Comparison of Essential Oil Components and In Vitro Antioxidant Activity of Zanthoxylum nitidum from Different Parts
Abstract
1. Introduction
2. Materials and Methods
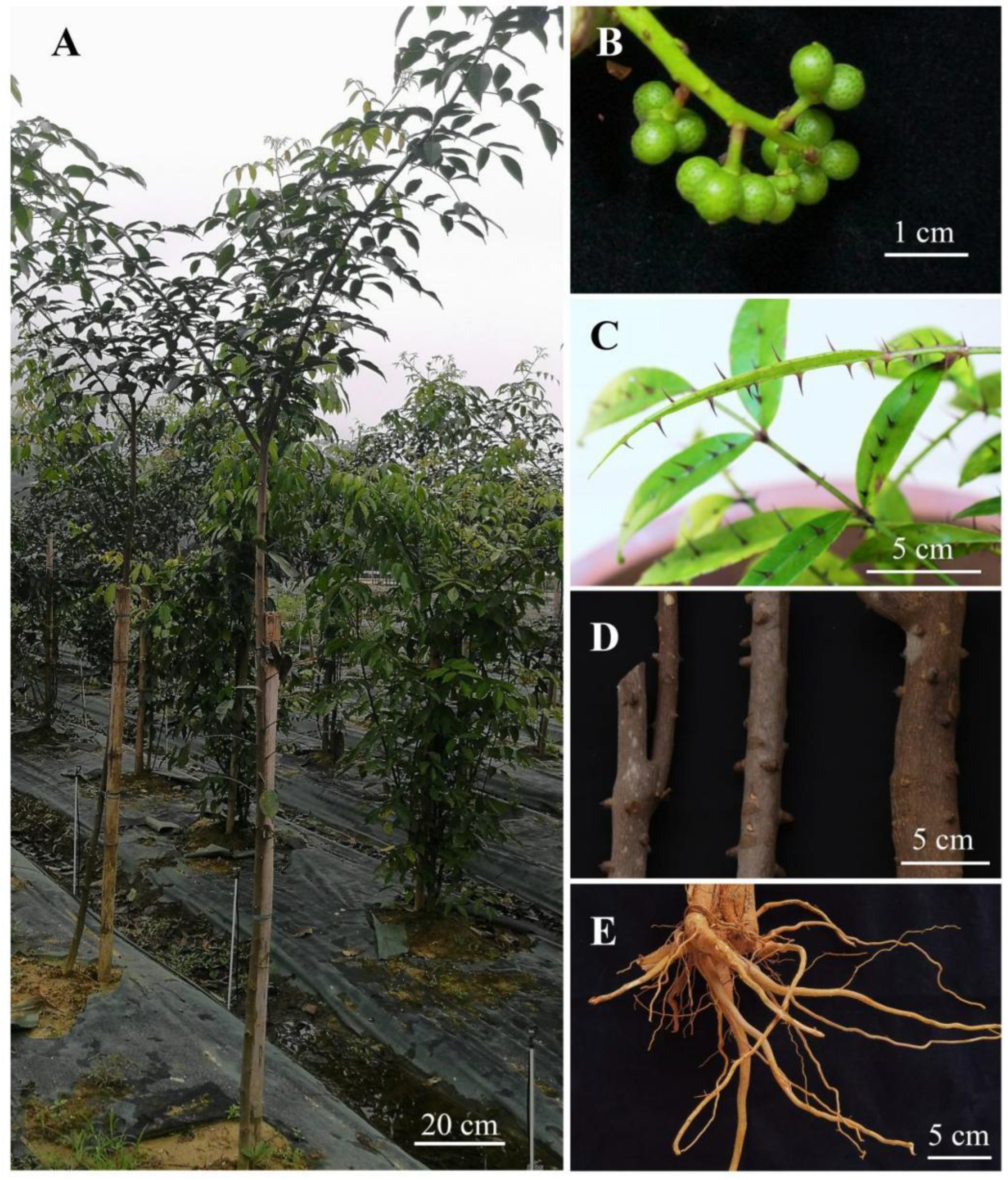
2.1. Experimental Materials
2.2. Essential Oil Extraction and Content Determination
2.3. GC-MS Analysis
2.4. Preparation of Essential Oil Solution
2.5. Total Antioxidant Capacity Determination (FRAP)
2.6. Total Antioxidant Capacity Determination (ABTS)
2.7. DPPH Radical Scavenging Activity Assay
2.8. Hierarchical Clustering Analysis (HCA)
2.9. Principal Component Analysis (PCA)
2.10. Statistical Analysis
3. Results and Discussion
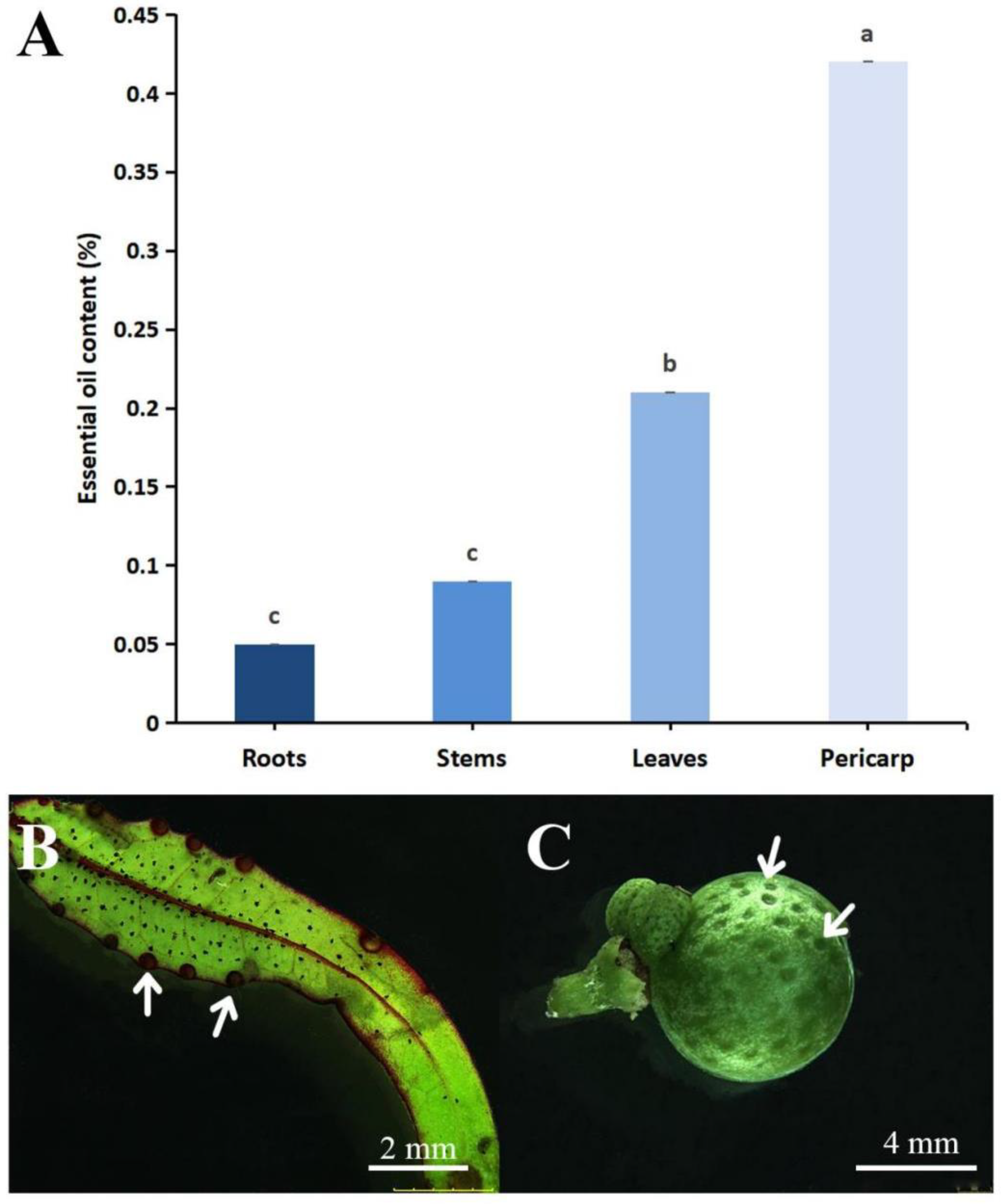
3.1. Comparison of Essential Oil Content
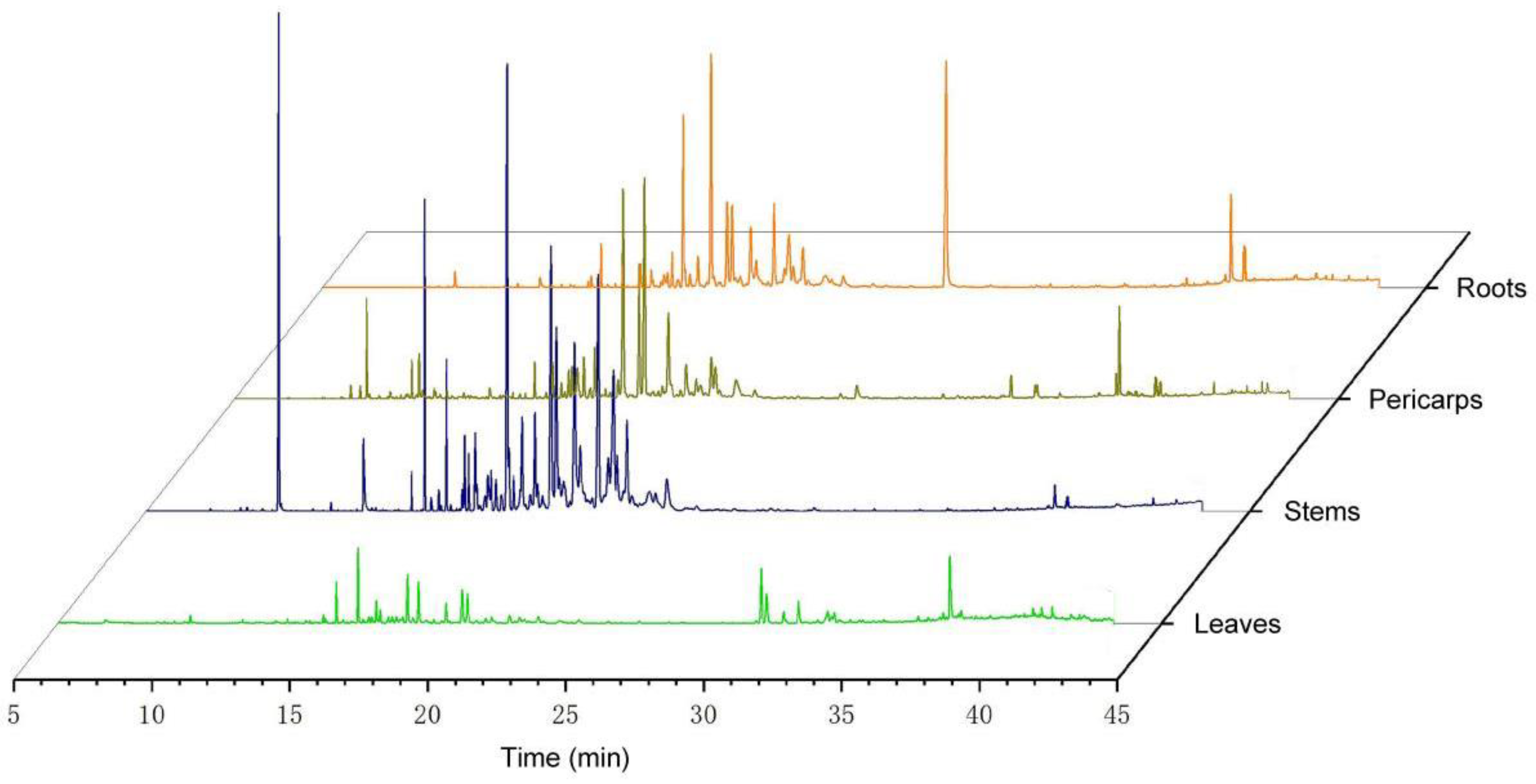
3.2. Chemical Composition
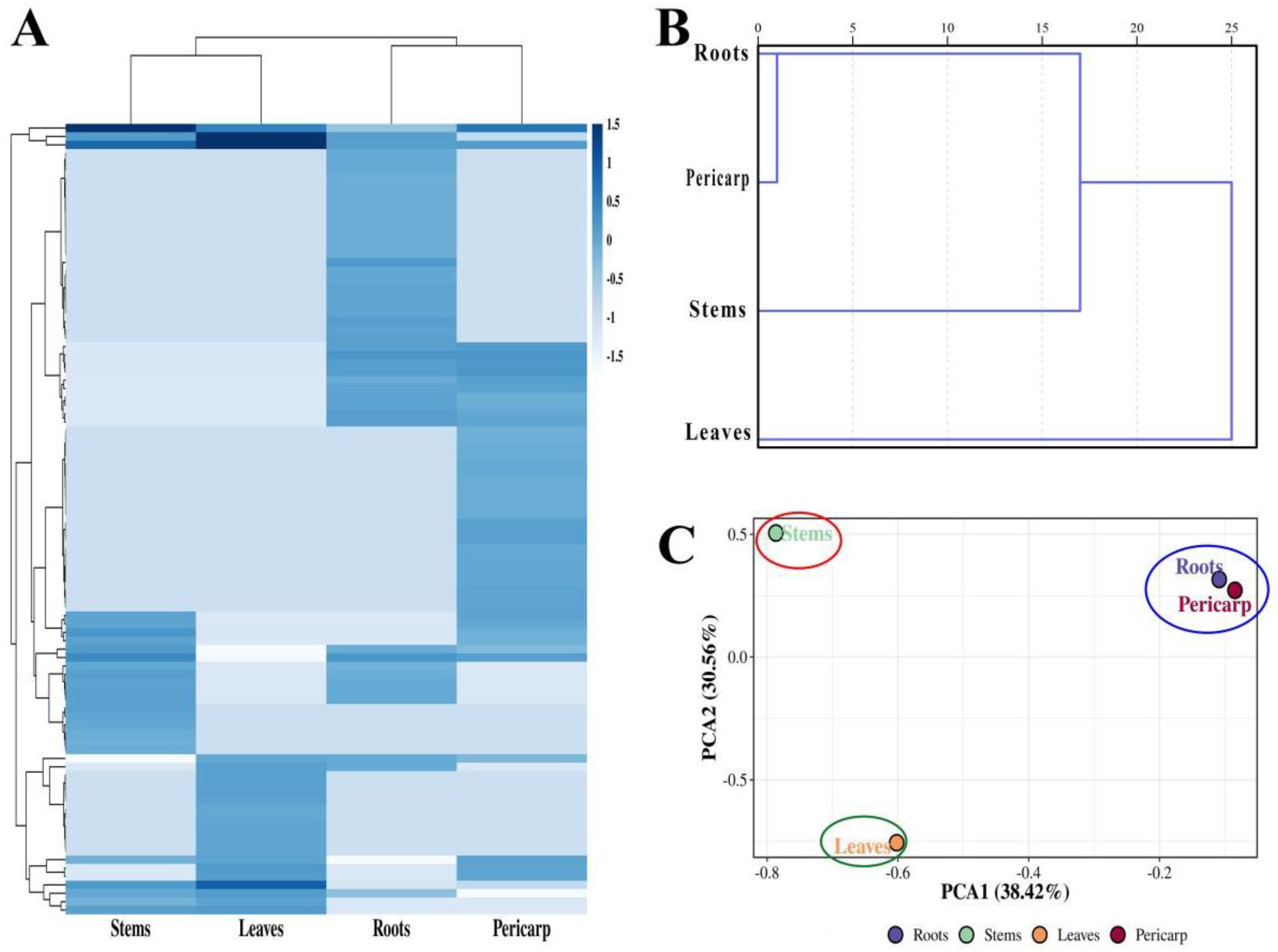
3.3. HCA and PCA of Z. nitidum
3.4. In Vitro Antioxidant Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Editorial Committee of Flora of China. Chinese Academy of Sciences, Flora of China; Science Press: Beijing, China, 2007; pp. 13–16. [Google Scholar]
- Yang, Y.; He, J.; Liu, Y.; Zeng, J.; Zeng, L.; He, R.; Guiang, M.M.; Li, Y.; Wu, H. Assessment of Chinese suitable habitats of Zanthoxylum nitidum in different climatic conditions by Maxent model, HPLC, and chemometric methods. Ind. Crop Prod. 2023, 196, 116515. [Google Scholar] [CrossRef]
