolate | Glucosinolates are naturally occurring sulfur-containing chemicals, principally found in plants from the Brassicaceae family, such as mustard, broccoli, and cabbage. The structure consists of a glucose molecule (C6H11O5) attached to a sulfur-containing group (SO3) and a variable side chain (R), which is responsible for the compound’s specific properties. When the plant tissue is disrupted, either by mechanical damage or decomposition, glucosinolates break down enzymatically into biologically active products, primarily isothiocyanates, through the action of the enzyme myrosinase. The breakdown of glucosinolates into isothiocyanates and other products like thiocyanates, nitriles, and epithionitriles contributes to the plant’s defense mechanism against herbivores, pests, and pathogens [6]. |
Isothiocyanate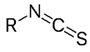 | Isothiocyanates are the principal active products released from the breakdown of glucosinolates. The chemical structure consists of a nitrogen atom (N) double-bonded to a carbon (C) atom, which is also double-bonded to a sulfur (S) atom. The side chain (R) of the isothiocyanate molecule is derived from the original glucosinolate and varies depending on the specific Brassica species. These compounds have potent pesticidal properties and are known for their ability to disrupt the cellular processes of a wide range of pathogens, including fungi, bacteria, and nematodes. The presence of the isothiocyanate group (-N=C=S) is crucial for its antimicrobial activity. Isothiocyanates inhibit pathogen growth by affecting the enzymes involved in cellular metabolism and can act as effective biocides when released into the soil. The specificity and effectiveness of these compounds depend on the type and concentration of glucosinolates in the plant tissue, as well as the conditions under which they are released [2,23]. |
| Step | Description | Detailed Explanation with Citations |
|---|---|---|
| Tissue Disruption | Tissue damage or decomposition of biofumigant crops releases bioactive compounds. | When biofumigant crops like Brassica species (e.g., mustard, rapeseed) are damaged, either mechanically (through crushing or tilling) or through natural decomposition, the plant cells release glucosinolates, which, when broken down, produce various biologically active compounds, primarily isothiocyanates, and other breakdown products like nitriles and thiocyanates [25]. |
| Release of Isothiocyanates | Glucosinolates break down into isothiocyanates due to enzymatic action by myrosinase. | The breakdown of glucosinolates occurs via an enzyme called myrosinase, which is released when plant tissues are damaged. Myrosinase catalyzes the conversion of glucosinolates into isothiocyanates. The type of isothiocyanate produced depends on the side chain of the original glucosinolate [25]. |
| Inhibition of Pathogen Growth | Isothiocyanates, along with other breakdown products, interrupt the cellular processes of pathogens. | Isothiocyanates inhibit pathogen growth by disrupting various cellular processes. They can interfere with enzyme function, protein synthesis, and the integrity of the pathogen’s cellular membrane. For example, isothiocyanates bind to proteins and enzymes in microorganisms or pests, which inhibits their metabolic processes, leading to their death. These compounds can also disrupt fungal cell membranes, making them highly effective in controlling soil-borne diseases [24]. |
| Targeted Pest Control | The specificity of isothiocyanates provides targeted control of pests, pathogens, and nematodes without harming beneficial organisms. | The specificity and efficacy of biofumigants depend on the concentration and type of glucosinolates in the crop, which determines the type and potency of the isothiocyanates released. While these compounds are extremely toxic to pests and pathogens, they have minimal negative impact on beneficial organisms such as earthworms and plant roots, ensuring sustainable pest control [26]. |
