Transcriptional Profiling to Assess the Effects of Biological Stimulant Atlanticell Micomix on Tomato Seedlings Under Salt Stress
Abstract
1. Introduction
2. Results
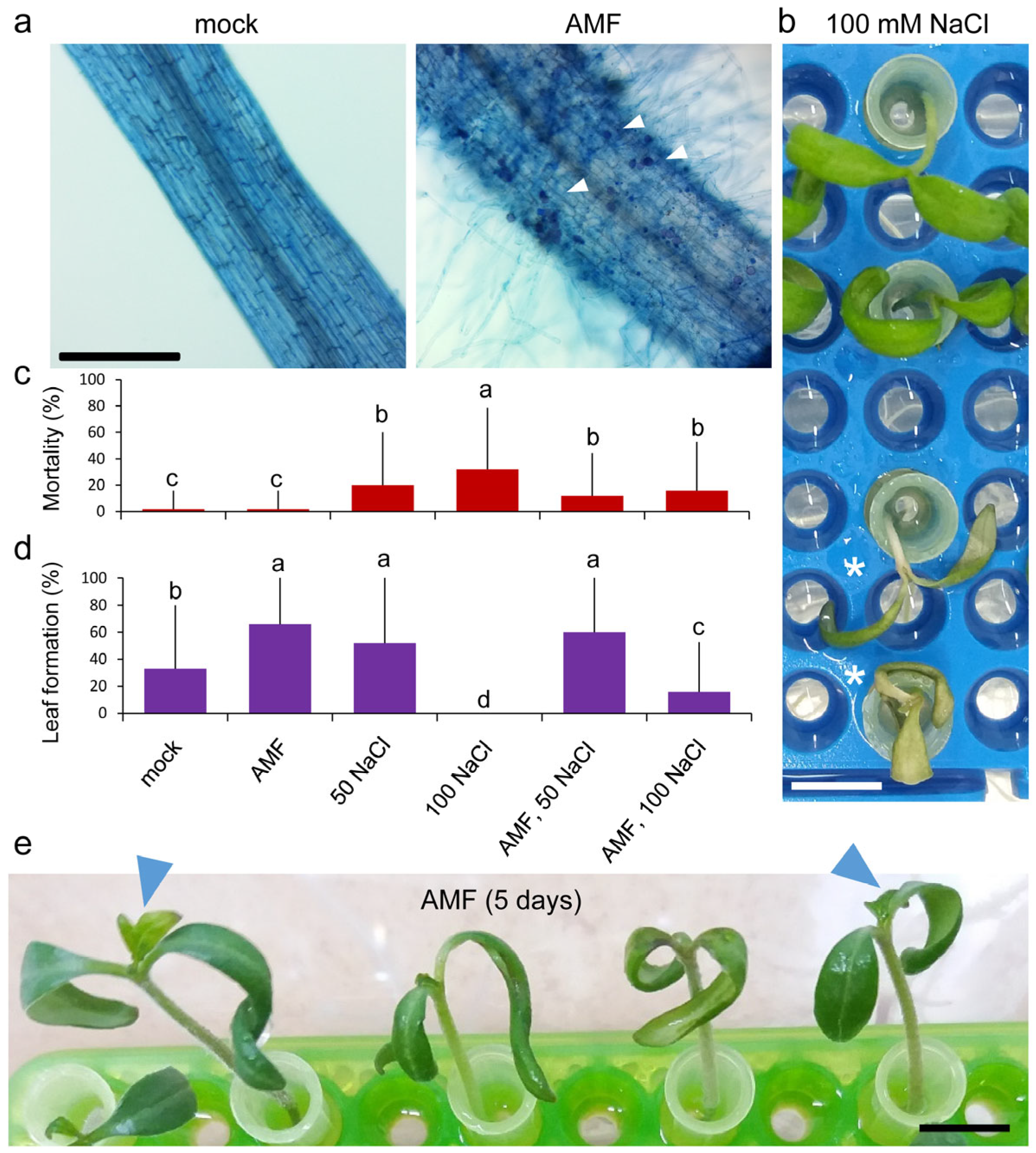
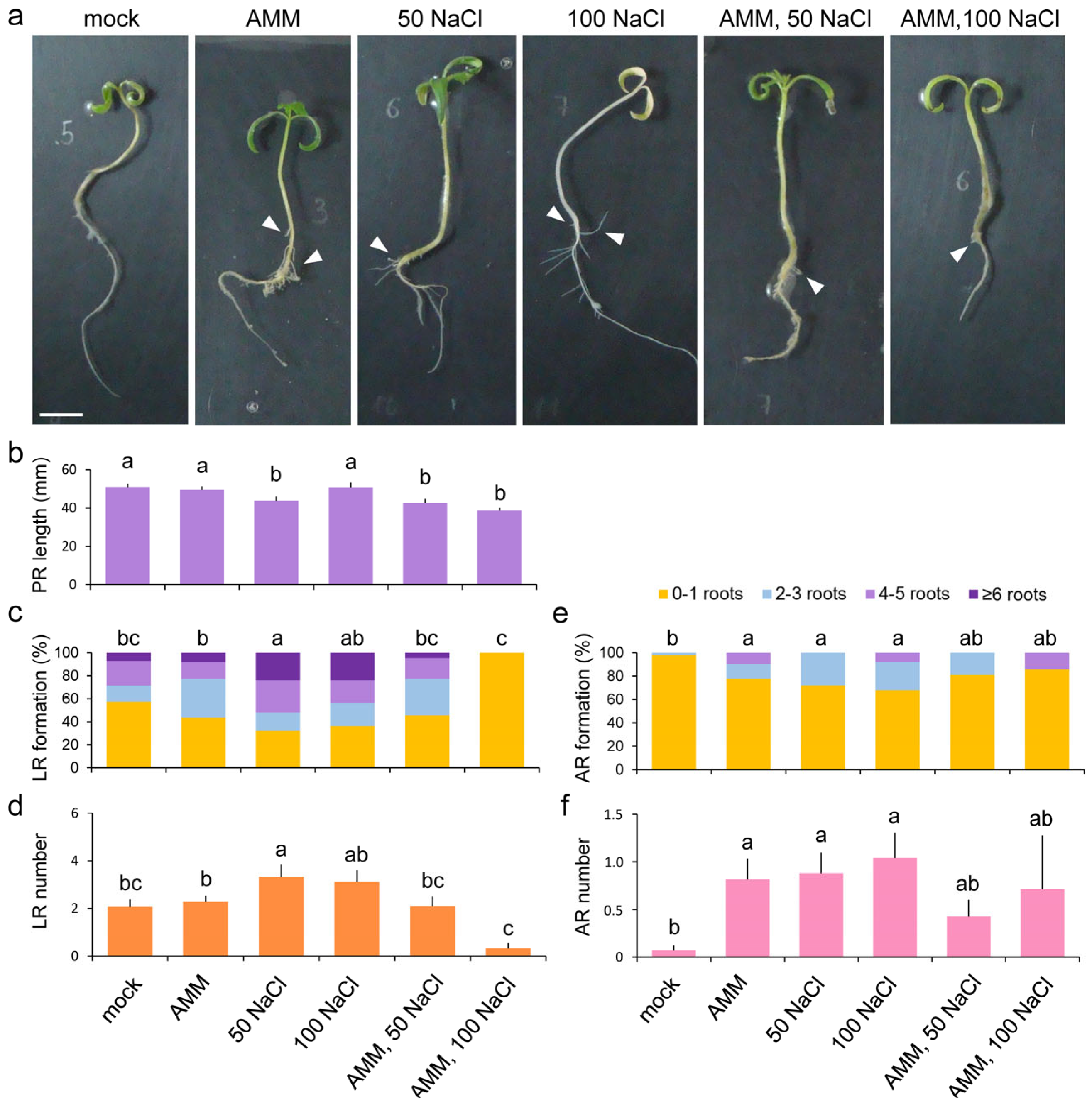
2.1. Growth Responses of Young Tomato Seedlings to Atlanticell Micomix and Salt Stress
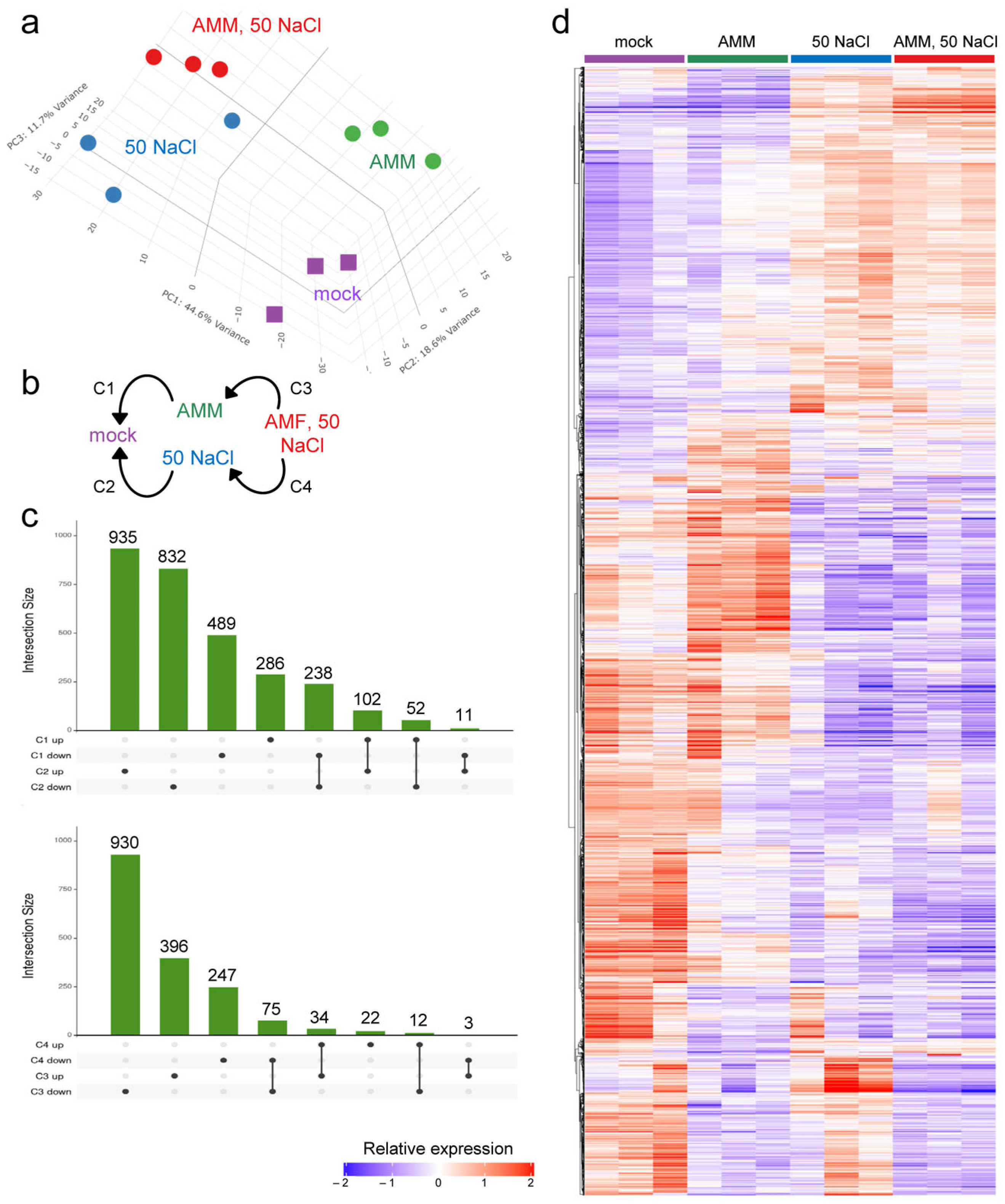
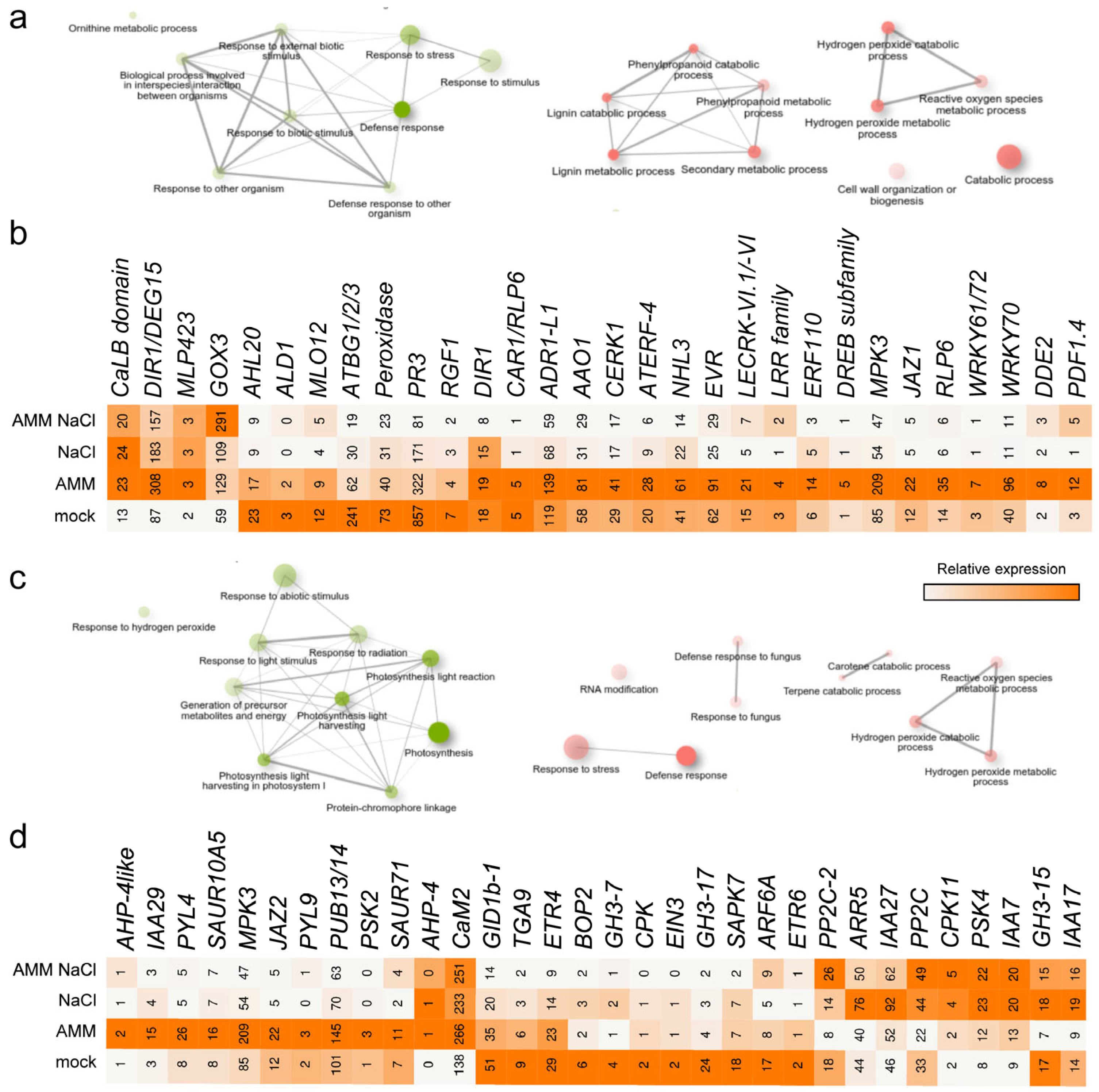
2.2. Gene Expression Analysis in Response to AMM and Moderate Salinity
2.3. Regulatory Responses to AMM and Moderate Salinity
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Culture of Tomato Seedlings
4.3. Sample Collection and RNA Extraction
4.4. RNA-Seq Analysis
4.5. Reverse Transcription-Quantitative PCR (RT-qPCR)
4.6. Microscopy Observation
4.7. Trait Measurement
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | arbuscular mycorrhizal fungi |
| AMM | Atlanticell Micomix |
| AR | adventitious root |
| CPM | counts per million |
| DEG | differential expressed gene |
| GO | gene ontology |
| JA | jasmonic acid |
| LR | lateral root |
| NCED | 9-cis-epoxycarotenoid dioxygenase |
| ncRNA | non-coding RNA |
