Sasa veitchii Extract Mitigates Mycophenolate Mofetil-Induced Human Palatal Cell Proliferation Inhibition by Downregulating microRNA-4680-3p
Abstract
1. Introduction
2. Results
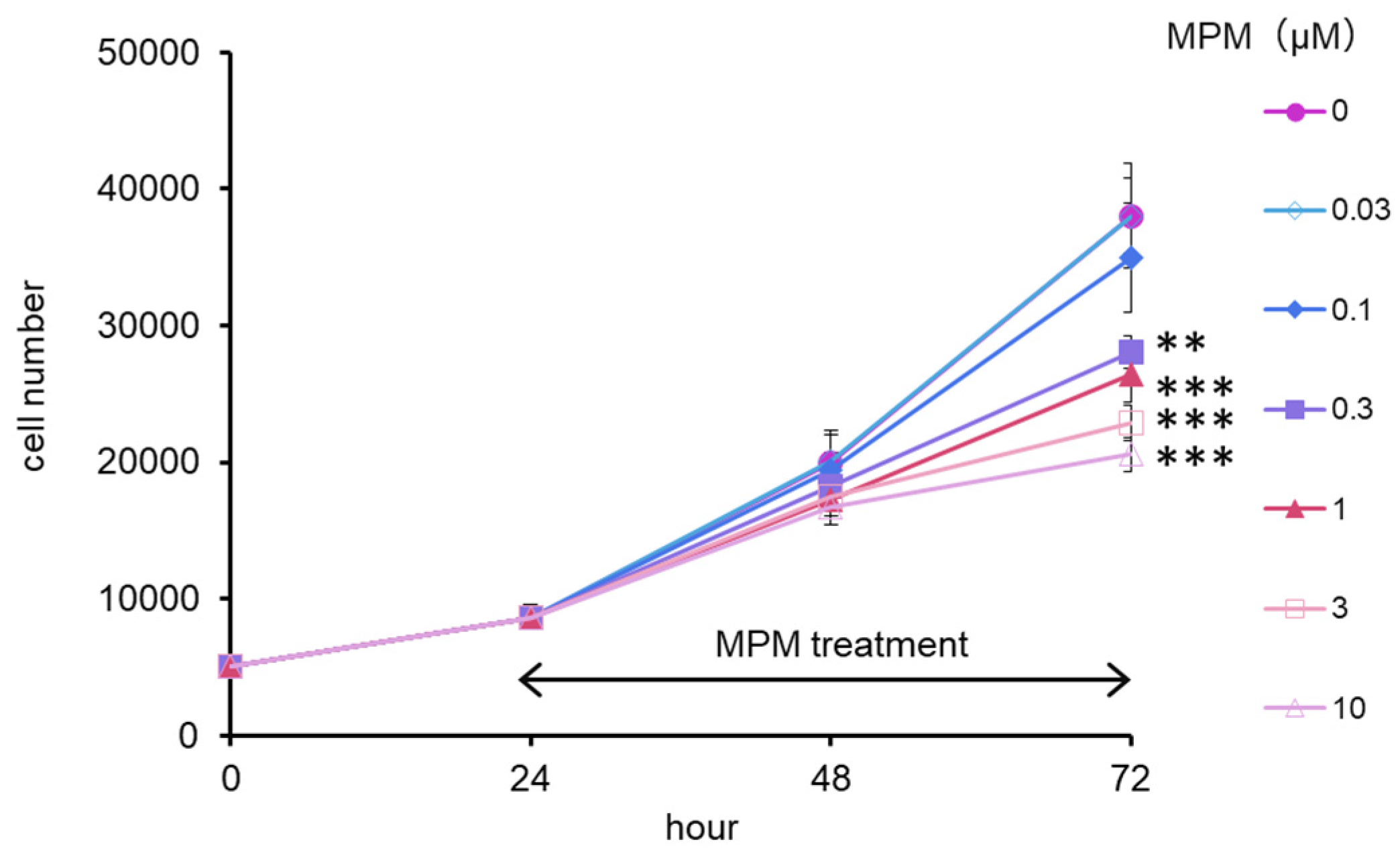
2.1. MPM Inhibited HEPM Cell Proliferation in a Dose- and Time-Dependent Manner
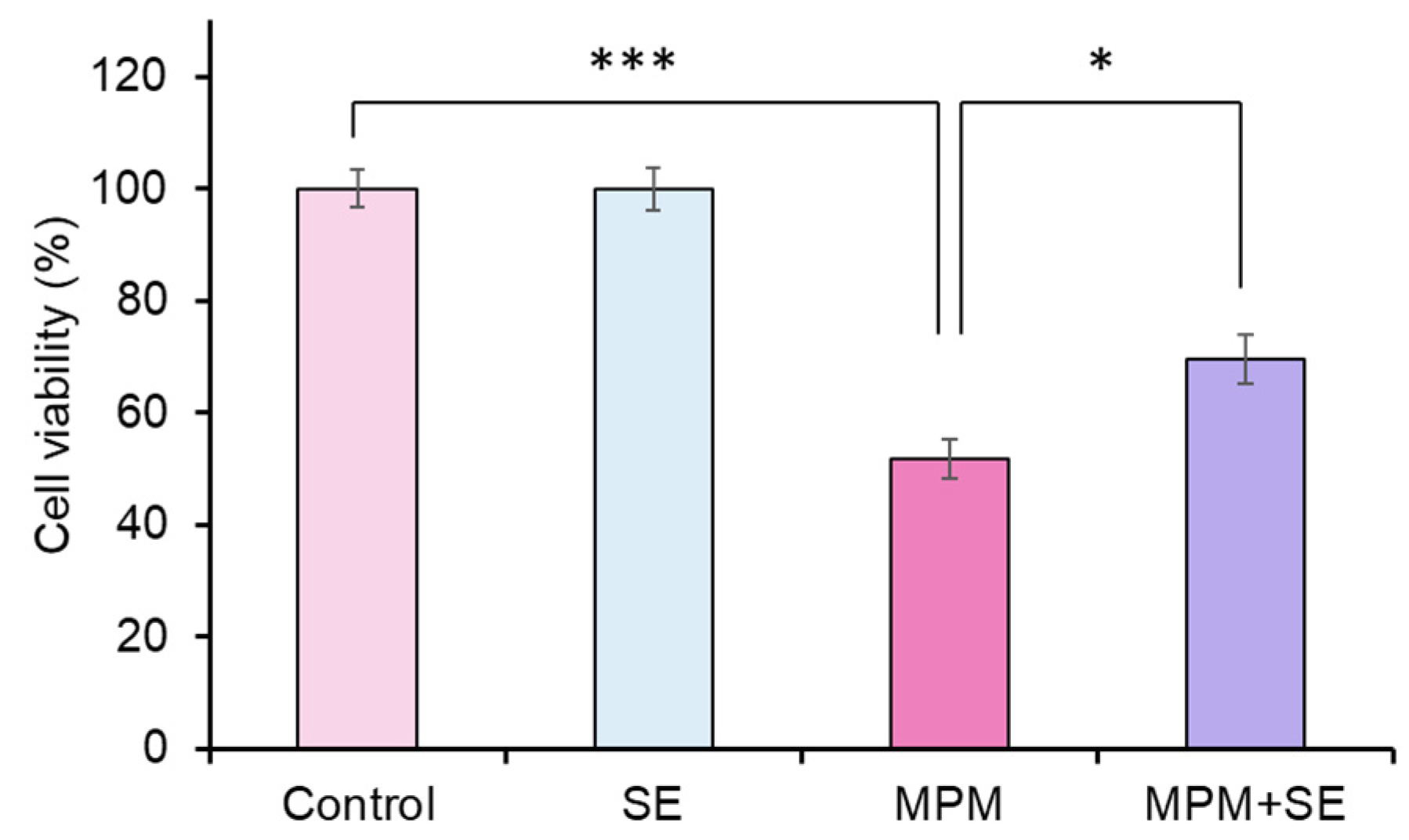
2.2. SE Alleviated MPM-Induced Proliferation Inhibition in HEPM Cells
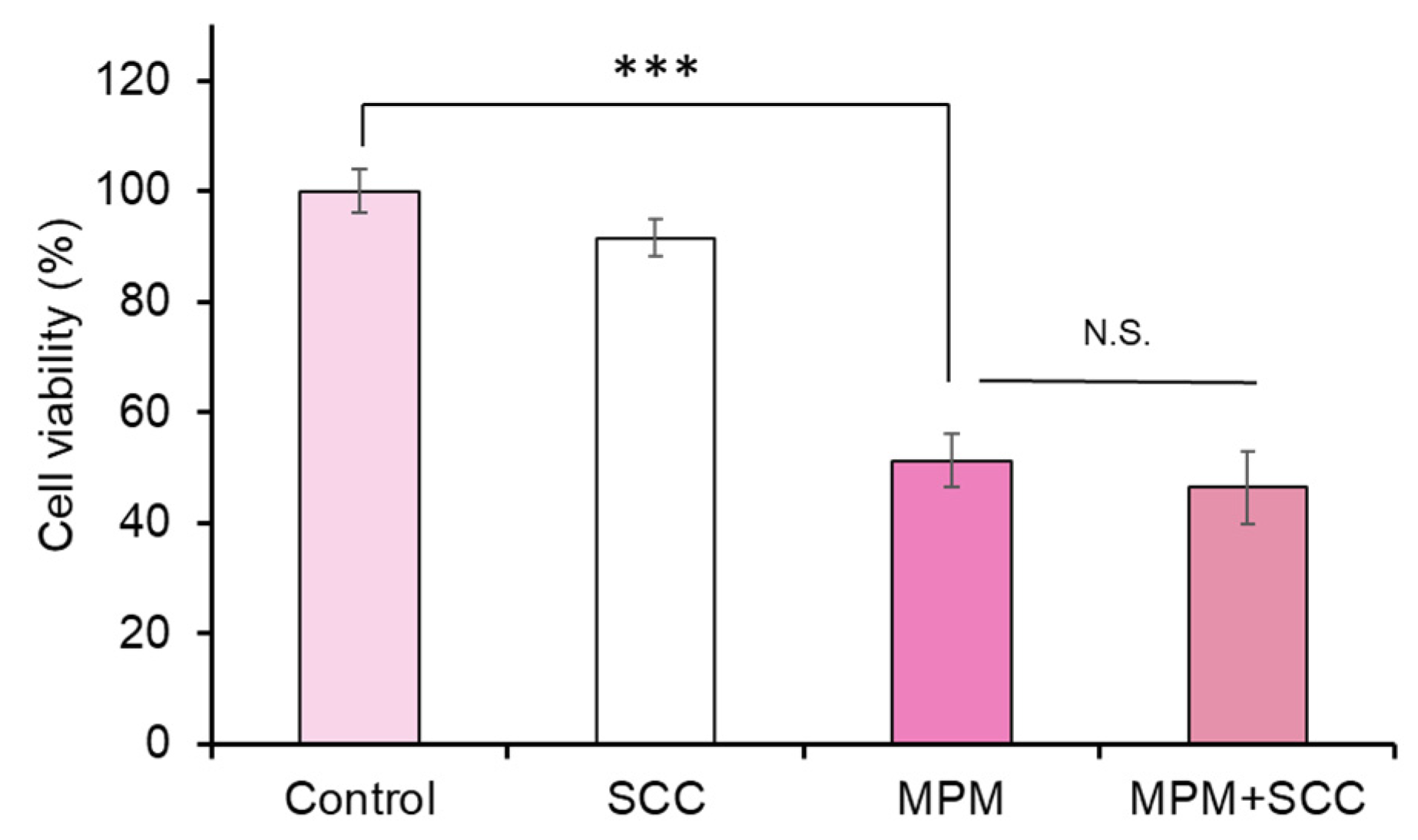
2.3. Co-Treatment with Sodium Copper Chlorophyllin (SCC) Failed to Recover MPM-Induced Cell Proliferation Reduction in HEPM Cells
2.4. SE Alleviated MPM-Induced Cell Cycle Arrest in HEPM Cells
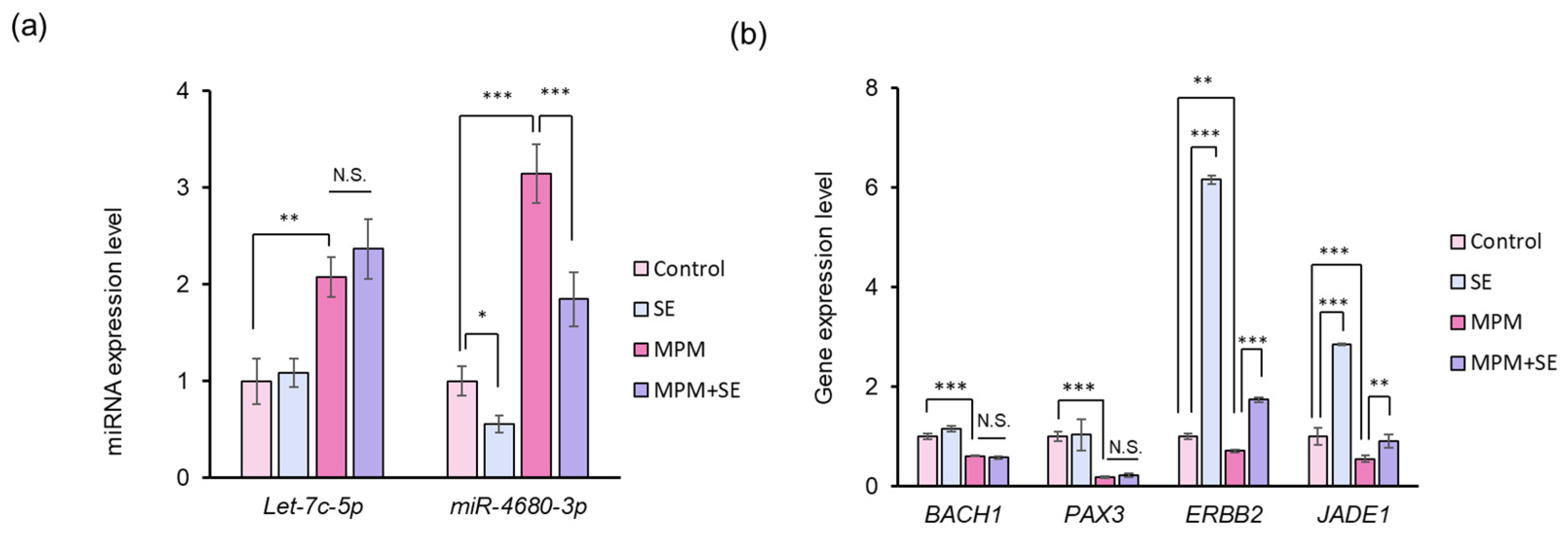
2.5. SE Downregulated miR-4680-3p and Upregulated Its Downstream Genes in HEPM Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of SE
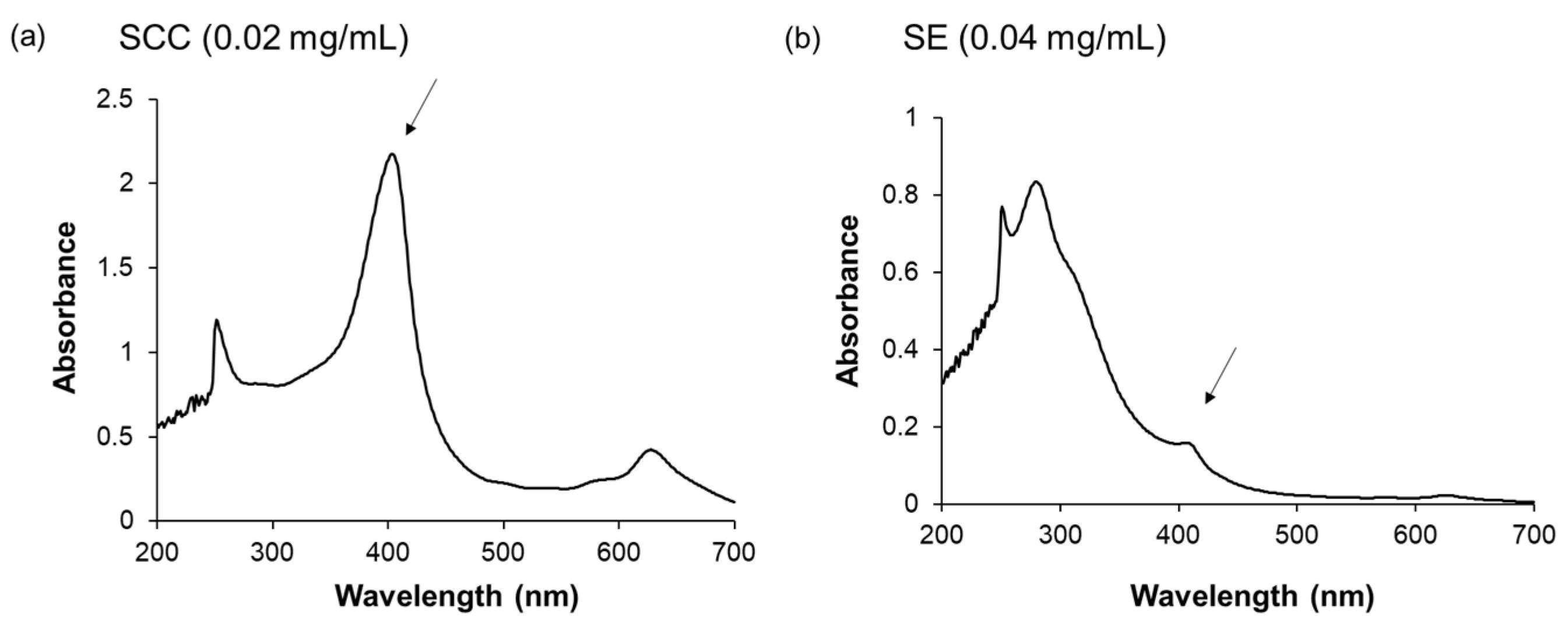
4.3. Infrared Absorption Spectrometry Analysis
4.4. Cell Proliferation Assay
4.5. Bromodeoxyuridine (BrdU) Incorporation Assay
4.6. Western Blot Analysis
4.7. Quantitative RT-PCR
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| atRA | all-trans-retinoic acid |
| CDK | cyclin-dependent kinases |
| CL | Cleft lip |
| CP | Cleft palate |
| CL/P | Cleft lip with or without cleft palate |
| EMT | epithelial-mesenchymal transition |
| HEPM | human embryonic palatal mesenchymal |
| HPLC | High performance liquid chromatography |
| miRNA | microRNA |
| MPM | Mycophenolate mofetil |
| Pax | Paired box gene |
| SE | Sasa veitchii extract |
| SCC | sodium copper chlorophyllin |
| TGF | transforming growth factor |
| WNT | Wingless/integrase-1 |
References
- Sadler, T.W. Establishing the Embryonic Axes: Prime Time for Teratogenic Insults. J. Cardiovasc. Dev. Dis. 2017, 4, 15. [Google Scholar] [CrossRef]
- Van Gelder, M.M.; van Rooij, I.A.; de Jong-van den Berg, L.T.; Roeleveld, N. Teratogenic mechanisms associated with prenatal medication exposure. Therapie 2014, 69, 13–24. [Google Scholar] [PubMed]