- Lin, Q.; Pu, H.; Guan, H.; Ma, C.; Zhang, Y.; Ding, W.; Cheng, X.; Ji, L.; Wang, Z.; Wang, C. Rapid identification and pharmacokinetic studies of multiple active alkaloids in rat plasma through UPLC-Q-TOF-MS and UPLC-MS/MS after the oral administration of Zanthoxylum nitidum extract. J. Pharm. Biomed. 2020, 186, 113232. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ma, R.; Yang, Y.; Mo, Z.; Pu, X.; Li, C. Zanthoxylum nitidum (Roxb.) DC: Traditional uses, phytochemistry, pharmacological activities and toxicology. J. Ethnopharmacol. 2020, 260, 112946. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Wang, F.-F.; Wang, C.-G.; Chen, Y.; Li, M.-S.; Zhu, Y.-K.; Huang, X.-C.; Fan, C.-W.; Wang, H.-S. The neurotrophic and antineuroinflammatory effects of phenylpropanoids from Zanthoxylum nitidum var. tomentosum (Rutaceae). Fitoterapia 2021, 153, 104990. [Google Scholar] [CrossRef]
- He, J.; Zeng, J.; Zeng, L.; Yang, L.; Ma, Q.; Wu, H.; Yang, Y. Comparison of the bioactive components and antioxidant activities of wild-type Zanthoxylum nitidum roots from various regions of Southern China. Nat. Prod. Res. 2023, 38, 4399–4410. