Aquorin Bioluminescence-Based Ca2+ Imaging Reveals Differential Calcium Signaling Responses to Abiotic Stresses in Physcomitrella patens
Abstract
1. Introduction
2. Results
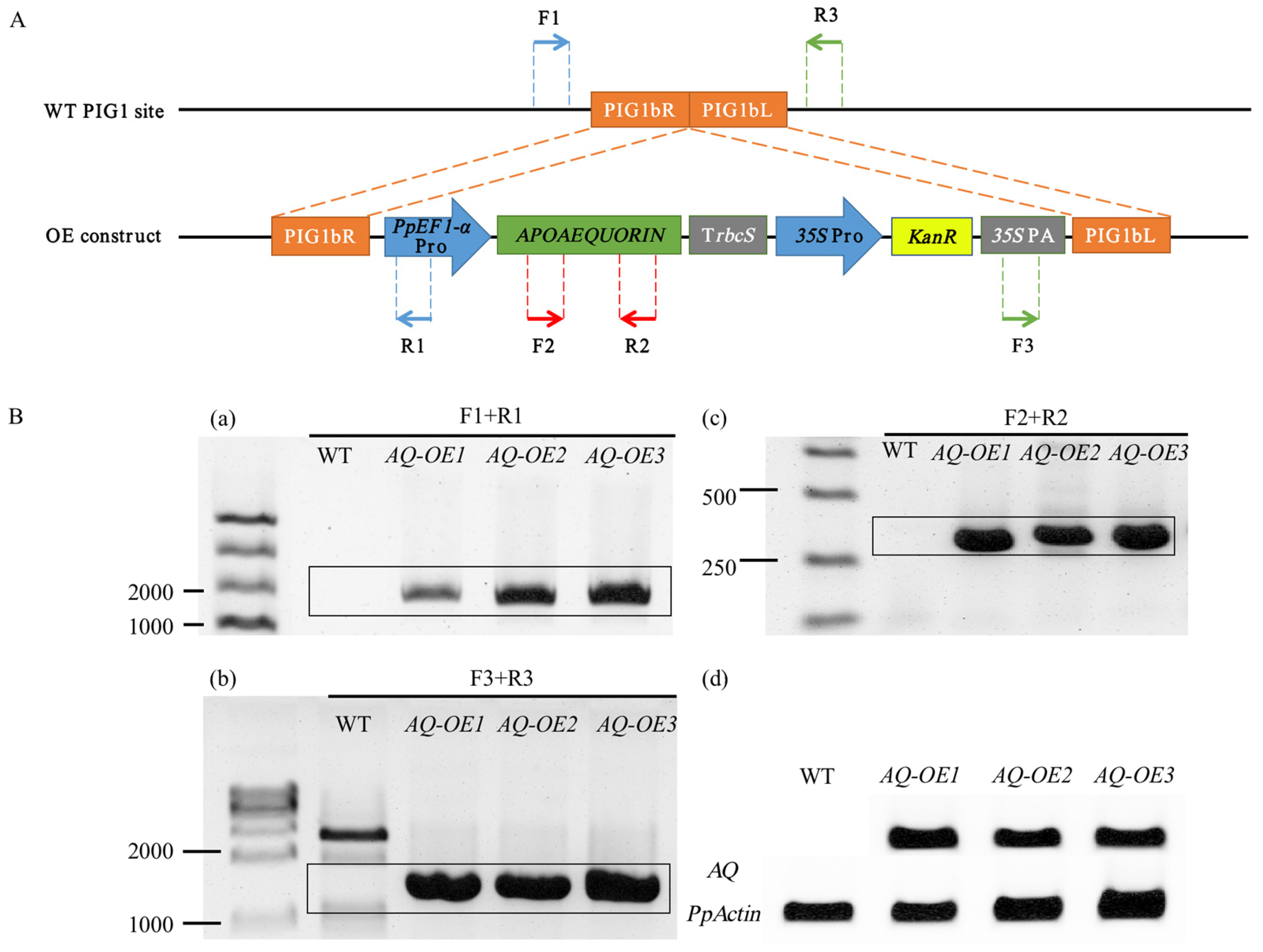
2.1. Production of Transgenic P. patens Expressing Apoaequorin
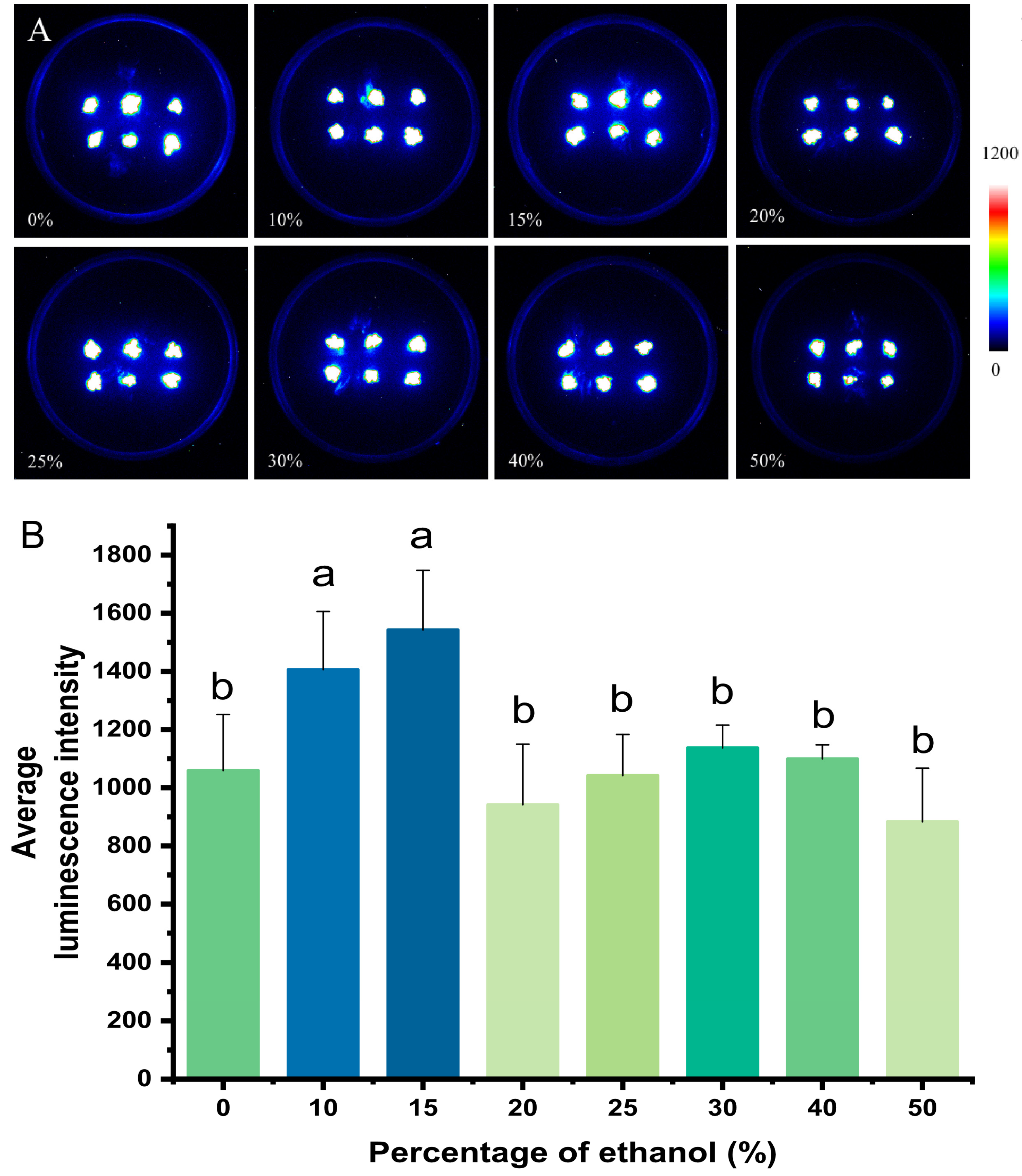
2.2. Optimization of the Discharging Solution for Luminescence Imaging in P. patens
2.3. Cold Induces an Immediate [Ca2+]i Spike in P. patens
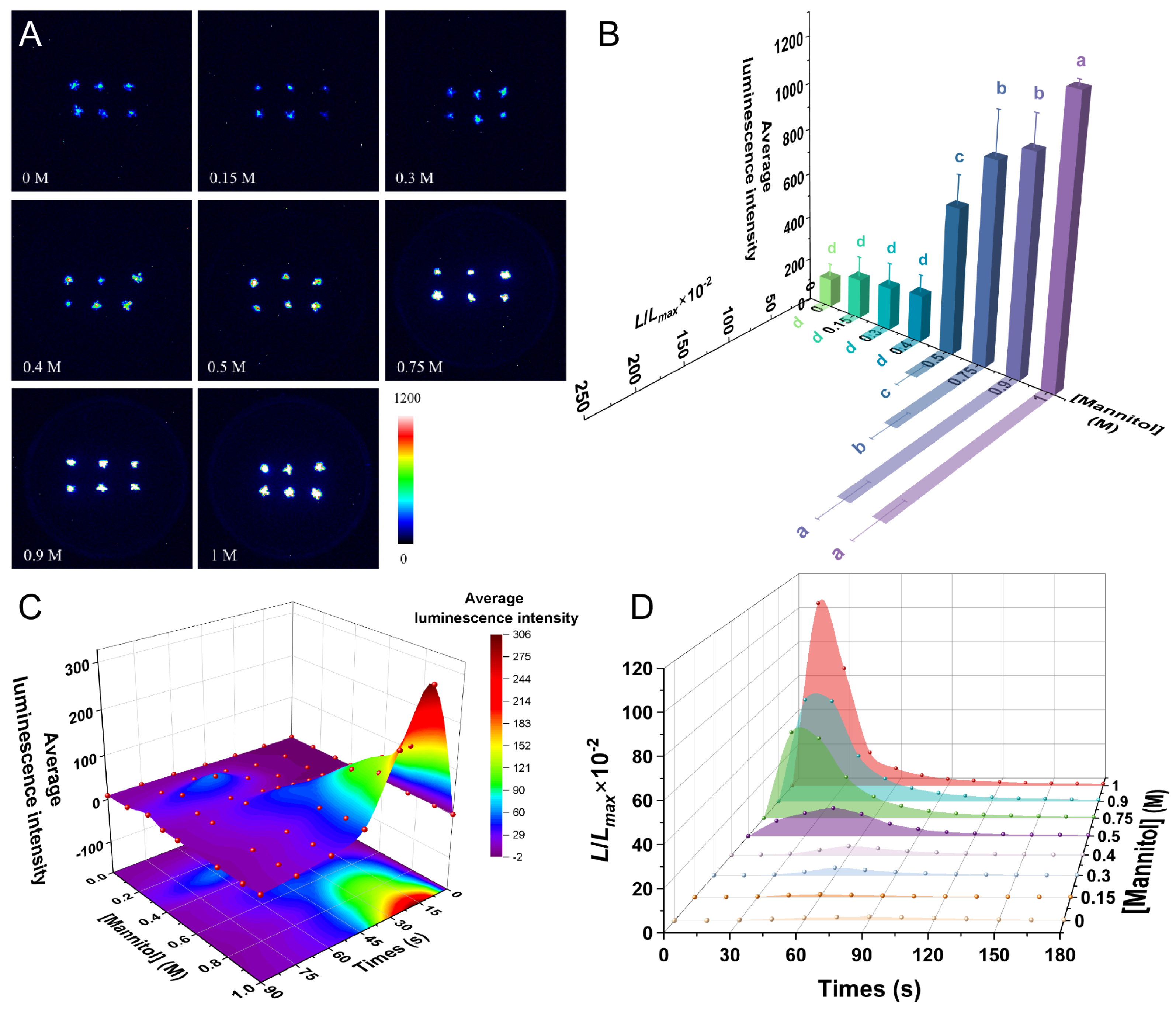
2.4. Drought Induces a [Ca2+]i Spike in P. patens
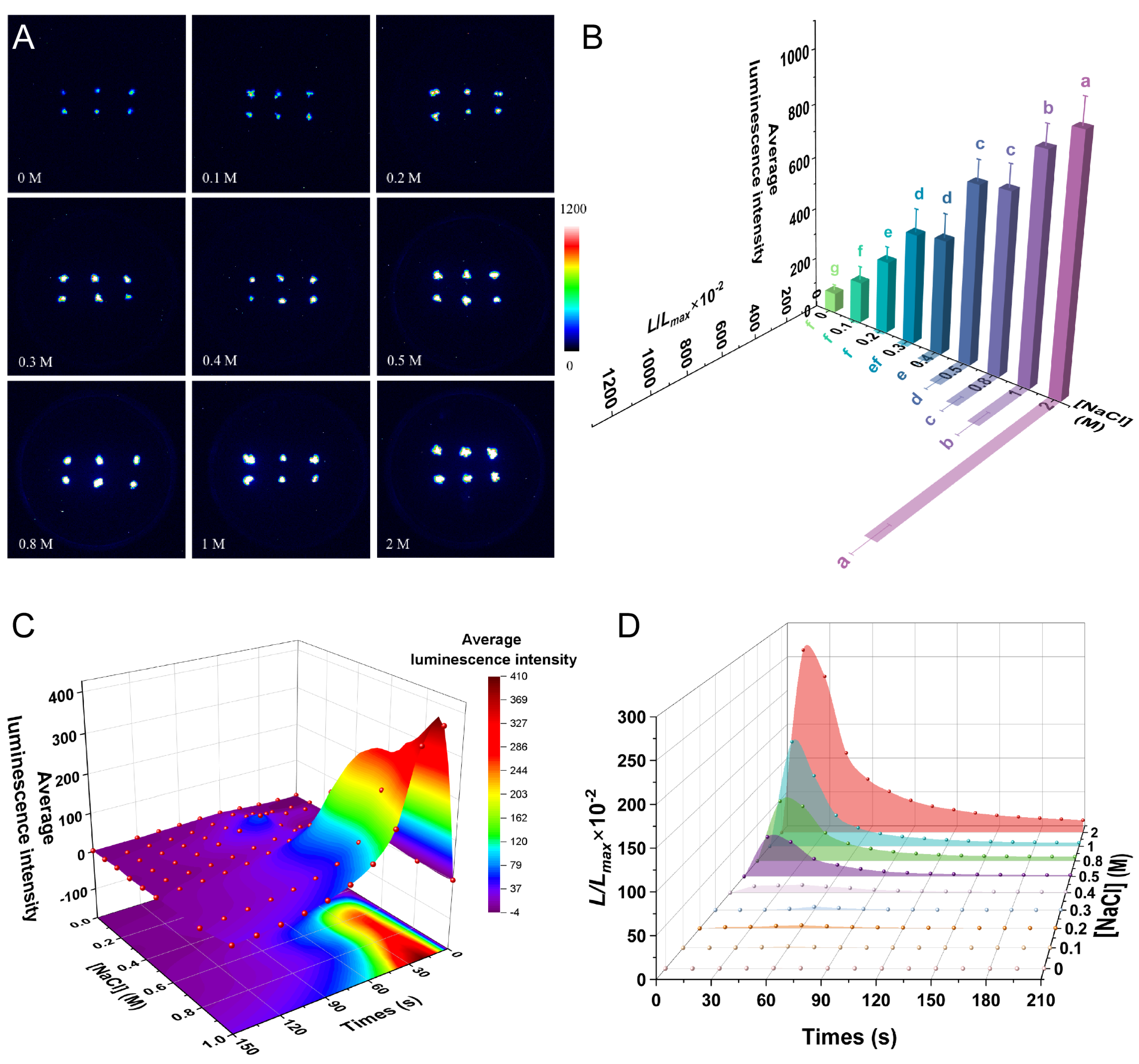
2.5. NaCl Induces a [Ca2+]i Spike in P. patens
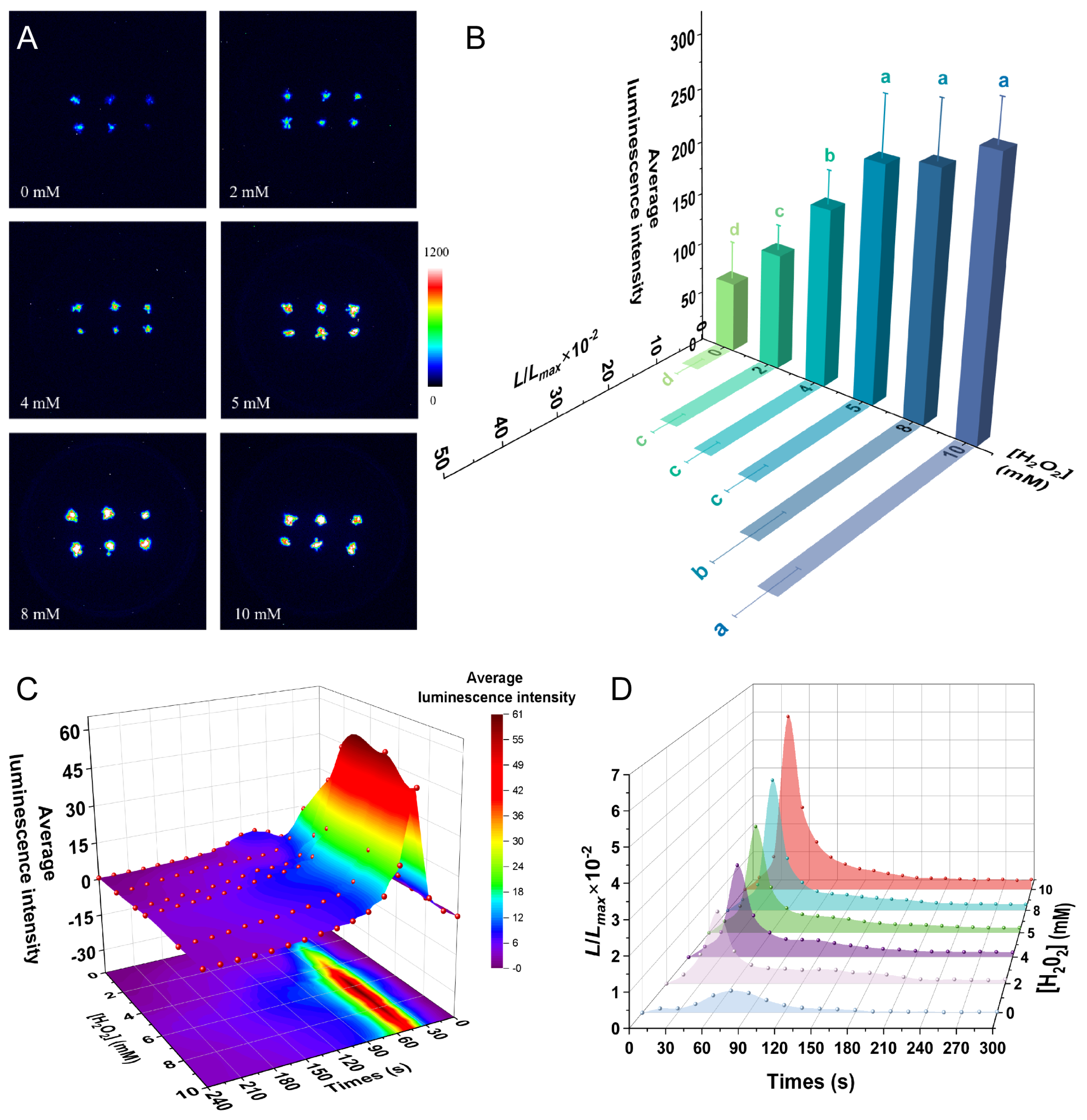
2.6. H2O2 Induced a [Ca2+]i Spike in P. patens
2.7. Different Sources of Ca2+ Induced by Various Stresses
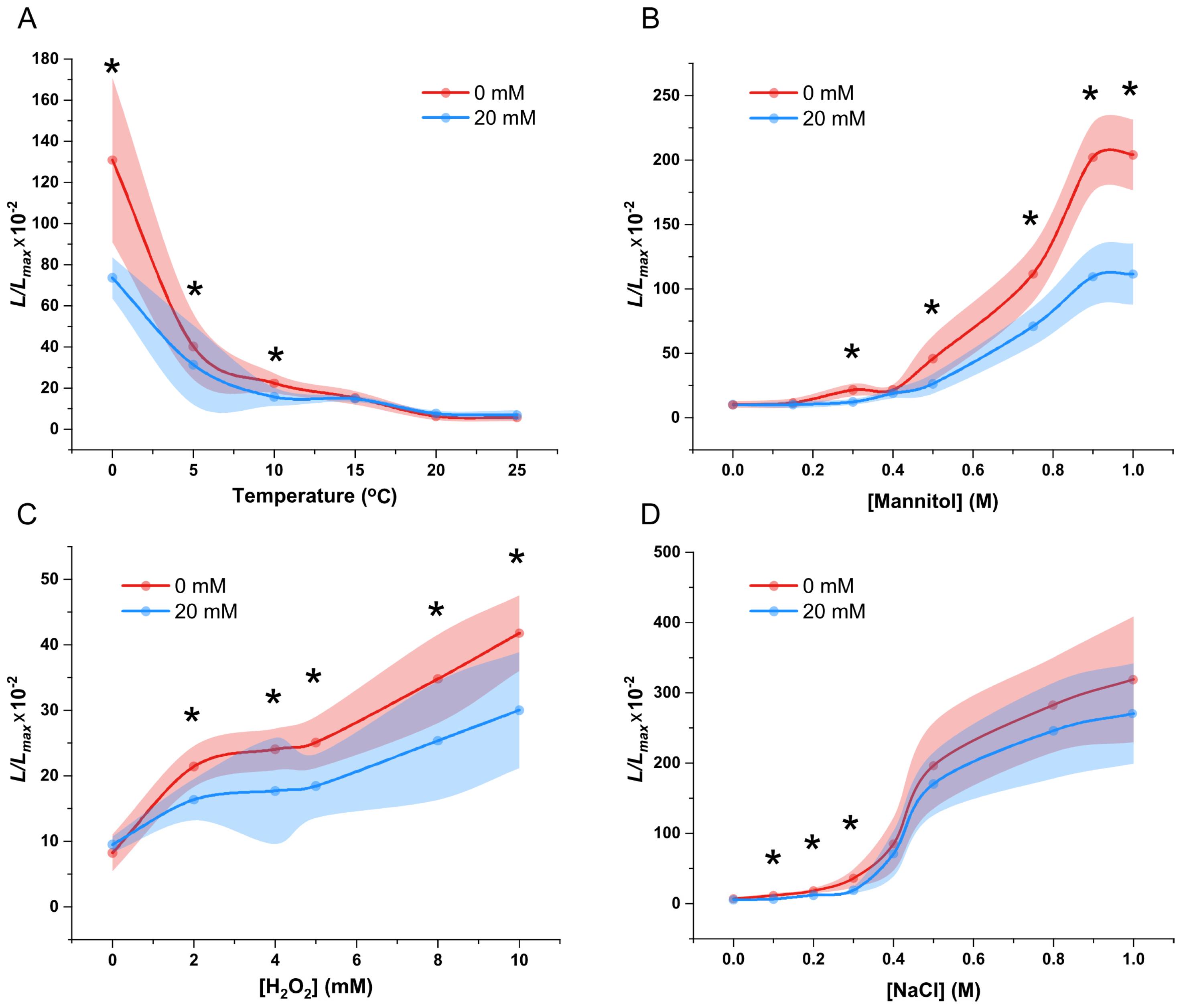
2.8. Saline Environment Impairs the Calcium Signaling in Response to Various Stresses in P. patens
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Vector Construction and Transformation of P. patens
4.3. Aequorin Bioluminescence–Based Ca2+ Imaging
4.4. Ca2+ Source Analysis
4.5. Various Stress Treatments Under Long-Term Salt Stress
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [Ca2+]i | cytosolic free Ca2+ concentration |
| ER | endoplasmic reticulum |
| GECI | genetically encoded calcium indicator |
| ROS | reactive oxygen species |