- Babai, A.; Irving, M. Orofacial Clefts: Genetics of Cleft Lip and Palate. Genes 2023, 14, 1603. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [PubMed]
- Blencowe, H.; Cousens, S.; Modell, B.; Lawn, J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i110–i121. [Google Scholar]
- Maldonado, E.; Murillo, J.; Barrio, C.; del Rio, A.; Perez-Miguelsanz, J.; Lopez-Gordillo, Y.; Partearroyo, T.; Paradas, I.; Maestro, C.; Martinez-Sanz, E.; et al. Occurrence of cleft-palate and alteration of Tgf-beta(3) expression and the mechanisms leading to palatal fusion in mice following dietary folic-acid deficiency. Cells Tissues Organs 2011, 194, 406–420. [Google Scholar] [CrossRef]
- Zhang, Y.; Kato, H.; Sato, H.; Yamaza, H.; Hirofuji, Y.; Han, X.; Masuda, K.; Nonaka, K. Folic acid-mediated mitochondrial activation for protection against oxidative stress in human dental pulp stem cells derived from deciduous teeth. Biochem. Biophys. Res. Commun. 2019, 508, 850–856. [Google Scholar]
- Won, H.J.; Kim, J.W.; Won, H.S.; Shin, J.O. Gene Regulatory Networks and Signaling Pathways in Palatogenesis and Cleft Palate: A Comprehensive Review. Cells 2023, 12, 1954. [Google Scholar] [CrossRef]
- Im, H.; Song, Y.; Kim, J.K.; Park, D.K.; Kim, D.S.; Kim, H.; Shin, J.O. Molecular Regulation of Palatogenesis and Clefting: An Integrative Analysis of Genetic, Epigenetic Networks, and Environmental Interactions. Int. J. Mol. Sci. 2025, 26, 1382. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, J.; Fanelli, C.; Wee, Y.; Bonds, J.; Schneider, P.; Mues, G.; D’Souza, R.N. Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero. Development 2017, 144, 3819–3828. [Google Scholar]
- Li, C.; Lan, Y.; Jiang, R. Molecular and Cellular Mechanisms of Palate Development. J. Dent. Res. 2017, 96, 1184–1191. [Google Scholar] [PubMed]