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Jia, H.; Tan, P.; Zeng, J.; Zeng, L.; Xie, T.; Geng, X.; He, H.; Bai, M.; et al. A comparative analysis of the composition of the primary bioactive molecules, antioxidant activities, and anti-inflammation abilities of the roots of Zanthoxylum nitidum harvested at different years of cultivation. Ind. Crop Prod. 2024, 221, 119426. [Google Scholar]
- Peng, Z.H.; Wu, M.H.; Xie, Z.J.; Yang, X.B.; Lai, M.X. Investigation of wild resource of Zanthoxylum nitidum. Pharm. Today 2018, 28, 500–504. [Google Scholar]
- China Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China (I); China Medical Science and Technology Press: Beijing, China, 2020; Volume 176. [Google Scholar]
- He, L.L.; Lin, Z.H.; Hu, Y.Z.; Qin, Q.Y.; Huang, G.W.; Hu, D.N. Dynamic accumulation research of active ingredient in different part and growth phase of caltivative Zanthoxylum nitidum by UPLC-DAD. Chin. J. Exp. Tradit. Med. Formula 2013, 19, 165–168. [Google Scholar]
- Ma, Q.; Wu, G.S.; Zheng, L.T.; Han, Z.Z.; Zhan, R.T.; Chen, W.W.; Niu, M. UPLC specific chromatogram study and pharmacodynamic composition analysis of roots and stems of Zanthoxylum nitidum. J. Chin. Med. Mater. 2020, 43, 2959–2965. [Google Scholar]
- Mackhaphonh, V. Studies on the Anti-Inflammatory and Analgesic Active of Different Portions and Low-Polarity Portion Chemical Constituents in the Leaves of Zanthoxylum nitidum DC; Guangxi Medical University: Nanning, China, 2013; pp. 15–20. [Google Scholar]