| FRET | fluorescence resonance energy transfer |
| YC | Yellow Cameleon |
| SOS | salt overly sensitive |
References
- Bisang, I.; Ehrlen, J.; Persson, C.; Hedenas, L. Family affiliation, sex ratio and sporophyte frequency in unisexual mosses. Bot. J. Linn. Soc. 2014, 174, 163–172. [Google Scholar] [CrossRef]
- Budke, J.M.; Bernard, E.C.; Gray, D.J.; Huttunen, S.; Piechulla, B.; Trigiano, R.N. Introduction to the special issue on bryophytes. Crit. Rev. Plant Sci. 2018, 37, 102–112. [Google Scholar] [CrossRef]
- Lindo, Z.; Nilsson, M.C.; Gundale, M.J. Bryophyte-cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob. Change Biol. 2013, 19, 2022–2035. [Google Scholar] [CrossRef]
- Lenton, T.M.; Dahl, T.W.; Daines, S.J.; Mills, B.J.W.; Ozaki, K.; Saltzman, M.R.; Porada, P. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl. Acad. Sci. USA 2016, 113, 9704–9709. [Google Scholar] [CrossRef]
- Chen, F.; Ludwiczuk, A.; Wei, G.; Chen, X.L.; Crandall-Stotler, B.; Bowman, J.L. Terpenoid secondary metabolites in bryophytes: Chemical diversity, biosynthesis and biological functions. Crit. Rev. Plant Sci. 2018, 37, 210–231. [Google Scholar] [CrossRef]
- Benitez, A.; Armijos, L.; Calva, J. Monitoring air quality with transplanted bryophytes in a neotropical Andean city. Life 2021, 11, 821. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Haas, F.B.; Gould, S.B.; Rensing, S.A. An overview of bioinformatics, genomics, and transcriptomics resources for bryophytes. J. Exp. Bot. 2022, 73, 4291–4305. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The danger and indeterminacy of forfeiting perching space of bryophytes from climate shift: A case study for 115 species in China. Environ. Monit. Assess. 2022, 194, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Taylor, J.L.; Jiang, Z.; Zhang, S.; Mei, F.; Wu, Y.; Wu, P.; Ni, J. Aequorin-based luminescence imaging reveals differential calcium signalling responses to salt and reactive oxygen species in rice roots. J. Exp. Bot. 2015, 66, 2535–2545. [Google Scholar] [CrossRef]
- Bickerton, P.; Sello, S.; Brownlee, C.; Pittman, J.K.; Wheeler, G.L. Spatial and temporal specificity of Ca2+ signalling in Chlamydomonas reinhardtii in response to osmotic stress. New Phytol. 2016, 212, 920–933. [Google Scholar] [CrossRef]
- White, P.J. Calcium channels in higher plants. Biochim. Biophys. Acta 2000, 1465, 171–189. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Brownlee, C. The generation of Ca2+ signals in plants. Annu. Rev. Plant Biol. 2004, 55, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Duan, Y.; Liu, C.; Xue, Q.; Guo, J.; Qi, T.; Kang, Z.; Guo, J. The calcium sensor TaCBL4 and its interacting protein TaCIPK5 are required for wheat resistance to stripe rust fungus. J. Exp. Bot. 2018, 69, 4443–4457. [Google Scholar] [CrossRef] [PubMed]
