Genome-Wide Identification of WOX Gene Family in Chimonanthus praecox and a Functional Analysis of CpWUS
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Properties of CpWOX Genes
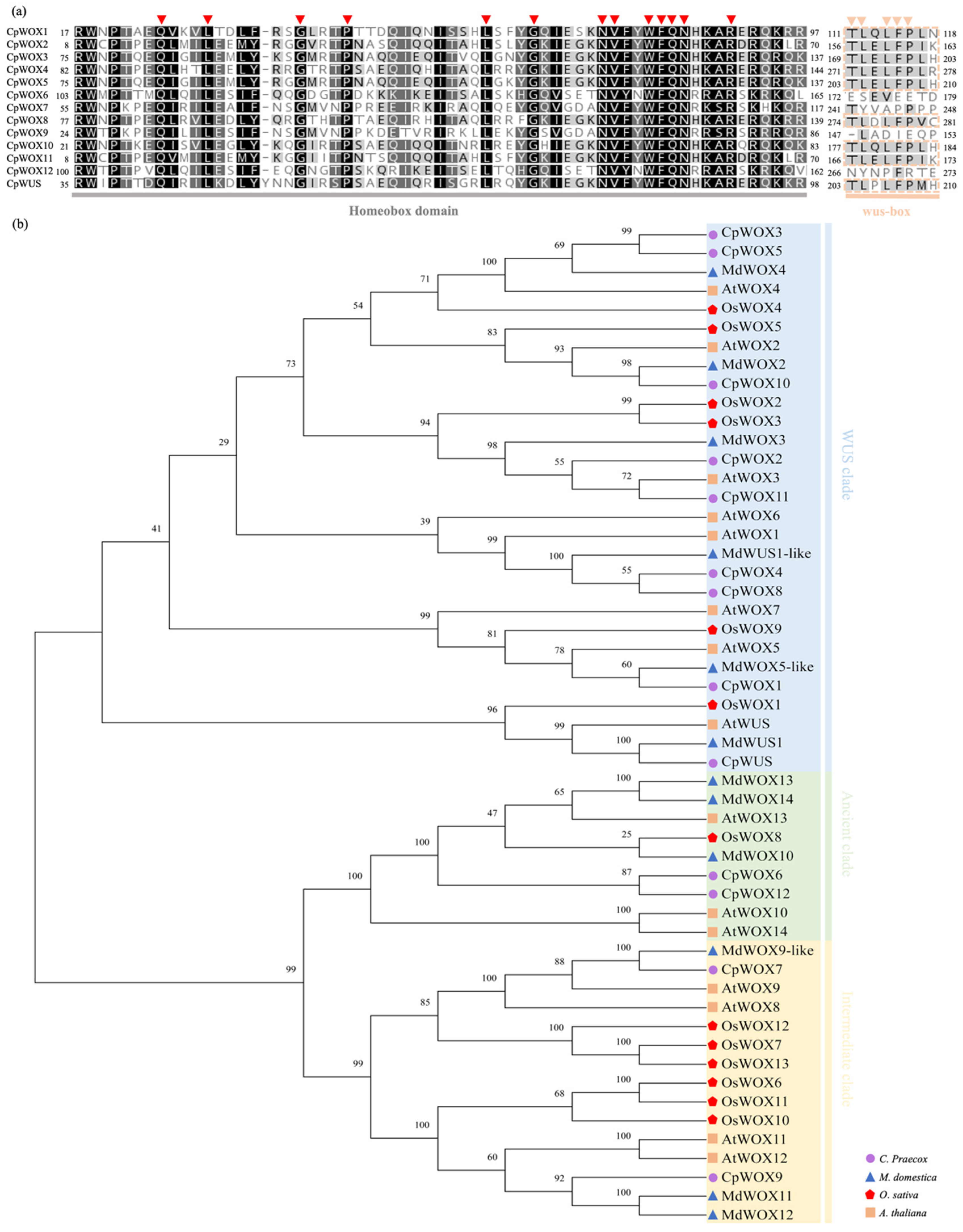
2.2. Multiple Sequence Alignment and Phylogenetic Tree Analysis of Wintersweet WOX Protein Family
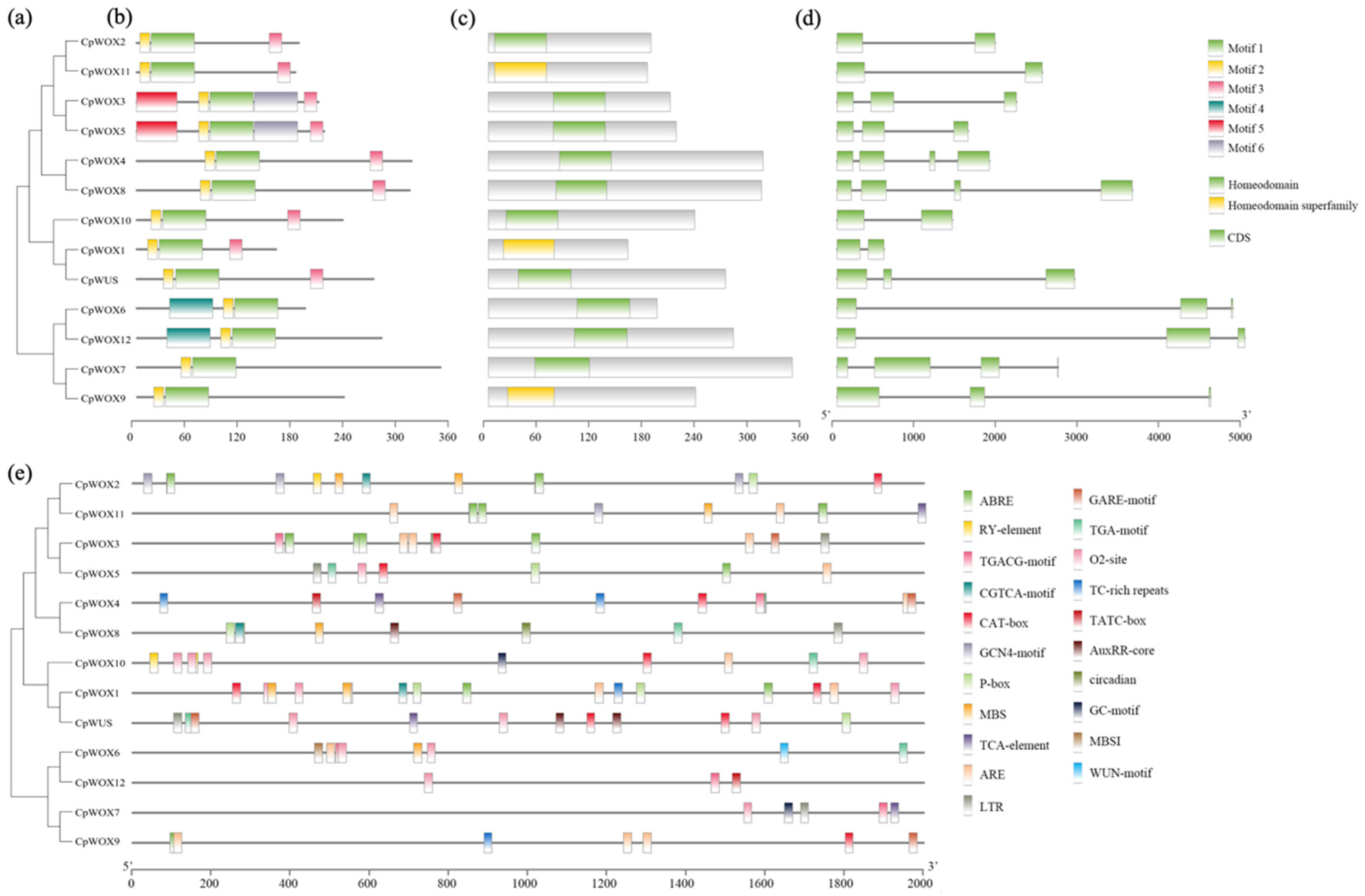
2.3. Conserved Motif, Domain, Gene Structure, and Promoter Cis-Acting Element Analysis of CpWOX
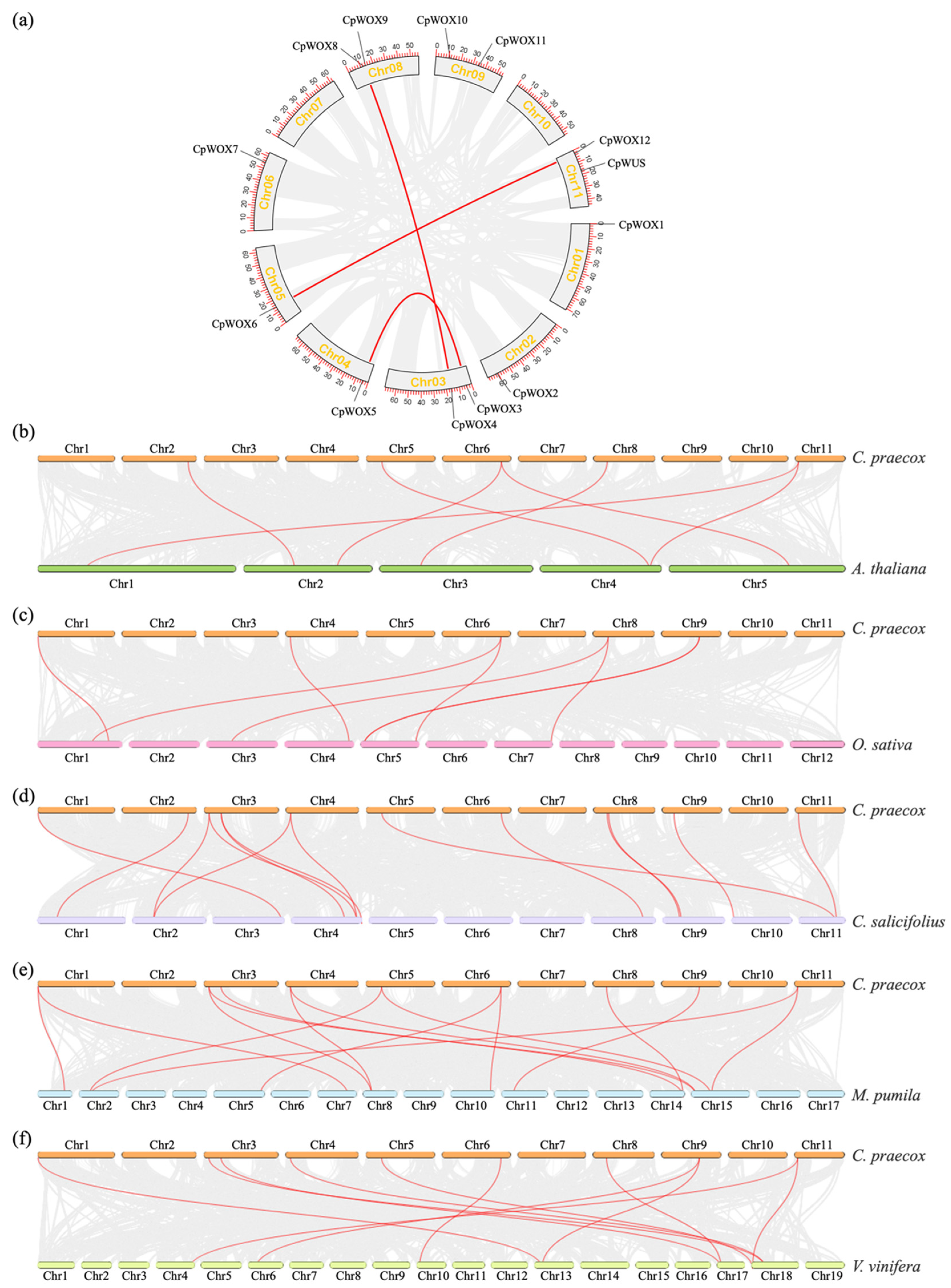
2.4. CpWOX Genes Localisation and Synteny Analysis
2.5. CpWUS Sequence Feature Analysis
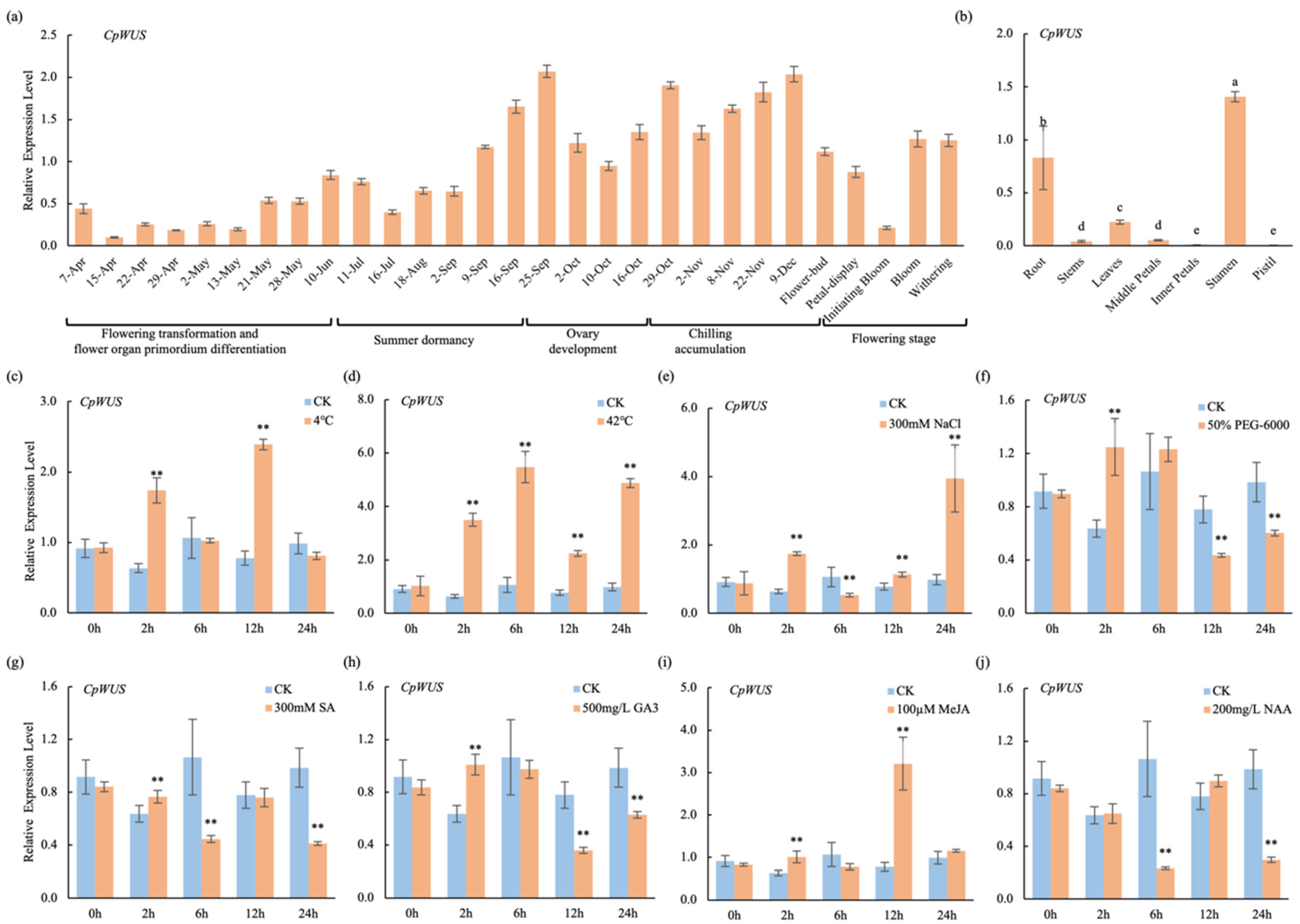
2.6. Expression Characteristics of CpWUS
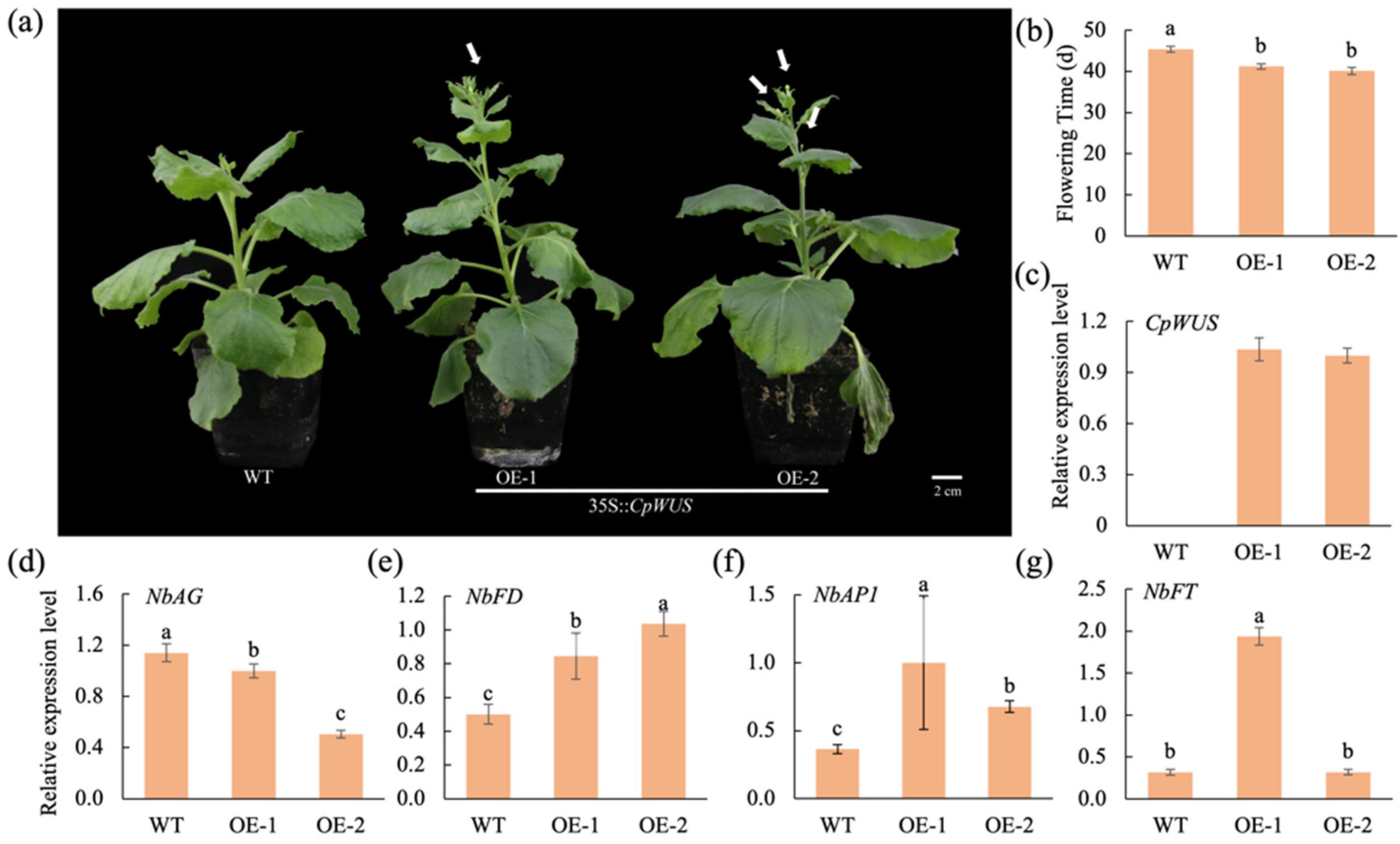
2.7. CpWUS Regulates Flowering Time in Nicotiana benthamiana
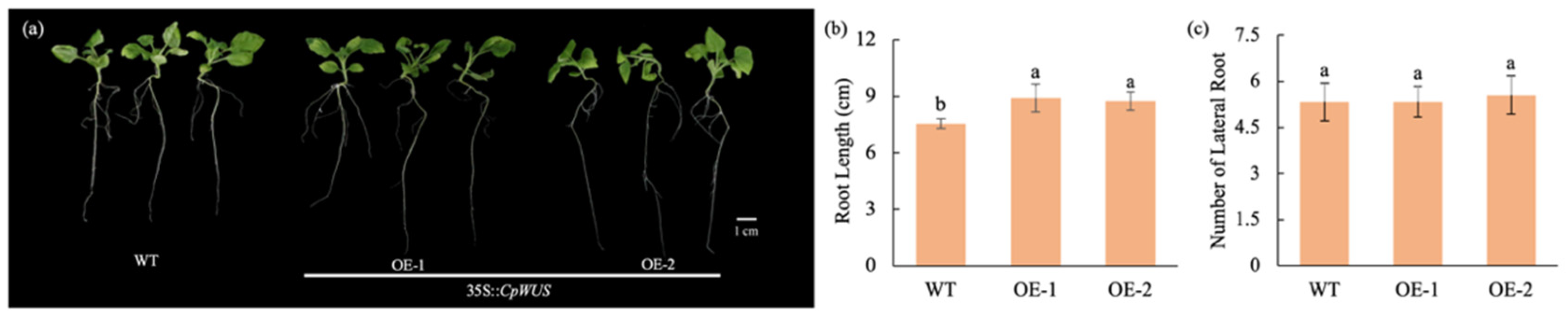
2.8. CpWUS Regulates Root Development in Nicotiana benthamian
3. Discussion
4. Materials and Methods
4.1. Identification of CpWOX Gene Family, Multiple Sequence Alignment, and Phylogenetic Tree Analysis
4.2. Gene Structure, Protein Structure, and Promoter Cis-Acting Element Analysis
4.3. Chromosomal Localisation and Synteny Analysis
4.4. Cloning of CpWOX
4.5. Transcriptional Self-Activation Activity and Subcellular Localisation
4.6. Gene Expression Analysis
4.7. Generation of Transgenic Tobacco
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gehring, W.J. Exploring the homeobox. Gene 1993, 135, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dolzblasz, A.; Nardmann, J.; Clerici, E.; Causier, B.; van der Graaff, E.; Chen, J.; Davies, B.; Werr, W.; Laux, T. Stem Cell Regulation by Arabidopsis WOX Genes. Mol. Plant 2016, 9, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Mayer, K.F.; Berger, J.; Jurgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Haecker, A.; Gross-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Xu, Y.; Lei, X.; Xie, Q.; Liu, Z.; Wang, Y.; Gao, C. Genome-wide identification of the WOX gene family in Populus davidiana×P.bolleana and functional analysis of PdbWOX4 in salt resistance. Plant Sci. 2025, 352, 112379. [Google Scholar] [CrossRef]
- Duan, L.; Hou, Z.; Zhang, W.; Liang, S.; Huangfu, M.; Zhang, J.; Yang, T.; Dong, J.; Che, D. Genome-wide analysis of the WOX gene family and function exploration of RhWOX331 in rose (R. ‘The Fairy’). Front. Plant Sci. 2024, 15, 1461322. [Google Scholar] [CrossRef]
- Shafique Khan, F.; Zeng, R.F.; Gan, Z.M.; Zhang, J.Z.; Hu, C.G. Genome-Wide Identification and Expression Profiling of the WOX Gene Family in Citrus sinensis and Functional Analysis of a CsWUS Member. Int. J. Mol. Sci. 2021, 22, 4919. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, J.; Liu, J.; Yin, J.; Zhang, D. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 2010, 52, 1016–1026. [Google Scholar] [CrossRef]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Burglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef]
- Kamiya, N.; Nagasaki, H.; Morikami, A.; Sato, Y.; Matsuoka, M. Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J. 2003, 35, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 2009, 21, 3493–3505. [Google Scholar] [CrossRef]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 2009, 19, 909–914. [Google Scholar] [CrossRef]