- Ali, F.; Alom, S.; Zaman, M.K. Ethnobotany, phytochemistry and pharmacological properties of Zanthoxylum nitidum: A systemic review. World J. Pharm. Res. 2022, 11, 175–195. [Google Scholar]
- Bhattacharya, S.; Zaman, M.K. Essential oil composition of fruits and leaves of Zanthoxylum nitidum grown in upper Assam region of India. Pharmacogn. Res. 2009, 1, 148–151. [Google Scholar]
- Tuyen, T.T.; Quan, P.M.; Thu Le, V.T.; Toan, T.Q. Chemical composition, antimicrobial, and cytotoxic activities of leaf, fruit, and branch essential oils obtained from Zanthoxylum nitidum grown in Vietnam. Nat. Prod. Commun. 2021, 16, 1934578X2098564. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Amoroso, V.; Acma, F.; Guiang, M.M.; Wu, H. Comparison of production of bioactive components in Zanthoxylum nitidum taproots from different regions in southern China. Biomed. Chromatogr. 2023, 37, e5602. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H. Research on the Extraction, Antibacterial and Insecticidal Activities of Citrus Essential Oil; Gannan Normal University: Ganzhou, China, 2018; pp. 42–58. [Google Scholar]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, phytochemical constituents, and antibacterial activity of essential oils from the leaves/twigs, branches, branch wood, and branch bark of sour orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef]