- Akaboshi, M.; Hashimoto, H.; Ishida, H.; Saijo, S.; Koizumi, N.; Sato, M.; Shimizu, T. The crystal structure of plant-specific calcium-binding protein AtCBL2 in complex with the regulatory domain of AtCIPK14. J. Mol. Biol. 2008, 377, 246–257. [Google Scholar] [CrossRef]
- Sze, H.; Liang, F.; Hwang, I.; Curran, A.C.; Harper, J.F. Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 433–462. [Google Scholar] [CrossRef]
- Hirschi, K. Vacuolar H+/Ca2+ transport: Who’s directing the traffic? Trends Plant Sci. 2001, 6, 100–104. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, P.J. Ca2+ talyzing initial responses to environmental stresses. Trends Plant Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef]
- Vigani, G.; Costa, A. Harnessing the new emerging imaging technologies to uncover the role of Ca2+ signalling in plant nutrient homeostasis. Plant Cell Environ. 2019, 42, 2885–2901. [Google Scholar] [CrossRef]
- Krogman, W.; Sparks, J.A.; Blancaflor, E. Cell type-specific imaging of calcium signaling in Arabidopsis thaliana seedling roots using GCaMP3. Int. J. Mol. Sci. 2020, 21, 6385. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Microdetermination of calcium by aequorin luminescence. Science 1963, 140, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Russell, A.J.; Knight, M.R.; Cove, D.J.; Knight, C.D.; Trewavas, A.J.; Wang, T.L. The