| PGPR | plant growth-promoting rhizobacteria |
| PR | primary root |
| ROS | reactive oxygen species |
| TF | transcription factor |
References
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate Change and Future of Agri-Food Production. Future Foods Glob. Trends Oppor. Sustain. Chall. 2022, 49–79. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing Climate Change Resilience in Agricultural Crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef] [PubMed]
- Wanders, N.; Wada, Y. Human and Climate Impacts on the 21st Century Hydrological Drought. J. Hydrol. 2015, 526, 208–220. [Google Scholar] [CrossRef]
- Essa, Y.H.; Hirschi, M.; Thiery, W.; El-Kenawy, A.M.; Yang, C. Drought Characteristics in Mediterranean under Future Climate Change. NPJ Clim. Atmos. Sci. 2023, 6, 133. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the Earth: A Review of Anthropogenic Soil Salinization and Plant-Based Strategies for Sustainable Mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential Risk Assessment of Soil Salinity to Agroecosystem Sustainability: Current Status and Management Strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef]
- Penuelas, J.; Coello, F.; Sardans, J. A Better Use of Fertilizers Is Needed for Global Food Security and Environmental Sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- González Guzmán, M.; Cellini, F.; Fotopoulos, V.; Balestrini, R.; Arbona, V. New Approaches to Improve Crop Tolerance to Biotic and Abiotic Stresses. Physiol. Plant. 2022, 174, e13547. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 511937. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput Plant Phenotyping for Developing Novel Biostimulants: From Lab to Field or from Field to Lab? Front. Plant Sci. 2018, 9, 408288. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 444124. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown under Limited Water Availability. Front. Plant Sci. 2019, 10, 450777. [Google Scholar] [CrossRef] [PubMed]
- Della Lucia, M.C.; Baghdadi, A.; Mangione, F.; Borella, M.; Zegada-Lizarazu, W.; Ravi, S.; Deb, S.; Broccanello, C.; Concheri, G.; Monti, A.; et al. Transcriptional and Physiological Analyses to Assess the Effects of a Novel Biostimulant in Tomato. Front. Plant Sci. 2022, 12, 781993. [Google Scholar] [CrossRef]
- Baghdadi, A.; Della Lucia, M.C.; Borella, M.; Bertoldo, G.; Ravi, S.; Zegada-Lizarazu, W.; Chiodi, C.; Pagani, E.; Hermans, C.; Stevanato, P.; et al. A Dual-Omics Approach for Profiling Plant Responses to Biostimulant Applications under Controlled and Field Conditions. Front. Plant Sci. 2022, 13, 983772. [Google Scholar] [CrossRef]
- Sorrentino, M.; Panzarová, K.; Spyroglou, I.; Spíchal, L.; Buffagni, V.; Ganugi, P.; Rouphael, Y.; Colla, G.; Lucini, L.; De Diego, N. Integration of Phenomics and Metabolomics Datasets Reveals Different Mode of Action of Biostimulants Based on Protein Hydrolysates in Lactuca sativa L. and Solanum lycopersicum L. Under Salinity. Front. Plant Sci. 2022, 12, 808711. [Google Scholar] [CrossRef]
- Lee, E.H.; Eo, J.K.; Ka, K.H.; Eom, A.H. Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular Mycorrhizas: A Promising Component of Plant Production Systems Provided Favorable Conditions for Their Growth. Front. Plant Sci. 2018, 9, 366362. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#home (accessed on 16 January 2024).