- Araki, T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 2001, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, Z.; Sun, B. KNUCKLES regulates floral meristem termination by controlling auxin distribution and cytokinin activity. Plant Cell 2024, 37, koae312. [Google Scholar] [CrossRef]
- Sablowski, R. Flowering and determinacy in Arabidopsis. J. Exp. Bot. 2007, 58, 899–907. [Google Scholar] [CrossRef]
- Costanzo, E.; Trehin, C.; Vandenbussche, M. The role of WOX genes in flower development. Ann. Bot. 2014, 114, 1545–1553. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Wang, X.; Li, C.; Ye, Z.; Zhang, J. UF, a WOX gene, regulates a novel phenotype of un-fused flower in tomato. Plant Sci. 2020, 297, 110523. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, A.; Han, H.; Li, T.; Geng, Y.; Liu, X.; Meyerowitz, E.M. HAIRY MERISTEM with WUSCHEL confines CLAVATA3 expression to the outer apical meristem layers. Science 2018, 361, 502–506. [Google Scholar] [CrossRef]
- Deyhle, F.; Sarkar, A.K.; Tucker, E.J.; Laux, T. WUSCHEL regulates cell differentiation during anther development. Dev. Biol. 2007, 302, 154–159. [Google Scholar] [CrossRef]
- Lenhard, M.; Bohnert, A.; Jürgens, G.; Laux, T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001, 105, 805–814. [Google Scholar] [CrossRef]
- Liu, X.; Kim, Y.J.; Muller, R.; Yumul, R.E.; Liu, C.; Pan, Y.; Cao, X.; Goodrich, J.; Chen, X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. Plant Cell 2011, 23, 3654–3670. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, Y.; Ng, K.H.; Ito, T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes. Dev. 2009, 23, 1791–1804. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Z.; Liu, X.; Che, G.; Gu, R.; Zhao, J.; Wang, Z.; Hou, Y.; Zhang, X. CsLFY is required for shoot meristem maintenance via interaction with WUSCHEL in cucumber (Cucumis sativus). New Phytol. 2018, 218, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, Y.; Toffano-Nioche, C.; Claisse, G.; Thareau, V.; Morin, H.; Laufs, P.; Moreau, H.; Kreis, M.; Lecharny, A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 2008, 8, 291. [Google Scholar] [CrossRef]
- Matsumoto, N.; Okada, K. A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of flowers. Gene Dev. 2001, 15, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, M.; Horstman, A.; Zethof, J.; Koes, R.; Rijpkema, A.S.; Gerats, T. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 2009, 21, 2269–2283. [Google Scholar] [CrossRef]
- Li, H.; Qi, M.; Sun, M.; Liu, Y.; Liu, Y.; Xu, T.; Li, Y.; Li, T. Tomato Transcription Factor SlWUS Plays an Important Role in Tomato Flower and Locule Development. Front. Plant Sci. 2017, 8, 457. [Google Scholar] [CrossRef]
- Niu, L.; Lin, H.; Zhang, F.; Watira, T.W.; Li, G.; Tang, Y.; Wen, J.; Ratet, P.; Mysore, K.S.; Tadege, M. LOOSE FLOWER, a WUSCHEL-like Homeobox gene, is required for lateral fusion of floral organs in Medicago truncatula. Plant J. 2015, 81, 480–492. [Google Scholar] [CrossRef]
- Souer, E.; van der Krol, A.; Kloos, D.; Spelt, C.; Bliek, M.; Mol, J.; Koes, R. Genetic control of branching pattern and floral identity during Petunia inflorescence development. Development 1998, 125, 733–742. [Google Scholar] [CrossRef]