- Letra, A.; Bjork, B.; Cooper, M.E.; Szabo-Rogers, H.; Deleyiannis, F.W.; Field, L.L.; Czeizel, A.E.; Ma, L.; Garlet, G.P.; Poletta, F.A.; et al. Association of AXIN2 with non-syndromic oral clefts in multiple populations. J. Dent. Res. 2012, 91, 473–478. [Google Scholar] [PubMed]
- Mostowska, A.; Hozyasz, K.K.; Wojcicki, P.; Lasota, A.; Dunin-Wilczynska, I.; Jagodzinski, P.P. Association of DVL2 and AXIN2 gene polymorphisms with cleft lip with or without cleft palate in a Polish population. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 943–950. [Google Scholar]
- Murray, S.A.; Oram, K.F.; Gridley, T. Multiple functions of Snail family genes during palate development in mice. Development 2007, 134, 1789–1797. [Google Scholar]
- Graf, D.; Malik, Z.; Hayano, S.; Mishina, Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016, 27, 129–139. [Google Scholar]
- Ueharu, H.; Mishina, Y. BMP signaling during craniofacial development: New insights into pathological mechanisms leading to craniofacial anomalies. Front. Physiol. 2023, 14, 1170511. [Google Scholar]
- Li, A.; Jia, P.; Mallik, S.; Fei, R.; Yoshioka, H.; Suzuki, A.; Iwata, J.; Zhao, Z. Critical microRNAs and regulatory motifs in cleft palate identified by a conserved miRNA-TF-gene network approach in humans and mice. Brief. Bioinform. 2020, 21, 1465–1478. [Google Scholar]
- Lee, K.S.; Choi, Y.J.; Cho, J.; Lee, H.; Lee, H.; Park, S.J.; Park, J.S.; Hong, Y.C. Environmental and Genetic Risk Factors of Congenital Anomalies: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Korean Med. Sci. 2021, 36, e183. [Google Scholar]
- Spinder, N.; Prins, J.R.; Bergman, J.E.H.; Smidt, N.; Kromhout, H.; Boezen, H.M.; de Walle, H.E.K. Congenital anomalies in the offspring of occupationally exposed mothers: A systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum. Reprod. 2019, 34, 903–919. [Google Scholar]
- Luteijn, J.M.; Brown, M.J.; Dolk, H. Influenza and congenital anomalies: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 809–823. [Google Scholar]