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with Arbuscular Mycorrhizal Fungi Improves the Nutritional Value of Tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Schubert, R.; Werner, S.; Cirka, H.; Rödel, P.; Moya, Y.T.; Mock, H.P.; Hutter, I.; Kunze, G.; Hause, B. Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production. Int. J. Mol. Sci. 2020, 21, 7029. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association with Two Different Arbuscular Mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 412387. [Google Scholar] [CrossRef] [PubMed]
- Khoshkhatti, N.; Eini, O.; Koolivand, D.; Pogiatzis, A.; Klironomos, J.N.; Pakpour, S. Differential Response of Mycorrhizal Plants to Tomato Bushy Stunt Virus and Tomato Mosaic Virus Infection. Microorganisms 2020, 8, 2038. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R. Sen Priming of Anti-Herbivore Defense in Tomato by Arbuscular Mycorrhizal Fungus and Involvement of the Jasmonate Pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef]
- Rivero, J.; Lidoy, J.; Llopis-Giménez, Á.; Herrero, S.; Flors, V.; Pozo, M.J. Mycorrhizal Symbiosis Primes the Accumulation of Antiherbivore Compounds and Enhances Herbivore Mortality in Tomato. J. Exp. Bot. 2021, 72, 5038–5050. [Google Scholar] [CrossRef]
- Song, Y.; Chen, D.; Lu, K.; Sun, Z.; Zeng, R. Enhanced Tomato Disease Resistance Primed by Arbuscular Mycorrhizal Fungus. Front. Plant Sci. 2015, 6, 163569. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root Metabolic Plasticity Underlies Functional Diversity in Mycorrhiza-Enhanced Stress Tolerance in Tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef]
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Di Fossalunga, A.S.; Ciancio, A.; Pentimone, I. Transcriptomic Responses to Water Deficit and Nematode Infection in Mycorrhizal Tomato Roots. Front. Microbiol. 2019, 10, 1807. [Google Scholar] [CrossRef]
- Duc, N.H.; Szentpéteri, V.; Mayer, Z.; Posta, K. Distinct Impact of Arbuscular Mycorrhizal Isolates on Tomato Plant Tolerance to Drought Combined with Chronic and Acute Heat Stress. Plant Physiol. Biochem. 2023, 201, 107892. [Google Scholar] [CrossRef]
- Leventis, G.; Tsiknia, M.; Feka, M.; Ladikou, E.V.; Papadakis, I.E.; Chatzipavlidis, I.; Papadopoulou, K.; Ehaliotis, C. Arbuscular Mycorrhizal Fungi Enhance Growth of Tomato under Normal and Drought Conditions, via Different Water Regulation Mechanisms. Rhizosphere 2021, 19, 100394. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato Responses to Salinity Stress: From Morphological Traits to Genetic Changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Omran, V.O.G.; Razavi, S.M.; Pirdashti, H.; Ranjbar, M. Piriformospora Indica Confers Salinity Tolerance on Tomato (Lycopersicon esculentum Mill.) through Amelioration of Nutrient Accumulation, K+/Na+ Homeostasis and Water Status. Plant Cell Rep. 2019, 38, 1151–1163. [Google Scholar] [CrossRef]
- Ghorbani, A.; Razavi, S.M.; Ghasemi Omran, V.O.; Pirdashti, H. Piriformospora Indica Inoculation Alleviates the Adverse Effect of NaCl Stress on Growth, Gas Exchange and Chlorophyll Fluorescence in Tomato (Solanum lycopersicum L.). Plant Biol. 2018, 20, 729–736. [Google Scholar] [CrossRef]
- Heidarianpour, M.B.; Aliasgharzad, N.; Olsson, P.A. Positive Effects of Co-Inoculation with Rhizophagus Irregularis and Serendipita Indica on Tomato Growth under Saline Conditions, and Their Individual Colonization Estimated by Signature Lipids. Mycorrhiza 2020, 30, 455–466. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Ouziad, F.; Hildebrandt, U.; Schmelzer, E.; Bothe, H. Differential Gene Expressions in Arbuscular Mycorrhizal-Colonized Tomato Grown under Heavy Metal Stress. J. Plant Physiol. 2005, 162, 634–649. [Google Scholar] [CrossRef]
- Kashyap, S.P.; Kumari, N.; Mishra, P.; Prasad Moharana, D.; Aamir, M.; Singh, B.; Prasanna, H.C. Transcriptional Regulation-Mediating ROS Homeostasis and Physio-Biochemical Changes in Wild Tomato (Solanum chilense) and Cultivated Tomato (Solanum lycopersicum) under High Salinity. Saudi J. Biol. Sci. 2020, 27, 1999–2009. [Google Scholar] [CrossRef]