- Kong, X.P.; Lu, S.C.; Tian, H.Y.; Ding, Z.J. WOX5 is Shining in the Root Stem Cell Niche. Trends Plant Sci. 2015, 20, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, S.L.; Zhang, Q.; Song, H.Z.; Zhou, D.X.; Zhao, Y. Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J. Exp. Bot. 2017, 68, 2787–2798. [Google Scholar] [CrossRef]
- Liu, J.C.; Sheng, L.H.; Xu, Y.Q.; Li, J.Q.; Yang, Z.N.; Huang, H.; Xu, L. WOX11 and 12 Are Involved in the First-Step Cell Fate Transition during de Novo Root Organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Garg, T.; Singh, Z.; Chennakesavulu, K.; Dwivedi, A.K.; Varapparambathu, V.; Singh, R.S.; Mushahary, K.K.K.; Yadav, M.; Sircar, D.; Chandran, D.; et al. Genome-Wide High Resolution Expression Map and Functions of Key Cell Fate Determinants Reveal the Dynamics of Crown Root Development in Rice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lou, X.; Wang, J.; Wang, G.; He, D.; Shang, W.; Song, Y.; Wang, Z.; He, S. Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii. Int. J. Mol. Sci. 2024, 25, 7668. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, L.; Zhang, J.; Li, J.; Zheng, H.; Chen, J.; Lu, M. WUSCHEL-related Homeobox genes in Populus tomentosa: Diversified expression patterns and a functional similarity in adventitious root formation. BMC Genomics 2014, 15, 296. [Google Scholar] [CrossRef]
- Shang, J.; Tian, J.; Cheng, H.; Yan, Q.; Li, L.; Jamal, A.; Xu, Z.; Xiang, L.; Saski, C.A.; Jin, S.; et al. The chromosome-level wintersweet (Chimonanthus praecox) genome provides insights into floral scent biosynthesis and flowering in winter. Genome Biol. 2020, 21, 200. [Google Scholar] [CrossRef]