moss, Physcomitrella patens, transformed with apoaequorin cDNA responds to cold shock, mechanical perturbation and pH with transient increases in cytoplasmic calcium. Transgenic Res. 1996, 5, 167–170. [Google Scholar] [CrossRef]
- Mithöfer, A.; Mazars, C. Aequorin-based measurements of intracellular Ca2+-signatures in plant cells. Biol. Proced. Online 2002, 4, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991, 352, 524–526. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, Y.; Liang, G.; Liu, N.; Zhu, J.K. Aequorin-based luminescence imaging reveals stimulus- and tissue-specific Ca2+ dynamics in Arabidopsis plants. Mol. Plant 2013, 6, 444–455. [Google Scholar] [CrossRef]
- Kitagawa, M.; Fujita, T. Quantitative imaging of directional transport through plasmodesmata in moss protonemata via single-cell photoconversion of Dendra2. J. Plant Res. 2013, 126, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A.; Griesbeck, O.; Heim, R.; Tsien, R.Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA 1999, 96, 2135–2140. [Google Scholar] [CrossRef]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Horikawa, K.; Yamada, Y.; Matsuda, T.; Kobayashi, K.; Hashimoto, M.; Matsu-ura, T.; Miyawaki, A.; Michikawa, T.; Mikoshiba, K.; Nagai, T. Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat. Methods 2010, 7, 729–732. [Google Scholar] [CrossRef]