| Reduction in Synthetic Chemical Use | The natural release of bioactive compounds reduces the need for synthetic fumigants or pesticides. | By using biofumigant crops, farmers can reduce or eliminate the need for synthetic chemical pesticides, which can have negative environmental and health consequences. Biofumigation with plants like Brassica species provides a more environmentally friendly, sustainable approach to pest control while maintaining soil health. Additionally, the practice can improve soil quality over time by increasing organic matter content through crop decomposition [24] |
| Common Name | GSL (Glucosinolate) | ITC (Isothiocyanate) | Structure | Reference |
|---|---|---|---|---|
| Brown mustard (Brassica juncea) | Sinigrin | 2-propenyl-ITC (= allyl-ITC) | CH2=CH-CH2-N=C=S | [29] |
| Black mustard (Brassica nigra) | Sinigrin | 2-propenyl-ITC (= allyl-ITC) | CH2=CH-CH2-N=C=S | [30] |
| White mustard (Sinapis alba) | Sinalbin | 4-hydroxybenzyl-ITC | HO-C6H4-CH2-N=C=S (benzene ring) | [29] |
| Radish (Raphanus sativus) | Glucoraphenin | 4-methylsulfinyl-3-butenyl-ITC | CH3-SO-(CH2)-CH=CH-N=C=S | [31] |
| Rocket (Eruca sativa) | Glucoerucin | 4-methylthiobutyl-ITC | CH3-S-(CH2)3-N=C=S | [31] |
| Cabbage (Brassica oleracea) | Glucobrassicin | 3-indolylmethyl-ITC | C6H4-CH2-N=C=S | [32] |
| Broccoli (Brassica oleracea var. italica) | Glucoraphanin | 4-methylsulfinyl-3-butenyl-ITC | CH3-SO-(CH2)-CH=CH-N=C=S | [33] |
| Biofumigant Crops/Method of Application | Name of Plant Disease /Pest | Causal Agent | References |
|---|---|---|---|
| Brassica residues | Common scab disease of potato | Streptomyces scabies | [48] |
| B. juncea as cover crop | Root rot of pea | Aphanomyces euteiches | [49] |
| B. juncea leaf extracts and green manures | White potato cyst nematode | Globodera pallida | [50] |
| Brassica juncea as seed meal, seed powder, dry and fresh plants, and methanol extract | Damping off of vegetables | Rhizoctonia solani | [13] |
| B. napus as seed meal | Apple root rot | Rhizoctonia solani | [51] |
| Mustard as cover crop | Lettuce drop | Sclerotinia minor | [52] |
| B. juncea and B. napus residues | All diseases of wheat | Gaeumannomyces graminis var. tritici | [53] |
| B. oleracea residues | Damping off diseases in greenhouses | Pythium aphanidermatum | [54] |
| B. juncea as seed meal | Soil-borne pathogenic fungi of soyabean | Fusarium oxysporum, R. solani, Macrophomina phaseolina, Sclerotium rolfsii | [55] |
| B.carinata as seed meal | Sugar beet damping off | Pythium ultimum | [56] |
| B. oleracea residues | Cabbage yellows | F. oxysporum f.sp. conglutinans | [57] |
| Brassica as cover crop | Woody ornamentals | R. solani and Phytophthora | [14] |
| B.oleracea nicotianae, B. napus residues | Wilt disease in cauliflower | Verticillium dahliae | [58] |
| Brassica spp. As green manure | Soil-borne diseases of potato | Rhizoctonia solani, Phytophthora erythroseptica, Pythium ultimum, Sclerotinia sclerotiorum, and Fusarium sambucinam | [59] |
| B. napus as green manure | Root-knot nematode on potato | Meloidogyne chitwoodi | [60] |
| B. napus as seed meals | Apple replant disease | Phytophthora, Pythium and Rhizoctonia | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavana Praneetha, T.; Masih, S.A.; Addesso, R.; Maxton, A.; Sofo, A. Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases. Plants 2025, 14, 1200. https://doi.org/10.3390/plants14081200
Pavana Praneetha T, Masih SA, Addesso R, Maxton A, Sofo A. Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases. Plants. 2025; 14(8):1200. https://doi.org/10.3390/plants14081200
Chicago/Turabian StylePavana Praneetha, Tikkisetty, Sam A. Masih, Rosangela Addesso, Ann Maxton, and Adriano Sofo. 2025. "Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases" Plants 14, no. 8: 1200. https://doi.org/10.3390/plants14081200
APA StylePavana Praneetha, T., Masih, S. A., Addesso, R., Maxton, A., & Sofo, A. (2025). Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases. Plants, 14(8), 1200. https://doi.org/10.3390/plants14081200