- Li, Z.N.; Liu, N.; Zhang, W.; Wu, C.Y.; Jiang, Y.J.; Ma, J.; Li, M.Y.; Sui, S.Z. Integrated transcriptome and proteome analysis provides insight into chilling-induced dormancy breaking in. Hortic. Res. 2020, 7, 198. [Google Scholar] [CrossRef]
- Hou, H.F.; Tian, M.K.; Liu, N.; Huo, J.T.; Sui, S.Z.; Li, Z.N. Genome-wide analysis of MIKCC-type MADS-box genes and roles of CpFUL/SEP/AGL6 superclade in dormancy breaking and bud formation of Chimonanthus praecox. Plant Physiol. Biochem. 2023, 196, 893–902. [Google Scholar] [CrossRef]
- Wang, B.G.; Zhang, Q.; Wang, L.G.; Duan, K.; Pan, A.H.; Tang, X.M.; Sui, S.Z.; Li, M.Y. The AGL6-like Gene CpAGL6, a Potential Regulator of Floral Time and Organ Identity in Wintersweet (Chimonanthus praecox). J. Plant Growth Regul. 2011, 30, 343–352. [Google Scholar] [CrossRef]
- Lian, G.; Ding, Z.; Wang, Q.; Zhang, D.; Xu, J. Origins and evolution of WUSCHEL-related homeobox protein family in plant kingdom. Sci. World J. 2014, 2014, 534140. [Google Scholar] [CrossRef]
- Chukun, W.; Pengliang, H.; Yongmei, W.; Pengfei, W.; Dagang, H. Genome-wide Identification and Analysis of Apple WUSCHEL-related homeobox (WOX) Family Genes. Acta Hortic. Sin. 2019, 46, 1021–1032. [Google Scholar] [CrossRef]
- Katsir, L.; Davies, K.A.; Bergmann, D.C.; Laux, T. Peptide signaling in plant development. Curr. Biol. 2011, 21, R356–R364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Xia, Y.; Li, Z.; Jing, D.; Du, J.; Niu, N.; Ma, S.; Wang, J.; Song, Y.; et al. Identification of the WUSCHEL-Related Homeobox (WOX) Gene Family, and Interaction and Functional Analysis of TaWOX9 and TaWUS in Wheat. Int. J. Mol. Sci. 2020, 21, 1581. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, Q.; Qin, W.; Yang, Z.; Cheng, Y.; Lu, L.; Ge, X.; Zhang, C.; Wu, Z.; Li, F. Genome-wide analysis of WOX genes in upland cotton and their expression pattern under different stresses. BMC Plant Biol. 2017, 17, 113. [Google Scholar] [CrossRef]