- Nicoletti, D.; Appel, L.D.; Siedersberger Neto, P.; Guimaraes, G.W.; Zhang, L. Maternal smoking during pregnancy and birth defects in children: A systematic review with meta-analysis. Cad. Saude Publica 2014, 30, 2491–2529. [Google Scholar] [PubMed]
- Puho, E.H.; Szunyogh, M.; Metneki, J.; Czeizel, A.E. Drug treatment during pregnancy and isolated orofacial clefts in hungary. Cleft Palate Craniofacial J. 2007, 44, 194–202. [Google Scholar]
- Ebadifar, A.; Hamedi, R.; KhorramKhorshid, H.R.; Kamali, K.; Moghadam, F.A. Parental cigarette smoking, transforming growth factor-alpha gene variant and the risk of orofacial cleft in Iranian infants. Iran J. Basic Med. Sci. 2016, 19, 366–373. [Google Scholar] [PubMed]
- Junaid, M.; Narayanan, M.B.A.; Jayanthi, D.; Kumar, S.G.R.; Selvamary, A.L. Association between maternal exposure to tobacco, presence of TGFA gene, and the occurrence of oral clefts. A case control study. Clin. Oral Investig. 2018, 22, 217–223. [Google Scholar]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar]
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498. [Google Scholar]
- Shull, L.C.; Artinger, K.B. Epigenetic regulation of craniofacial development and disease. Birth Defects Res. 2024, 116, e2271. [Google Scholar]
- Li, L.; Shi, B.; Chen, J.; Li, C.; Wang, S.; Wang, Z.; Zhu, G. An E2F1/MiR-17-92 Negative Feedback Loop mediates proliferation of Mouse Palatal Mesenchymal Cells. Sci. Rep. 2017, 7, 5148. [Google Scholar]
- Wang, J.; Bai, Y.; Li, H.; Greene, S.B.; Klysik, E.; Yu, W.; Schwartz, R.J.; Williams, T.J.; Martin, J.F. MicroRNA-17-92, a direct Ap-2alpha transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 2013, 9, e1003785. [Google Scholar]
- Pan, Y.; Li, D.; Lou, S.; Zhang, C.; Du, Y.; Jiang, H.; Zhang, W.; Ma, L.; Wang, L. A functional polymorphism in the pre-miR-146a gene is associated with the risk of nonsyndromic orofacial cleft. Hum. Mutat. 2018, 39, 742–750. [Google Scholar] [PubMed]