- Meyer, J.S.; Tullis, G.; Pierret, C.; Spears, K.M.; Morrison, J.A.; Kirk, M.D. Detection of calcium transients in embryonic stem cells and their differentiated progeny. Cell Mol. Neurobiol. 2009, 29, 1191–1203. [Google Scholar] [CrossRef][Green Version]
- Kleist, T.; Cartwright, H.; Perera, A.; Christianson, M.; Lemaux, P.; Luan, S. Genetically encoded calcium indicators for fluorescence imaging in the moss Physcomitrella: GCaMP3 provides a bright new look. Plant Biotechnol. J. 2017, 15, 1235–1237. [Google Scholar] [CrossRef]
- Zhang, Y.; Rozsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef]
- Rebouillat, J.; Dievart, A.; Verdeil, L.; Escoute, J.; Giese, G.; Breitler, C.; Gantet, P.; Espeout, S.; Guiderdoni, E.; Perin, C. Molecular genetics of rice root development. Rice 2009, 2, 15–34. [Google Scholar] [CrossRef]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 550. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014, 24, 632–635. [Google Scholar] [CrossRef]
- Storti, M.; Costa, A.; Golin, S.; Zottini, M.; Morosinotto, T.; Alboresi, A. Systemic calcium wave propagation in Physcomitrella patens. Plant Cell Physiol. 2018, 59, 1377–1384. [Google Scholar] [CrossRef]