- Liu, W.Z.; Hu, Z.H. Comparative anatomy of secretory cavities in leaves of the rutaceae in China. Acta Phytotax Sin. 1998, 36, 119–127. [Google Scholar]
- Liu, W.Z.; Hu, Z.H. Studies on the secretory cavity of stems in Rutaceae. Acta Bot. Boreal. Occident. Sin. 1999, 19, 456–460. [Google Scholar]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; Andolfi, A. Essential oils in Citrus fruit ripening and postharvest quality. Horticulturae 2022, 8, 396. [Google Scholar] [CrossRef]
- Metcalfe, G.R.; Chalk, L. Anatomy of the Dicotyledons; Clarendon Press: Oxford, UK, 1950; Volume I, pp. 305–320. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Frag. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Secondary Metabolites. Plant Physiology, Development and Metabolism; Springer: Singapore, 2023; pp. 765–808. [Google Scholar]
- Cirak, C.; Radusiene, J.; Stanius, Z.; Camas, N.; Caliskan, O.; Odabas, M.S. Secondary metabolites of Hypericum orientale L. growing in Turkey: Variation among populations and plant parts. Acta Physiol. Plant 2012, 34, 1313–1320. [Google Scholar] [CrossRef]
- Biradar, S.R.; Rachetti, B.D. Extraction of some secondary metabolites & thin layer chromatography from different parts of Centella asiatica L. (URB). Am. J. Life Sci. 2013, 1, 243–247. [Google Scholar]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Kong, D.-X.; Huang, R.-S.; Liang, H.-L.; Xu, C.-G.; Wu, H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crop Prod. 2013, 47, 92–101. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Su, R.; Ruan, G.; Du, F.; Li, G. Current application of chemometrics in traditional Chinese herbal medicine research. J. Chromatogr. B 2016, 1026, 27–35. [Google Scholar] [CrossRef]
- Ding, R.; Yu, L.; Wang, C.; Zhong, S.; Gu, R. Quality assessment of traditional Chinese medicine based on data fusion combined with machine learning: A review. Crit. Rev. Anal. Chem. 2023, 54, 2618–2635. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.-Z. Comparative analysis of essential oil components of three Phlomis species in Qinling mountains of China. J. Pharm. Biomed. 2008, 47, 213–217. [Google Scholar] [CrossRef]
- Chen, P.; Yu, J.; Lu, F.; Lin, M.; Cheng, H. Differentiating parts of Cinnamomum cassia using LC-qTOF-MS in conjunction with principal component analysis. Biomed. Chromatogr. 2016, 30, 1449–1457. [Google Scholar] [CrossRef]