- Suzuki, A.; Li, A.; Gajera, M.; Abdallah, N.; Zhang, M.; Zhao, Z.; Iwata, J. MicroRNA-374a, -4680, and -133b suppress cell proliferation through the regulation of genes associated with human cleft palate in cultured human palate cells. BMC Med. Genom. 2019, 12, 93. [Google Scholar]
- Fu, C.; Lou, S.; Zhu, G.; Fan, L.; Yu, X.; Zhu, W.; Ma, L.; Wang, L.; Pan, Y. Identification of New miRNA-mRNA Networks in the Development of Non-syndromic Cleft Lip With or Without Cleft Palate. Front. Cell Dev. Biol. 2021, 9, 631057. [Google Scholar]
- Chen, H.; Chen, B. Clinical mycophenolic acid monitoring in liver transplant recipients. World J. Gastroenterol. 2014, 20, 10715–10728. [Google Scholar]
- Narayanaswami, P.; Sanders, D.B.; Thomas, L.; Thibault, D.; Blevins, J.; Desai, R.; Krueger, A.; Bibeau, K.; Liu, B.; Guptill, J.T.; et al. Comparative effectiveness of azathioprine and mycophenolate mofetil for myasthenia gravis (PROMISE-MG): A prospective cohort study. Lancet Neurol. 2024, 23, 267–276. [Google Scholar]
- Perez-Aytes, A.; Ledo, A.; Boso, V.; Saenz, P.; Roma, E.; Poveda, J.L.; Vento, M. In utero exposure to mycophenolate mofetil: A characteristic phenotype? Am. J. Med. Genet. A 2008, 146A, 1–7. [Google Scholar]
- Lin, Y.; Song, T.; Ronde, E.M.; Ma, G.; Cui, H.; Xu, M. The important role of MDM2, RPL5, and TP53 in mycophenolic acid-induced cleft lip and palate. Medicine 2021, 100, e26101. [Google Scholar]
- Yoshioka, H.; Horita, H.; Tsukiboshi, Y.; Kurita, H.; Ogata, A.; Ogata, K. Cleft Palate Induced by Mycophenolate Mofetil Is Associated with miR-4680-3p and let-7c-5p in Human Palate Cells. Noncoding RNA 2025, 11, 12. [Google Scholar]
- Available online: https://www.sunchlon.co.jp/wp/wp-content/uploads/2021/11/%E6%B7%BB%E4%BB%98%E6%96%87%E6%9B%B8%E3%82%B5%E3%83%B3%E3%82%AF%E3%83%AD%E3%83%B3%E8%8B%B1%E8%AA%9E.pdf (accessed on 27 March 2025).