- Price, A.H.; Taylor, A.; Ripley, S.J.; Griffiths, A.; Trewavas, A.J.; Knight, M.R. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 1994, 6, 1301–1310. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Zhang, S.; Zhao, H.; Yang, W.; Siedow, J.N.; Pei, Z.M. Both NaCl and H2O2 long-term stresses affect basal cytosolic Ca2+ levels but only NaCl alters cytosolic Ca2+ signatures in Arabidopsis. Front. Plant Sci. 2018, 9, 1390. [Google Scholar] [CrossRef]
- Queirós, F.; Rodrigues, J.A.; Almeida, J.M.; Almeida, D.P.; Fidalgo, F. Differential responses of the antioxidant defence system and ultrastructure in a salt-adapted potato cell line. Plant Physiol. Biochem. 2011, 49, 1410–1419. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Li, N.; Li, S.X.; Guo, J.H.; Li, X.N. Parental salt priming improves the low temperature tolerance in wheat offspring via modulating the seed proteome. Plant Sci. 2022, 324, 111428. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, P.; Feng, G.; Hou, W.; Liu, T.; Gai, Z.; Shen, Y.; Qiu, X.; Li, X. Salt priming induces low-temperature tolerance in sugar beet via xanthine metabolism. Plant Physiol. Biochem. 2023, 201, 107810. [Google Scholar] [CrossRef]
- Nishiyama, T.; Hiwatashi, Y.; Sakakibara, I.; Kato, M.; Hasebe, M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 2000, 7, 9–17. [Google Scholar] [CrossRef]
- Okano, Y.; Aono, N.; Hiwatashi, Y.; Murata, T.; Nishiyama, T.; Ishikawa, T.; Kubo, M.; Hasebe, M. A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 16321–16326. [Google Scholar] [CrossRef]