- Peng, J.; Fan, X.Y.; Wang, D.L.; Xu, B.; Zhang, Z.K.; Xiao, W.Q.; Yang, T.Z.; Li, P.Y.; Du, S.Y. Differences of Nauclea officinalis in different parts based on quantitative analysis of components and fingerprint by chemical pattern recognition. Chin. Tradit. Herb. Drug 2023, 54, 3281–3291. [Google Scholar]
- Zhang, F.; Han, X.; Li, D.; Liu, Z. Differences of malonyl ginsenosides and neutral ginsenosides in different parts of Panax ginseng and Panax notoginseng by high performance liquid chromatography and chemometric analysis. China Food Addit. 2024, 35, 238–245. [Google Scholar]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Chapter 5: Potential antioxidant activity of terpenes. In Terpenes and Terpenoids: Recent Advances; IntechOpen: London, UK, 2021; pp. 53–62. [Google Scholar]
- Kokilananthan, S.; Bulugahapitiya, V.P.; Manawadu, H.; Gangabadage, C.S. Sesquiterpenes and monoterpenes from different varieties of guava leaf essential oils and their antioxidant potential. Heliyon 2022, 8, e12104. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
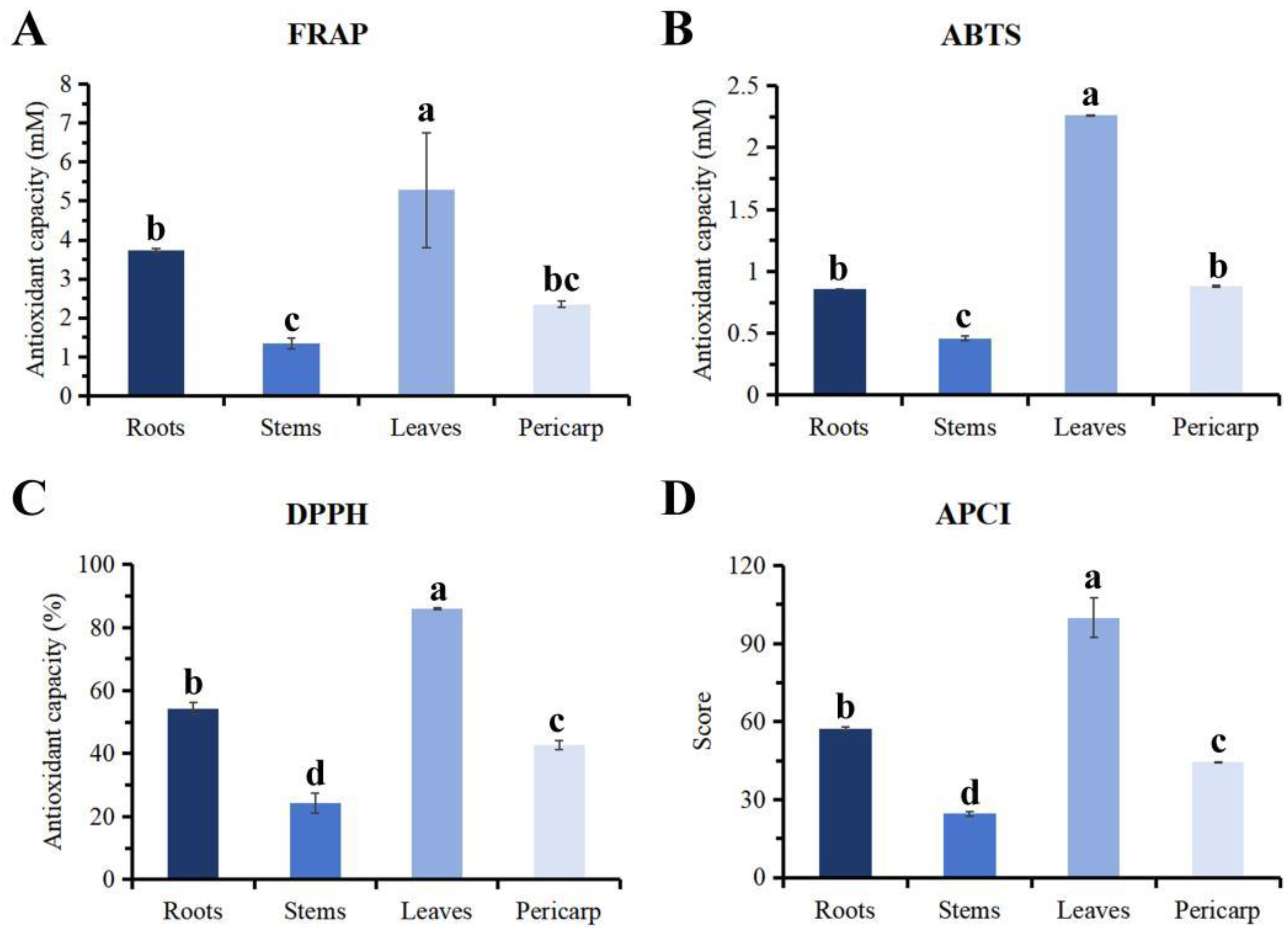
| Samples | FRAP (mM) | ABTS (mM) | DPPH (%) | Antioxidant Potency Composite Index (APCI) | Sort |
|---|---|---|---|---|---|
| Roots | 3.74 ± 0.042 b | 0.86 ± 0.0012 b | 54.36 ± 1.85 b | 57.37 ± 0.79 b | 2 |