- Kimura, I.; Kagawa, S.; Tsuneki, H.; Tanaka, K.; Nagashima, F. Multitasking bamboo leaf-derived compounds in prevention of infectious, inflammatory, atherosclerotic, metabolic, and neuropsychiatric diseases. Pharmacol. Ther. 2022, 235, 108159. [Google Scholar]
- Yoshioka, H.; Nonogaki, T.; Fukaya, S.; Ichimaru, Y.; Nagatsu, A.; Yoshikawa, M.; Fujii, H.; Nakao, M. Sasa veitchii extract protects against carbon tetrachloride-induced hepatic fibrosis in mice. Environ. Health Prev. Med. 2018, 23, 49. [Google Scholar]
- Kojima, S.; Hakamata, M.; Asanuma, T.; Suzuki, R.; Tsuruda, J.I.; Nonoyama, T.; Lin, Y.; Fukatsu, H.; Koide, N.; Umezawa, K. Cellular Anti-Inflammatory and Antioxidant Activities of Bamboo Sasa albomarginata Leaf Extract and Its Constituent Coumaric Acid Methyl Ester. Sci. World J. 2022, 2022, 8454865. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, J.; Mikami, Y.; Horiuchi, A.; Yano, A.; Amano, F.; Shibata, S.; Ogata, A.; Ogata, K.; Nagatsu, A.; Miura, N.; et al. Sasa veitchii extract exhibits antitumor effect against murine pancreatic adenocarcinoma in vivo and in vitro. Tradit. Kampo Med. 2025, 12, 11–20. [Google Scholar] [CrossRef]
- Yoshioka, H.; Wu, S.; Moriishi, T.; Tsukiboshi, Y.; Yokota, S.; Miura, N.; Yoshikawa, M.; Inagaki, N.; Matsushita, Y.; Nakao, M. Sasa veitchii extract alleviates nonalcoholic steatohepatitis in methionine–choline deficient diet-induced mice by regulating peroxisome proliferator-activated receptor alpha. Tradit. Kampo Med. 2023, 10, 259–268. [Google Scholar] [CrossRef]
- Sakagami, H.; Tomomura, M. Dental Application of Natural Products. Medicines 2018, 5, 21. [Google Scholar] [CrossRef]
- Tsukiboshi, Y.; Mikami, Y.; Horita, H.; Ogata, A.; Noguchi, A.; Yokota, S.; Ogata, K.; Yoshioka, H. Protective effect of Sasa veitchii extract against all-trans-retinoic acid-induced inhibition of proliferation of cultured human palate cells. Nagoya J. Med. Sci. 2024, 86, 223–236. [Google Scholar]
- Yoshioka, H.; Tsukiboshi, Y.; Horita, H.; Kurita, H.; Ogata, A.; Ogata, K.; Horiguchi, H. Sasa veitchii extract alleviates phenobarbital-induced cell proliferation inhibition by upregulating transforming growth factor-beta 1. Tradit. Kampo Med. 2024, 11, 192–199. [Google Scholar] [CrossRef]
- Sun, X.X.; Dai, M.S.; Lu, H. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J. Biol. Chem. 2008, 283, 12387–12392. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, H.; Wang, Y.; Tian, J.; Li, R.; Zhou, X. Evaluation of the inhibitory effect of tacrolimus combined with mycophenolate mofetil on mesangial cell proliferation based on the cell cycle. Int. J. Mol. Med. 2020, 46, 1582–1592. [Google Scholar] [CrossRef]
- Takebe, N.; Cheng, X.; Fandy, T.E.; Srivastava, R.K.; Wu, S.; Shankar, S.; Bauer, K.; Shaughnessy, J.; Tricot, G. IMP dehydrogenase inhibitor mycophenolate mofetil induces caspase-dependent apoptosis and cell cycle inhibition in multiple myeloma cells. Mol. Cancer Ther. 2006, 5, 457–466. [Google Scholar] [CrossRef]
- Yoshioka, H.; Horita, H.; Tsukiboshi, Y.; Kurita, H.; Mikami, Y.; Ogata, K.; Ogata, A. Mycophenolate mofetil reduces cell viability associated with the miR-205-PAX9 pathway in human lip fibroblast cells. Biomed. Res. 2025, 46, 1–8. [Google Scholar] [CrossRef]
- Schoen, C.; Aschrafi, A.; Thonissen, M.; Poelmans, G.; Von den Hoff, J.W.; Carels, C.E.L. MicroRNAs in Palatogenesis and Cleft Palate. Front. Physiol. 2017, 8, 165. [Google Scholar]
- Zhou, J.; Zhang, M.; Zhang, M.; Tan, M.; Ji, Y.; Shu, S.; Liang, Y. MiRNA-470-5p suppresses epithelial-mesenchymal transition of embryonic palatal shelf epithelial cells by targeting Fgfr1 during palatogenesis. Exp. Biol. Med. 2023, 248, 1124–1133. [Google Scholar]
- Zhang, W.; Shen, Z.; Xing, Y.; Zhao, H.; Liang, Y.; Chen, J.; Zhong, X.; Shi, L.; Wan, X.; Zhou, J.; et al. MiR-106a-5p modulates apoptosis and metabonomics changes by TGF-beta/Smad signaling pathway in cleft palate. Exp. Cell Res. 2020, 386, 111734. [Google Scholar]