- Cove, D.J.; Perroud, P.F.; Charron, A.J.; McDaniel, S.F.; Khandelwal, A.; Quatrano, R.S. Transformation of the moss Physcomitrella patens using direct DNA uptake by protoplasts. Cold Spring Harb. Protoc. 2009, 2009, pdb.prot5143. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Ding, K.; Yu, Z.; Zhang, Y.; Ni, J.; Wu, Y. Aquorin Bioluminescence-Based Ca2+ Imaging Reveals Differential Calcium Signaling Responses to Abiotic Stresses in Physcomitrella patens. Plants 2025, 14, 1178. https://doi.org/10.3390/plants14081178
Shen J, Ding K, Yu Z, Zhang Y, Ni J, Wu Y. Aquorin Bioluminescence-Based Ca2+ Imaging Reveals Differential Calcium Signaling Responses to Abiotic Stresses in Physcomitrella patens. Plants. 2025; 14(8):1178. https://doi.org/10.3390/plants14081178
Chicago/Turabian StyleShen, Jiamin, Kexin Ding, Zhiming Yu, Yuzhen Zhang, Jun Ni, and Yuhuan Wu. 2025. "Aquorin Bioluminescence-Based Ca2+ Imaging Reveals Differential Calcium Signaling Responses to Abiotic Stresses in Physcomitrella patens" Plants 14, no. 8: 1178. https://doi.org/10.3390/plants14081178
APA StyleShen, J., Ding, K., Yu, Z., Zhang, Y., Ni, J., & Wu, Y. (2025). Aquorin Bioluminescence-Based Ca2+ Imaging Reveals Differential Calcium Signaling Responses to Abiotic Stresses in Physcomitrella patens. Plants, 14(8), 1178. https://doi.org/10.3390/plants14081178