- Figueira-Galán, D.; Heupel, S.; Duelli, G.; Tomasi Morgano, M.; Stapf, D.; Requena, N. Exploring the Synergistic Effects of Biochar and Arbuscular Mycorrhizal Fungi on Phosphorus Acquisition in Tomato Plants by Using Gene Expression Analyses. Sci. Total Environ. 2023, 884, 163506. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Argin, S.; Brinza, M.; Yildirim, E. Drought Stress Amelioration in Tomato (Solanum lycopersicum L.) Seedlings by Biostimulant as Regenerative Agent. Front. Plant Sci. 2023, 14, 1211210. [Google Scholar] [CrossRef] [PubMed]
- Patani, A.; Prajapati, D.; Ali, D.; Kalasariya, H.; Yadav, V.K.; Tank, J.; Bagatharia, S.; Joshi, M.; Patel, A. Evaluation of the Growth-Inducing Efficacy of Various Bacillus Species on the Salt-Stressed Tomato (Lycopersicon esculentum Mill.). Front. Plant Sci. 2023, 14, 1168155. [Google Scholar] [CrossRef] [PubMed]
- Altimira, F.; Godoy, S.; Arias-Aravena, M.; Vargas, N.; González, E.; Dardón, E.; Montenegro, E.; Viteri, I.; Tapia, E. Reduced Fertilization Supplemented with Bacillus Safensis RGM 2450 and Bacillus Siamensis RGM 2529 Promotes Tomato Production in a Sustainable Way. Front. Plant Sci. 2024, 15, 1451887. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, Y.; Di, Y.; Qiu, Y.; Ji, Z.; Zhou, T.; Shen, S.; Du, N.; Zhang, T.; Dong, X.; et al. Plant Growth-Promoting Rhizobacteria Pseudomonas Aeruginosa HG28-5 Improves Salt Tolerance by Regulating Na+/K+ Homeostasis and ABA Signaling Pathway in Tomato. Microbiol. Res. 2024, 283, 127707. [Google Scholar] [CrossRef]
- Giovannini, L.; Pagliarani, C.; Cañizares, E.; Sillo, F.; Chitarra, W.; De Rose, S.; Zampieri, E.; Ioannou, A.; Spanos, A.; Vita, F.; et al. Mycorrhization and Chemical Seed Priming Boost Tomato Stress Tolerance by Changing Primary and Defence Metabolic Pathways. J. Exp. Bot. 2024, erae457. [Google Scholar] [CrossRef]
- Mekkaoui, F.; Ait-El-Mokhtar, M.; Zaari Jabri, N.; Amghar, I.; Essadssi, S.; Hmyene, A. The Use of Compost and Arbuscular Mycorrhizal Fungi and Their Combination to Improve Tomato Tolerance to Salt Stress. Plants 2024, 13, 2225. [Google Scholar] [CrossRef]
- Soussani, F.E.; Boutasknit, A.; Ben-Laouane, R.; Benkirane, R.; Baslam, M.; Meddich, A. Arbuscular Mycorrhizal Fungi and Compost-Based Biostimulants Enhance Fitness, Physiological Responses, Yield, and Quality Traits of Drought-Stressed Tomato Plants. Plants 2023, 12, 1856. [Google Scholar] [CrossRef]
- Arcidiacono, M.; Pellegrino, E.; Nuti, M.; Ercoli, L. Field Inoculation by Arbuscular Mycorrhizal Fungi with Contrasting Life-History Strategies Differently Affects Tomato Nutrient Uptake and Residue Decomposition Dynamics. Plant Soil 2023, 500, 105–127. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boopathi, T.; Manivannan, P. Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants. J. Fungi 2021, 7, 303. [Google Scholar] [CrossRef]
- Li, Y.; Laterrière, M.; Lay, C.Y.; Klabi, R.; Masse, J.; St-Arnaud, M.; Yergeau, É.; Lupwayi, N.Z.; Gan, Y.; Hamel, C. Effects of Arbuscular Mycorrhizal Fungi Inoculation and Crop Sequence on Root-Associated Microbiome, Crop Productivity and Nutrient Uptake in Wheat-Based and Flax-Based Cropping Systems. Appl. Soil Ecol. 2021, 168, 104136. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C.; Ma, F. Functions of Arbuscular Mycorrhizal Fungi in Horticultural Crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; Van den Boom, T.; Weber, E. Phänologische Entwicklungsstadien von Gemüsepflanzen: II. Fruchtgemüse Und Hülsenfrüchte. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 1995, 47, 217–232. [Google Scholar]
- Shui, D.; Sun, J.; Xiong, Z.; Zhang, S.; Shi, J. Comparative Identification of WRKY Transcription Factors and Transcriptional Response to Ralstonia Solanacearum in Tomato. Gene 2024, 912, 148384. [Google Scholar] [CrossRef] [PubMed]