- Yoshioka, H.; Ramakrishnan, S.S.; Shim, J.; Suzuki, A.; Iwata, J. Excessive All-Trans Retinoic Acid Inhibits Cell Proliferation Through Upregulated MicroRNA-4680-3p in Cultured Human Palate Cells. Front. Cell Dev. Biol. 2021, 9, 618876. [Google Scholar]
- Yoshioka, H.; Jun, G.; Suzuki, A.; Iwata, J. Dexamethasone Suppresses Palatal Cell Proliferation through miR-130a-3p. Int. J. Mol. Sci. 2021, 22, 12453. [Google Scholar] [CrossRef] [PubMed]
- Tsukiboshi, Y.; Horita, H.; Mikami, Y.; Noguchi, A.; Yokota, S.; Ogata, K.; Yoshioka, H. Involvement of microRNA-4680-3p against phenytoin-induced cell proliferation inhibition in human palate cells. J. Toxicol. Sci. 2024, 49, 1–8. [Google Scholar]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Su, J.L.; Chen, P.S.; Johansson, G.; Kuo, M.L. Function and regulation of let-7 family microRNAs. Microrna 2012, 1, 34–39. [Google Scholar]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Avraham, R.; Yarden, Y. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 2011, 12, 104–117. [Google Scholar]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [PubMed]
- Timms, J.F.; White, S.L.; O’Hare, M.J.; Waterfield, M.D. Effects of ErbB-2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene 2002, 21, 6573–6586. [Google Scholar] [PubMed]
- Yang, Y.; Suzuki, A.; Iwata, J.; Jun, G. Secondary Genome-Wide Association Study Using Novel Analytical Strategies Disentangle Genetic Components of Cleft Lip and/or Cleft Palate in 1q32.2. Genes 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Borgal, L.; Rinschen, M.M.; Dafinger, C.; Hoff, S.; Reinert, M.J.; Lamkemeyer, T.; Lienkamp, S.S.; Benzing, T.; Schermer, B. Casein kinase 1 alpha phosphorylates the Wnt regulator Jade-1 and modulates its activity. J. Biol. Chem. 2014, 289, 26344–26356. [Google Scholar]
- Siriwardana, N.S.; Meyer, R.D.; Panchenko, M.V. The novel function of JADE1S in cytokinesis of epithelial cells. Cell Cycle 2015, 14, 2821–2834. [Google Scholar]
- Havasi, A.; Haegele, J.A.; Gall, J.M.; Blackmon, S.; Ichimura, T.; Bonegio, R.G.; Panchenko, M.V. Histone acetyl transferase (HAT) HBO1 and JADE1 in epithelial cell regeneration. Am. J. Pathol. 2013, 182, 152–162. [Google Scholar]
- Panchenko, M.V. Structure, function and regulation of jade family PHD finger 1 (JADE1). Gene 2016, 589, 1–11. [Google Scholar]
- Han, J.; Lachance, C.; Ricketts, M.D.; McCullough, C.E.; Gerace, M.; Black, B.E.; Cote, J.; Marmorstein, R. The scaffolding protein JADE1 physically links the acetyltransferase subunit HBO1 with its histone H3-H4 substrate. J. Biol. Chem. 2018, 293, 4498–4509. [Google Scholar]
- Chitalia, V.C.; Foy, R.L.; Bachschmid, M.M.; Zeng, L.; Panchenko, M.V.; Zhou, M.I.; Bharti, A.; Seldin, D.C.; Lecker, S.H.; Dominguez, I.; et al. Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat. Cell Biol. 2008, 10, 1208–1216. [Google Scholar]
- Tauriello, D.V.; Maurice, M.M. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle 2010, 9, 3700–3709. [Google Scholar]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [PubMed]
- Dashwood, R. Chlorophylls as anticarcinogens (review). Int. J. Oncol. 1997, 10, 721–727. [Google Scholar] [PubMed]
- Suparmi, S.; Fasitasari, M.; Martosupono, M.; Mangimbulude, J.C. Comparisons of Curative Effects of Chlorophyll from Sauropus androgynus (L) Merr Leaf Extract and Cu-Chlorophyllin on Sodium Nitrate-Induced Oxidative Stress in Rats. J. Toxicol. 2016, 2016, 8515089. [Google Scholar] [PubMed]
- Cortes-Eslava, J.; Gomez-Arroyo, S.; Villalobos-Pietrini, R.; Espinosa-Aguirre, J.J. Antimutagenicity of coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. Toxicol. Lett. 2004, 153, 283–292. [Google Scholar]
- Aizenbud, D.; Peri-Front, Y.; Nagler, R.M. Salivary analysis and antioxidants in cleft lip and palate children. Arch. Oral Biol. 2008, 53, 517–522. [Google Scholar]
- Li, R.; Huang, C.; Ho, J.C.H.; Leung, C.C.T.; Kong, R.Y.C.; Li, Y.; Liang, X.; Lai, K.P.; Tse, W.K.F. The use of glutathione to reduce oxidative stress status and its potential for modifying the extracellular matrix organization in cleft lip. Free Radic Biol. Med. 2021, 164, 130–138. [Google Scholar]
