Enhanced Production by Terra-Sorb® Symbiotic Biostimulant in Two Model Species Under Nitrogen Stress
Abstract
1. Introduction
2. Results
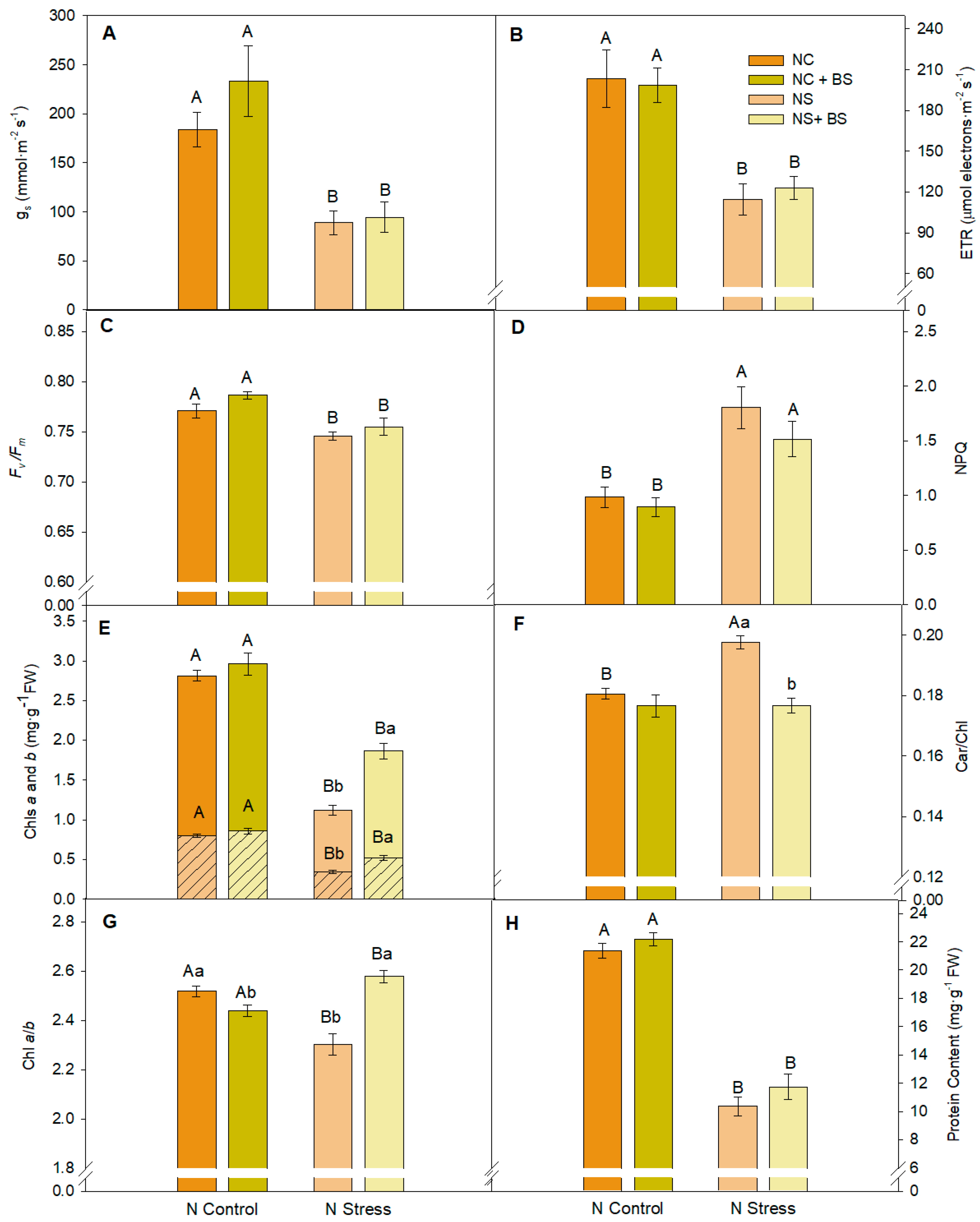
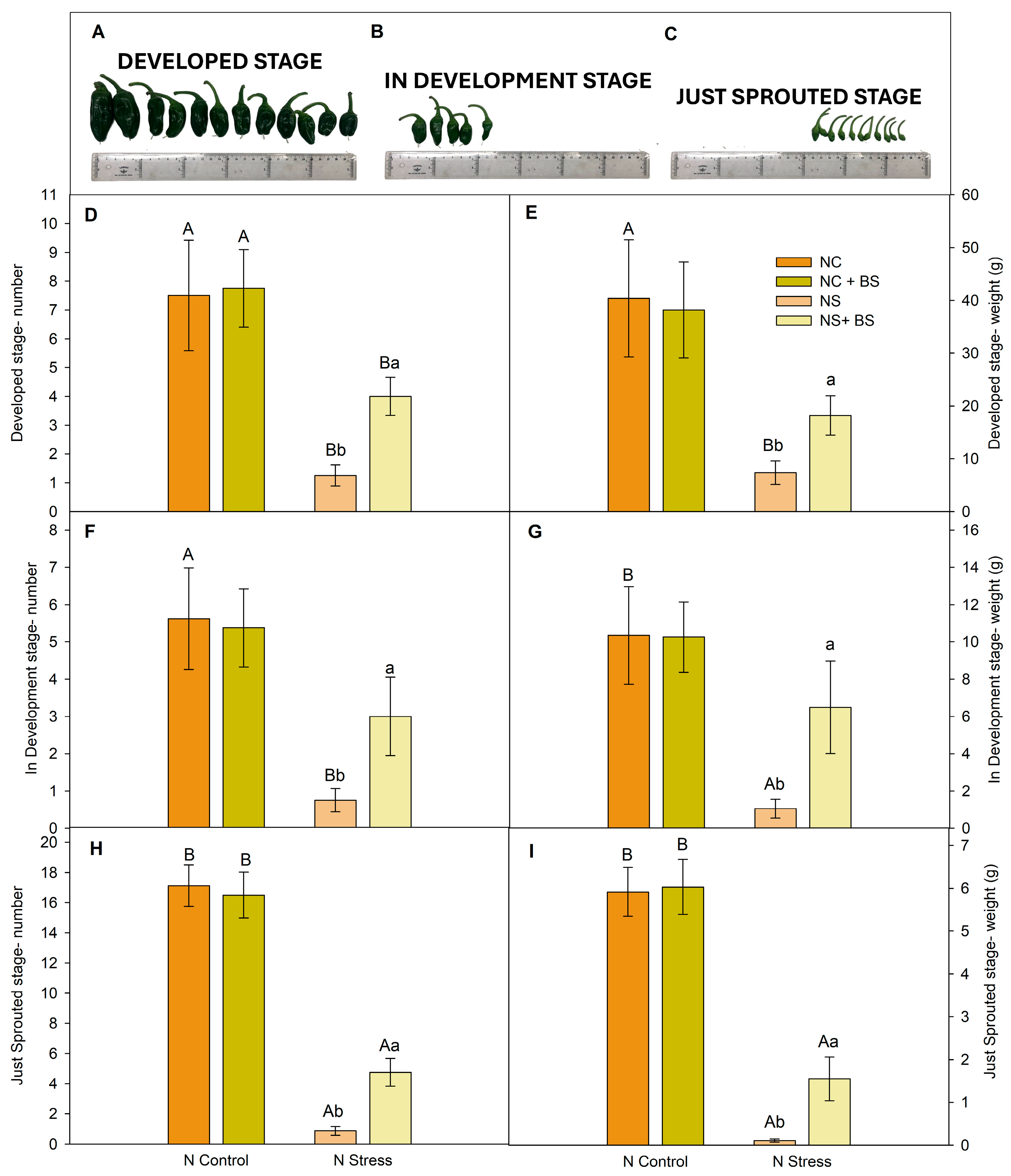
2.1. Nitrogen Stress Drastically Reduced Photosynthetic Activity, Pepper Production, and Plant Vegetative Biomass, but BS Application Promoted a Recovery of Both Vegetative and Reproductive Structures in Pepper Plants
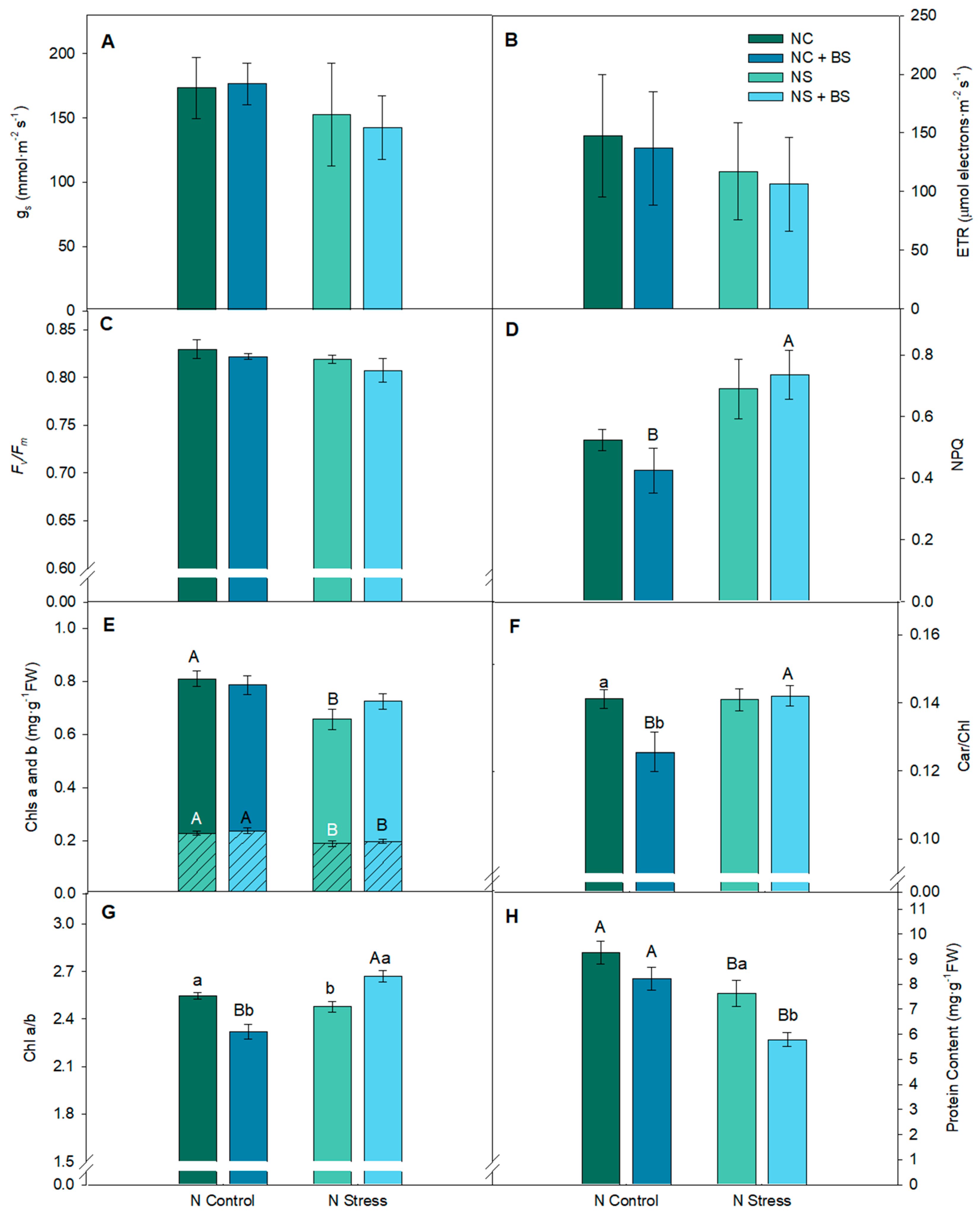
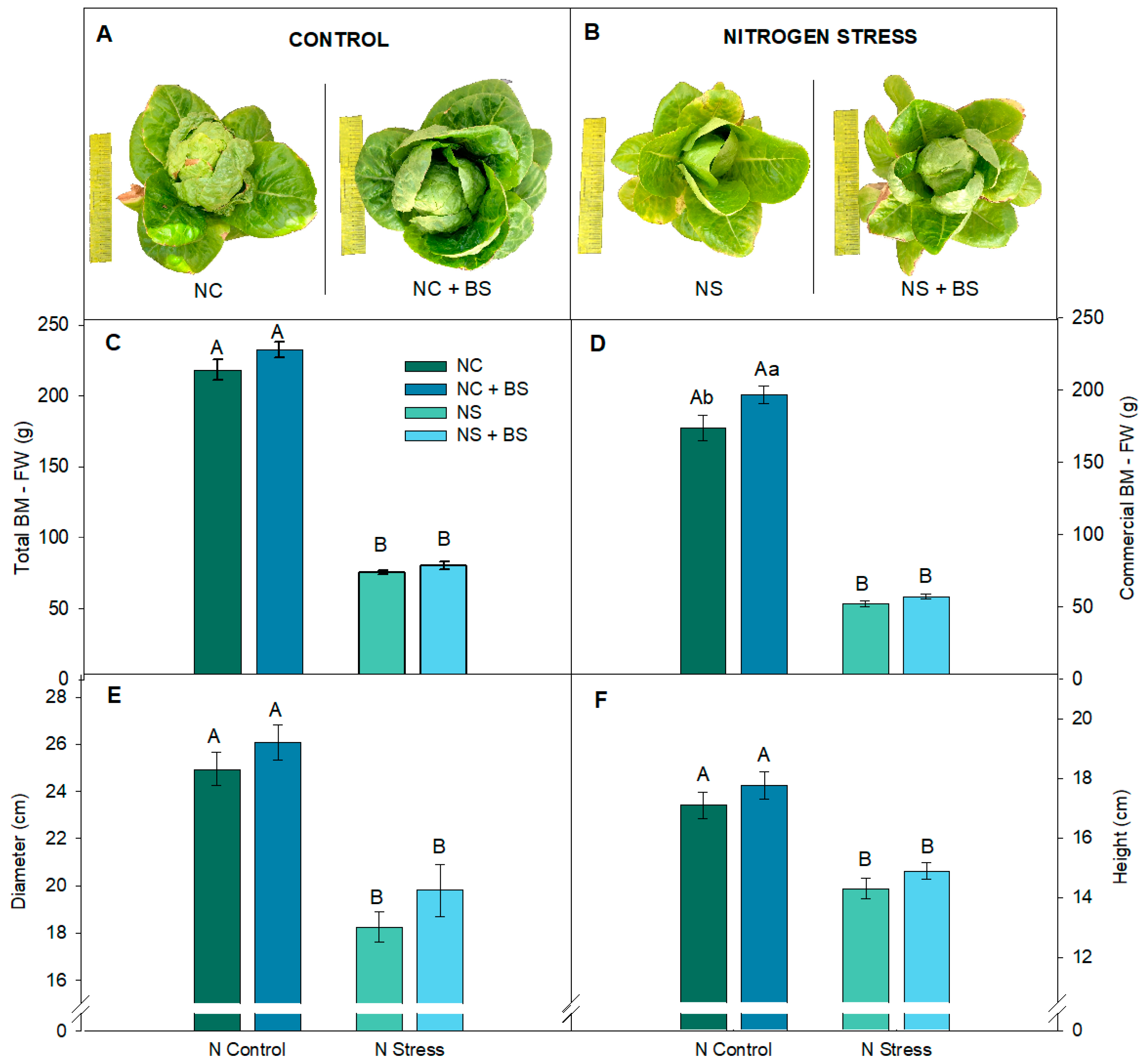
2.2. In Lettuce Plants, BS Application Promoted Lower Need for Photoprotection (In NC), Lower Need for Protein Content (In NS), and Significantly Enhanced Commercial Production in Lettuce Plants
3. Discussion
| Biostimulant | Crop | Application | Stress | Results | References | |
|---|---|---|---|---|---|---|
| PHs | Amino acids, soluble sugars, and phenols | Jute | Foliar | Full Nutrient Stress | Increased yield, enhanced CO2 assimilation, chlorophyll content, and nitrogen distribution | [53] |
| Basil | Root | Full Nutrient Stress | Enhanced yield and functional quality attributes | [54] | ||
| Lettuce | Foliar | Nitrogen Stress | Improved yield, chlorophyll content, N-uptake efficiency, and HAA | [55] | ||
| Spinach | Foliar | Nitrogen Stress | Improved yield, chlorophyll content, N-uptake efficiency, and LAA | [55] | ||
| Amino acids | Tomato | Root | Nutrient Stress | Enhanced primary and lateral root growth, by stimulating the biosynthesis of salicylic acid under stress | [56] | |
| Tomato | Root | Nitrogen Stress | Tendency to improve total fruit production | [37] | ||
| Free L-α-amino acids and organic matter | Lettuce | Root | Nitrogen Stress | Enhanced photosynthesis, total N, and increased biomass production | [31] | |
| Seaweed extract | Lettuce | Foliar | Nitrogen Stress | Increased photochemical efficiency and marketable fresh yield | [57] | |
| Tomato | Foliar | Nutrient Stress (NPK) | Decreased SOD and POD; maintained fruit quality and yield | [36] | ||