| Stems | 1.34 ± 0.13 c | 0.46 ± 0.018 c | 24.21 ± 3.15 d | 24.63 ± 0.91 d | 4 |
| Leaves | 5.28 ± 1.47 a | 2.26 ± 0.0061 a | 85.95 ± 0.26 a | 100.00 ± 7.67 a | 1 |
| Pericarp | 2.35 ± 0.082 bc | 0.88 ± 0.0092 b | 42.72 ± 1.38 c | 44.38 ± 0.29 c | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Y.; He, H.; Yang, L.; Zeng, J.; Bai, M.; Wu, H. Comparison of Essential Oil Components and In Vitro Antioxidant Activity of Zanthoxylum nitidum from Different Parts. Plants 2025, 14, 1194. https://doi.org/10.3390/plants14081194
Yang Y, Li Y, He H, Yang L, Zeng J, Bai M, Wu H. Comparison of Essential Oil Components and In Vitro Antioxidant Activity of Zanthoxylum nitidum from Different Parts. Plants. 2025; 14(8):1194. https://doi.org/10.3390/plants14081194
Chicago/Turabian StyleYang, Yang, Yanqun Li, Hanjun He, Leilei Yang, Jiaxin Zeng, Mei Bai, and Hong Wu. 2025. "Comparison of Essential Oil Components and In Vitro Antioxidant Activity of Zanthoxylum nitidum from Different Parts" Plants 14, no. 8: 1194. https://doi.org/10.3390/plants14081194
APA StyleYang, Y., Li, Y., He, H., Yang, L., Zeng, J., Bai, M., & Wu, H. (2025). Comparison of Essential Oil Components and In Vitro Antioxidant Activity of Zanthoxylum nitidum from Different Parts. Plants, 14(8), 1194. https://doi.org/10.3390/plants14081194