- Wang, J.; Yue, Y.D.; Jiang, H.; Tang, F. Rapid Screening for Flavone C-Glycosides in the Leaves of Different Species of Bamboo and Simultaneous Quantitation of Four Marker Compounds by HPLC-UV/DAD. Int. J. Anal. Chem. 2012, 2012, 205101. [Google Scholar]
- Wang, J.; Yue, Y.D.; Tang, F.; Sun, J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa. textilis McClure. Molecules 2012, 17, 12297–12311. [Google Scholar] [CrossRef]
- Chung, D.J.; Wang, C.J.; Yeh, C.W.; Tseng, T.H. Inhibition of the Proliferation and Invasion of C6 Glioma Cells by Tricin via the Upregulation of Focal-Adhesion-Kinase-Targeting MicroRNA-7. J. Agric. Food Chem. 2018, 66, 6708–6716. [Google Scholar]
- Xia, S.F.; Qiu, Y.Y.; Chen, L.M.; Jiang, Y.Y.; Huang, W.; Xie, Z.X.; Tang, X.; Sun, J. Myricetin alleviated hepatic steatosis by acting on microRNA-146b/thyroid hormone receptor b pathway in high-fat diet fed C57BL/6J mice. Food Funct. 2019, 10, 1465–1477. [Google Scholar]
- Bai, Y.; Liu, X.; Chen, Q.; Chen, T.; Jiang, N.; Guo, Z. Myricetin ameliorates ox-LDL-induced HUVECs apoptosis and inflammation via lncRNA GAS5 upregulating the expression of miR-29a-3p. Sci. Rep. 2021, 11, 19637. [Google Scholar]
- Najafipour, R.; Momeni, A.M.; Mirmazloomi, Y.; Moghbelinejad, S. Vitexin Induces Apoptosis in MCF-7 Breast Cancer Cells through the Regulation of Specific miRNAs Expression. Int. J. Mol. Cell Med. 2022, 11, 197–206. [Google Scholar] [PubMed]
- Zaki, A.; Mohsin, M.; Khan, S.; Khan, A.; Ahmad, S.; Verma, A.; Ali, S.; Fatma, T.; Syed, M.A. Vitexin mitigates oxidative stress, mitochondrial damage, pyroptosis and regulates small nucleolar RNA host gene 1/DNA methyltransferase 1/microRNA-495 axis in sepsis-associated acute lung injury. Inflammopharmacology 2024, 33, 1435–1454. [Google Scholar]
- Jang, M.G.; Ko, H.C.; Kim, S.J. Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genom. 2020, 42, 817–825. [Google Scholar]
- Kheiry, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V. Does p-coumaric acid improve cardiac injury following LPS-induced lung inflammation through miRNA-146a activity? Avicenna J. Phytomedicine 2020, 10, 50–57. [Google Scholar]
- Jiang, Z.Q.; Li, M.H.; Qin, Y.M.; Jiang, H.Y.; Zhang, X.; Wu, M.H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef]
- Yoshioka, H.; Tominaga, S.; Amano, F.; Wu, S.; Torimoto, S.; Moriishi, T.; Tsukiboshi, Y.; Yokota, S.; Miura, N.; Inagaki, N.; et al. Juzentaihoto alleviates cisplatin-induced renal injury in mice. Tradit. Kampo Med. 2024, 11, 147–155. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horita, H.; Tsukiboshi, Y.; Ogata, K.; Ogata, A.; Kurita, H.; Yamashita, S.; Yamashita, H.; Inagaki, N.; Horiguchi, H.; Yoshioka, H. Sasa veitchii Extract Mitigates Mycophenolate Mofetil-Induced Human Palatal Cell Proliferation Inhibition by Downregulating microRNA-4680-3p. Plants 2025, 14, 1150. https://doi.org/10.3390/plants14071150
Horita H, Tsukiboshi Y, Ogata K, Ogata A, Kurita H, Yamashita S, Yamashita H, Inagaki N, Horiguchi H, Yoshioka H. Sasa veitchii Extract Mitigates Mycophenolate Mofetil-Induced Human Palatal Cell Proliferation Inhibition by Downregulating microRNA-4680-3p. Plants. 2025; 14(7):1150. https://doi.org/10.3390/plants14071150
Chicago/Turabian StyleHorita, Hanane, Yosuke Tsukiboshi, Kenichi Ogata, Aya Ogata, Hisaka Kurita, Shuji Yamashita, Hirotaka Yamashita, Naoki Inagaki, Hyogo Horiguchi, and Hiroki Yoshioka. 2025. "Sasa veitchii Extract Mitigates Mycophenolate Mofetil-Induced Human Palatal Cell Proliferation Inhibition by Downregulating microRNA-4680-3p" Plants 14, no. 7: 1150. https://doi.org/10.3390/plants14071150
APA StyleHorita, H., Tsukiboshi, Y., Ogata, K., Ogata, A., Kurita, H., Yamashita, S., Yamashita, H., Inagaki, N., Horiguchi, H., & Yoshioka, H. (2025). Sasa veitchii Extract Mitigates Mycophenolate Mofetil-Induced Human Palatal Cell Proliferation Inhibition by Downregulating microRNA-4680-3p. Plants, 14(7), 1150. https://doi.org/10.3390/plants14071150