- Xiaoxu, L.; Cun, G.; Wenxuan, P.; Wanfeng, L.; Yinxia, Z.; Nan, S.; Xinxi, H.; Cheng, L.; Liangtao, X.; Junping, G. Genome-wide identification and systemic analysis of WOX family genes in tobacco. Acta Tabacaria Sin. 2021, 27, 90–100. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, Z.; Xu, T.; Yao, S.; Zhang, M.; Cheng, X.; Zhao, Y.; Ji, K. Identification and Expression Patterns of WOX Transcription Factors under Abiotic Stresses in Pinus massoniana. Int. J. Mol. Sci. 2024, 25, 1627. [Google Scholar] [CrossRef]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef]
- Hao, Q.; Zhang, L.; Yang, Y.; Shan, Z.; Zhou, X.A. Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean. Plants 2019, 8, 215. [Google Scholar] [CrossRef]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Y.; Zhu, N.; Zhao, Y. The rice WUSCHEL-related homeobox genes are involved in reproductive organ development, hormone signaling and abiotic stress response. Gene 2014, 549, 266–274. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Sun, M.; Chen, S.; Ma, H.; Lin, J.; Sun, Y.; Zhong, M. Molecular characterization and gene expression analysis of tomato WOX transcription factor family under abiotic stress and phytohormone treatment. J. Plant Biochem. Biotechnol. 2021, 30, 973–986. [Google Scholar] [CrossRef]
- Lv, J.; Feng, Y.; Jiang, L.; Zhang, G.; Wu, T.; Zhang, X.; Xu, X.; Wang, Y.; Han, Z. Genome-wide identification of WOX family members in nine Rosaceae species and a functional analysis of MdWOX13-1 in drought resistance. Plant Sci. 2023, 328, 111564. [Google Scholar] [CrossRef]