| Melatonin | Soybean | Root | Nitrogen Stress | Increased biomass, stem diameter, total leaf area, root nodule number, SOD, GPX and CAT | [58] | |
| Cucumber | Foliar | Nitrogen Stress | Enhanced activity of enzymes involved in N metabolism and N assimilation efficiency | [59] | ||
| Others | Betaines, alginic acid, and kaidrin | Lettuce | Foliar | Nitrogen Stress | Increased yield | [60] |
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Growth Quantification
4.3. Photosynthesis Related Parameters
4.4. Total Soluble Proteins
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BM | total biomass |
| BS | biostimulants |
| Car | total carotenoids |
| Chla | chlorophyll a |
| Chlb | chlorophyll b |
| ETR | electron transport rate |
| FW | fresh weight |
| Fv/Fm | maximum efficiency of PSII |
| gs | stomatal conductance |
| LHC | light-harvesting complex |
| NC | Nitrogen Control Treatment |
| NPQ | non-photochemical quenching |
| NS | Nitrogen Deficiency Stress Treatment |
| PGPB | Plant Growth-Promoting Bacteria |
| PHs | protein hydrolysates |
| ROS | reactive oxygen species |
| ΦPSII | relative efficiency of PSII |
References
- Larramendy, M.; Soloneski, S. Organic Fertilizers History, Production and Applications; IntechOpener: London, UK, 2019; pp. 23–25. [Google Scholar]
- Dar, G.H.; Bhat, R.A.; Mehmood, M.A.; Hakeem, K.R. (Eds.) Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- European Environment Agency. Food in a Green Light—A Systems Approach to Sustainable Food. Available online: https://www.eea.europa.eu/publications/food-in-a-green-light (accessed on 15 April 2024).
- European Commission. Farm to Fork Strategy. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 13 April 2024).
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Burgos, S. Advancing Climate Action on Agriculture and Food Security; FAO: Rome, Italy, 2024; pp. 1–5. [Google Scholar]