- Carella, P.; Kempthorne, C.J.; Wilson, D.C.; Isaacs, M.; Cameron, R.K. Exploring the Role of DIR1, DIR1-like and Other Lipid Transfer Proteins during Systemic Immunity in Arabidopsis. Physiol. Mol. Plant Pathol. 2017, 97, 49–57. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Wang, K.; Ryu, C.M.; Kaundal, A.; Mysore, K.S. Glycolate Oxidase Modulates Reactive Oxygen Species–Mediated Signal Transduction during Nonhost Resistance in Nicotiana Benthamiana and Arabidopsis. Plant Cell 2012, 24, 336–352. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 Functions as a Node of Convergence for Jasmonic Acid– and Auxin-Mediated Signaling in Jasmonic Acid–Induced Leaf Senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef]
- Gonzalez-Guzman, M.; Rodriguez, L.; Lorenzo-Orts, L.; Pons, C.; Sarrion-Perdigones, A.; Fernandez, M.A.; Peirats-Llobet, M.; Forment, J.; Moreno-Alvero, M.; Cutler, S.R.; et al. Tomato PYR/PYL/RCAR Abscisic Acid Receptors Show High Expression in Root, Differential Sensitivity to the Abscisic Acid Agonist Quinabactin, and the Capability to Enhance Plant Drought Resistance. J. Exp. Bot. 2014, 65, 4451–4464. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate ZIM-Domain Protein Gene SlJAZ2 Regulates Plant Morphology and Accelerates Flower Initiation in Solanum lycopersicum Plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Dere, S. Mitigating the Adverse Effects of Salt Stress on Pepper Plants Through Arbuscular Mycorrhizal Fungi (AMF) and Beneficial Bacterial (PGPR) Inoculation. Horticulturae 2024, 10, 1150. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant Growth-Promoting Rhizobacteria: Salt Stress Alleviators to Improve Crop Productivity for Sustainable Agriculture Development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Babalola, O.O.; Adejumo, T.O. Harnessing of Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi in Agroecosystem Sustainability. CABI Agric. Biosci. 2023, 4, 26. [Google Scholar] [CrossRef]
- Reyes-Impellizzeri, S.; Moreno, A.A. The Endoplasmic Reticulum Role in the Plant Response to Abiotic Stress. Front. Plant Sci. 2021, 12, 755447. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Di Stasio, E.; Cirillo, V.; Silletti, S.; Ventorino, V.; Pepe, O.; Raimondi, G.; Maggio, A. Root inoculation with Azotobacter chroococcum 76A enhances tomato plants adaptation to salt stress under low N conditions. BMC Plant Biol. 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Larriba, E.; Nicolás-Albujer, M.; Sánchez-García, A.B.; Pérez-Pérez, J.M. Identification of Transcriptional Networks Involved in De Novo Organ Formation in Tomato Hypocotyl Explants. Int. J. Mol. Sci. 2022, 23, 16112. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of Salinity Stress on Crop Plants: Improving Salt Tolerance through Genetic and Molecular Dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Rivera, P.; Moya, C.; O’Brien, J.A. Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings. Plants 2022, 11, 807. [Google Scholar] [CrossRef]
- Liang, S.M.; Li, Q.S.; Liu, M.Y.; Hashem, A.; Al-Arjani, A.B.F.; Alenazi, M.M.; Abd_Allah, E.F.; Muthuramalingam, P.; Wu, Q.S. Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (Aquaporins and SOSs) of Tomato under Salt Stress. J. Fungi 2022, 8, 1305. [Google Scholar] [CrossRef]
- Chen, G.; Yang, A.; Wang, J.; Ke, L.; Chen, S.; Li, W. Effects of the Synergistic Treatments of Arbuscular Mycorrhizal Fungi and Trehalose on Adaptability to Salt Stress in Tomato Seedlings. Microbiol. Spectr. 2024, 12, e03404-23. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Short-Term Salt Stress Strongly Affects Dynamic Photosynthesis, but Not Steady-State Photosynthesis, in Tomato (Solanum lycopersicum). Environ. Exp. Bot. 2018, 149, 109–119. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia Pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Holsteens, K.; De Jaegere, I.; Wynants, A.; Prinsen, E.L.J.; Van de Poel, B. Mild and Severe Salt Stress Responses Are Age-Dependently Regulated by Abscisic Acid in Tomato. Front. Plant Sci. 2022, 13, 982622. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 Controls Strigolactone Biosynthesis, Shoot Branching and Mycorrhiza-Induced Apocarotenoid Formation in Tomato. Plant J. 2010, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Tóth, P.; Haider, I.; Pozo, M.J.; Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The Tomato CAROTENOID CLEAVAGE DIOXYGENASE 8 (SlCCD 8) Regulates Rhizosphere Signaling, Plant Architecture and Affects Reproductive Development through Strigolactone Biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate Regulates Leaf Senescence and Tolerance to Cold Stress: Crosstalk with Other Phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Jiao, C.; Li, K.; Zuo, Y.; Gong, J.; Guo, Z.; Shen, Y. CALMODULIN1 and WRKY53 Function in Plant Defense by Negatively Regulating the Jasmonic Acid Biosynthesis Pathway in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 7718. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Larriba, E.; Sánchez-García, A.B.; Martínez-Andújar, C.; Albacete, A.; Pérez-Pérez, J.M. Tissue-Specific Metabolic Reprogramming during Wound-Induced Organ Formation in Tomato Hypocotyl Explants. Int. J. Mol. Sci. 2021, 22, 10112. [Google Scholar] [CrossRef]
- Hosmani, P.S.; Flores-Gonzalez, M.; van de Geest, H.; Maumus, F.; Bakker, L.V.; Schijlen, E.; van Haarst, J.; Cordewener, J.; Sanchez-Perez, G.; Peters, S.; et al. An Improved de Novo Assembly and Annotation of the Tomato Reference Genome Using Single-Molecule Sequencing, Hi-C Proximity Ligation and Optical Maps. bioRxiv 2019, 767764. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R Package Rsubread Is Easier, Faster, Cheaper and Better for Alignment and Quantification of RNA Sequencing Reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Sol Genomics Network. Available online: https://solgenomics.net/ (accessed on 16 January 2024).
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Findeiß, S.; Steiner, L.; Marz, M.; Stadler, P.F.; Prohaska, S.J. Proteinortho: Detection of (Co-)Orthologs in Large-Scale Analysis. BMC Bioinform. 2011, 12, 124. [Google Scholar] [CrossRef]
- Morpheus. Available online: https://software.broadinstitute.org/morpheus/ (accessed on 16 January 2024).
- Tseng, K.C.; Wu, N.Y.; Chow, C.N.; Zheng, H.Q.; Chou, C.Y.; Yang, C.W.; Wang, M.J.; Chang, S.B.; Chang, W.C. JustRNA: A Database of Plant Long Noncoding RNA Expression Profiles and Functional Network. J. Exp. Bot. 2023, 74, 4949–4958. [Google Scholar] [CrossRef]
- Justamante, M.S.; Ibáñez, S.; Peidró, A.; Pérez-Pérez, J.M. A Genome-Wide Association Study Identifies New Loci Involved in Wound-Induced Lateral Root Formation in Arabidopsis Thaliana. Front. Plant Sci. 2019, 10, 311. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justamante, M.S.; Larriba, E.; Zavala-González, E.A.; Aranda-Martínez, A.; Pérez-Pérez, J.M. Transcriptional Profiling to Assess the Effects of Biological Stimulant Atlanticell Micomix on Tomato Seedlings Under Salt Stress. Plants 2025, 14, 1198. https://doi.org/10.3390/plants14081198
Justamante MS, Larriba E, Zavala-González EA, Aranda-Martínez A, Pérez-Pérez JM. Transcriptional Profiling to Assess the Effects of Biological Stimulant Atlanticell Micomix on Tomato Seedlings Under Salt Stress. Plants. 2025; 14(8):1198. https://doi.org/10.3390/plants14081198
Chicago/Turabian StyleJustamante, María Salud, Eduardo Larriba, Ernesto Alejandro Zavala-González, Almudena Aranda-Martínez, and José Manuel Pérez-Pérez. 2025. "Transcriptional Profiling to Assess the Effects of Biological Stimulant Atlanticell Micomix on Tomato Seedlings Under Salt Stress" Plants 14, no. 8: 1198. https://doi.org/10.3390/plants14081198
APA StyleJustamante, M. S., Larriba, E., Zavala-González, E. A., Aranda-Martínez, A., & Pérez-Pérez, J. M. (2025). Transcriptional Profiling to Assess the Effects of Biological Stimulant Atlanticell Micomix on Tomato Seedlings Under Salt Stress. Plants, 14(8), 1198. https://doi.org/10.3390/plants14081198