- Hereu-Morales, J.; Vinardell, S.; Valderrama, C. Towards Climate Neutrality in the Spanish N-Fertilizer Sector: A Study Based on Radiative Forcing. Sci. Total Environ. 2024, 946, 174131. [Google Scholar] [CrossRef] [PubMed]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 567388. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sierras, N.; Botta, A.; Staasing, L.; Martinez, M.J.; Bru, R. Understanding the Effect of Amino Acids Based Biostimulant by an Enantiomeric Analysis of Their Active Principles and a Proteomic Profiling Approach. Acta Hortic. 2016, 1148, 93–100. [Google Scholar] [CrossRef]
- Magnabosco, P.; Masi, A.; Shukla, R.; Bansal, V.; Carletti, P. Advancing the Impact of Plant Biostimulants to Sustainable Agriculture through Nanotechnologies. Chem. Biol. Technol. Agric. 2023, 10, 117. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the Environmental Impact of Chemically-Produced Protein Hydrolysate from Leather Waste vs. Enzymatically-Produced Protein Hydrolysate from Legume Grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Acin-Albiac, M.; García-Jiménez, B.; Marín Garrido, C.; Borda Casas, E.; Velasco-Alvarez, J.; Serra, N.S.; Acedo, A. Lettuce Soil Microbiome Modulated by an L-α-Amino Acid-Based Biostimulant. Agriculture 2023, 13, 344. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant Properties of Protein Hydrolysates: Recent Advances and Future Challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Potential Use of Bacillus spp. as an Effective Biostimulant against Abiotic Stresses in Crops—A Review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Castillo, P.; Escalante, M.; Gallardo, M.; Alemano, S.; Abdala, G. Effects of Bacterial Single Inoculation and Co-Inoculation on Growth and Phytohormone Production of Sunflower Seedlings under Water Stress. Acta Physiol. Plant. 2013, 35, 2299–2309. [Google Scholar] [CrossRef]
- da Silva, M.S.R.A.; Dos Santos, B.M.S.; da Silva, C.S.R.A.; da Silva, C.S.R.A.; Antunes, L.F.S.; Dos Santos, R.M.; Santos, C.H.B.; Rigobelo, E.C. Humic Substances in Combination With Plant Growth-Promoting Bacteria as an Alternative for Sustainable Agriculture. Front Microbiol. 2021, 12, 719653. [Google Scholar] [CrossRef]
- Barnawal, D.; Maji, D.; Bharti, N.; Chanotiya, C.S.; Kalra, A. ACC Deaminase-Containing Bacillus subtilis Reduces Stress Ethylene-Induced Damage and Improves Mycorrhizal Colonization and Rhizobial Nodulation in Trigonella Foenum-Graecum Under Drought Stress. J. Plant Growth Regul. 2013, 32, 809–822. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondì, C.; De Pasquale, C.; Guarino, V.; Iacuzzi, N.; Capodici, G.; et al. Combined Effects of Biostimulants, N Level and Drought Stress on Yield, Quality and Physiology of Greenhouse-Grown Basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB Combined with Variable N Doses Affects Growth, Yield-Related Traits, N-Fertilizer Efficiency and Nutritional Status of Lettuce Grown under Controlled Condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Martinez Esteso, M.J.; Vilella-Antón, M.; Sellés-Marchart, S.; Martínez-Márquez, A.; Botta-Catala, A.; Piñol-Dastis, R.; Bru-Martinez, R. A DIGE Proteomic Analysis of Wheat Flag Leaf Treated with TERRA-SORB® Foliar, a Free Amino Acid High Content Biostimulant. J. Integr. OMICS 2016, 6, 188. [Google Scholar] [CrossRef]
- Beckman, J. Economic and Food Security Impacts of Agricultural Input Reduction Under the European Union Green Deal’s Farm to Fork and Biodiversity Strategies. 2020. Available online: https://ageconsearch.umn.edu/record/307277/?v=pdf (accessed on 13 April 2024).
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Shewangizaw, B.; Kassie, K.; Assefa, S.; Lemma, G.; Gete, Y.; Getu, D.; Getanh, L.; Shegaw, G.; Manaze, G. Tomato Yield, and Water Use Efficiency as Affected by Nitrogen Rate and Irrigation Regime in the Central Low Lands of Ethiopia. Sci. Rep. 2024, 14, 13307. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Effect of l-amino Acid-based Biostimulants on Nitrogen Use Efficiency (NUE) in Lettuce Plants. J. Sci. Food Agric. 2022, 102, 7098–7106. [Google Scholar] [CrossRef]
- Attar, A.Z.; Ahmed, T.; Kato, A.; Saadaoui, I.; Shabala, S. Understanding Impact of Heat, Drought, and Salinity Stresses on Growth and Physiological Attributes of Chenopodium Album under Field Conditions. Plant Growth Regul. 2023, 100, 107–118. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-Oxidative Stress Markers as a Measure of Abiotic Stress-Induced Leaf Senescence: Advantages and Limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef]
- Huang, Z.-A.; Jiang, D.-A.; Yang, Y.; Sun, J.-W.; Jin, S.-H. Effects of Nitrogen Deficiency on Gas Exchange, Chlorophyll Fluorescence, and Antioxidant Enzymes in Leaves of Rice Plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant Prevents Yield Loss and Reduces Oxidative Damage in Tomato Plants Grown on Reduced NPK Nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Mesa, T.; Polo, J.; Casadesús, A.; Gómez, Í.; Munné-Bosch, S. Application of a Biostimulant (Pepton) Based in Enzymatic Hydrolyzed Animal Protein Combined with Low Nitrogen Priming Boosts Fruit Production Without Negatively Affecting Quality in Greenhouse-Grown Tomatoes. Front. Plant Sci. 2022, 13, 828267. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, J.; Shen, Y. Advances from Chlorophyll Biosynthesis to Photosynthetic Adaptation, Evolution and Signaling. Plant Stress 2024, 12, 100470. [Google Scholar] [CrossRef]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-Induced Drought Tolerance in Grapevine Is Associated with Physiological and Biochemical Changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of Nitrogen in Agriculture and Environment: Agronomic, Eco-Physiological and Molecular Approaches to Improve Nitrogen Use Efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine Mitigates Fertilizer Requirements of Agricultural Crops: Case Study with Cucumber as a High Fertilizer Demanding Crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Ronga, D.; Parisi, M.; Pentangelo, A.; Mori, M.; Di Mola, I. Effects of Nitrogen Management on Biomass Production and Dry Matter Distribution of Processing Tomato Cropped in Southern Italy. Agronomy 2019, 9, 855. [Google Scholar] [CrossRef]
- Evans, C.C.; Qaderi, M.M. Supplemental Nitrogen Alleviates the Negative Effects of Higher Temperature on the Vegetative Growth of Canola Regardless of Carbon Dioxide Concentration. Plant Stress 2024, 13, 100521. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Sas-Paszt, L.; Głuszek, S.; Górnik, K.; Anjum, M.A.; Saleh, A.A.; Abada, H.S.; Awad, R.M. Effect of Some Biostimulants on the Vegetative Growth, Yield, Fruit Quality Attributes and Nutritional Status of Apple. Horticulturae 2023, 9, 32. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Kaushal, P.; Ali, N.; Saini, S.; Pati, P.K.; Pati, A.M. Physiological and Molecular Insight of Microbial Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1041413. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. Int. J. Mol. Sci. 2025, 26, 1129. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Canchignia Martínez, H.; González, M.; Seeger, M. Review Biosynthesis of indole-3-acetic acid and plant growth promoting by bacteria. Cultiv. Trop 2016, 37, 33–39. [Google Scholar] [CrossRef]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The Use of Organic Biostimulants in Hot Pepper Plants to Help Low Input Sustainable Agriculture. Chem. Biol. Technol. Agric. 2015, 2, 11. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.C.; Colla, G.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Biostimulatory Action of Vegetal Protein Hydrolysate Compensates for Reduced Strength Nutrient Supply in a Floating Raft System by Enhancing Performance and Qualitative Features of “Genovese” Basil. Front. Plant Sci. 2022, 13, 906686. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Casadesús, A.; Pérez-Llorca, M.; Munné-Bosch, S.; Polo, J. An Enzymatically Hydrolyzed Animal Protein-Based Biostimulant (Pepton) Increases Salicylic Acid and Promotes Growth of Tomato Roots Under Temperature and Nutrient Stress. Front. Plant Sci. 2020, 11, 953. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Wang, H.; Ren, C.; Cao, L.; Jin, X.; Wang, M.; Zhang, M.; Zhao, Q.; Li, H.; Zhang, Y.; Yu, G. The Mechanisms Underlying Melatonin Improved Soybean Seedling Growth at Different Nitrogen Levels. Funct. Plant Biol. 2021, 48, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, R.; Li, S.; Ran, S.; Wang, J.; Zhou, Y.; Gao, H.; Zhong, F. The Mechanism of Melatonin Promotion on Cucumber Seedling Growth at Different Nitrogen Levels. Plant Physiol. Biochem. 2024, 206, 108263. [Google Scholar] [CrossRef]
- Amanda, A.; Ferrante, A.; Valagussa, M.; Piaggesi, A. Effect of Biostimulants on Quality of Baby Leaf Lettuce Grown Under Plastic Tunnel. Acta Hortic. 2009, 807, 407–412. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants Without Soil. Circular 1938, 347, 32. [Google Scholar]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148C, 350–382. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utgés-Minguell, L.; Sierras-Serra, N.; Marín, C.; Pintó-Marijuan, M. Enhanced Production by Terra-Sorb® Symbiotic Biostimulant in Two Model Species Under Nitrogen Stress. Plants 2025, 14, 1087. https://doi.org/10.3390/plants14071087
Utgés-Minguell L, Sierras-Serra N, Marín C, Pintó-Marijuan M. Enhanced Production by Terra-Sorb® Symbiotic Biostimulant in Two Model Species Under Nitrogen Stress. Plants. 2025; 14(7):1087. https://doi.org/10.3390/plants14071087
Chicago/Turabian StyleUtgés-Minguell, Laia, Nuria Sierras-Serra, Cándido Marín, and Marta Pintó-Marijuan. 2025. "Enhanced Production by Terra-Sorb® Symbiotic Biostimulant in Two Model Species Under Nitrogen Stress" Plants 14, no. 7: 1087. https://doi.org/10.3390/plants14071087
APA StyleUtgés-Minguell, L., Sierras-Serra, N., Marín, C., & Pintó-Marijuan, M. (2025). Enhanced Production by Terra-Sorb® Symbiotic Biostimulant in Two Model Species Under Nitrogen Stress. Plants, 14(7), 1087. https://doi.org/10.3390/plants14071087