- Cao, X.; He, Z.; Guo, L.; Liu, X. Epigenetic Mechanisms Are Critical for the Regulation of WUSCHEL Expression in Floral Meristems. Plant Physiol. 2015, 168, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jurgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wang, X.M.; Li, J.; Li, J.H.; Wu, J.S.; Walker, J.C.; Xu, Z.H.; Chong, K. Activation of the WUS gene induces ectopic initiation of floral meristems on mature stem surface in Arabidopsis thaliana. Plant Mol. Biol. 2005, 57, 773–784. [Google Scholar] [CrossRef]
- Pelayo, M.A.; Yamaguchi, N.; Ito, T. One factor, many systems: The floral homeotic protein AGAMOUS and its epigenetic regulatory mechanisms. Curr. Opin. Plant Biol. 2021, 61, 102009. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Yuan, C.; Han, Y.; Zheng, T.; Cheng, T.; Wang, J.; Zhang, Q. Interactions between WUSCHEL- and CYC2-like Transcription Factors in Regulating the Development of Reproductive Organs in Chrysanthemum morifolium. Int. J. Mol. Sci. 2019, 20, 1276. [Google Scholar] [CrossRef]
- Shiping, Y. Cloning and Functional Analysis of MdWUS Gene of Apple. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, W.; Fu, X.; Guo, C.; Cai, Y.; Huang, S.; Chen, L.; Yang, N. Screening and Verification of Reference Genes of WinterSweet (Chimonanthus praecox L.) in Real-time Quantitative PCR Analysis. Mol. Plant Breed. 2022, 1–14. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Sequence ID | Gene Name | Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| Cpra01G00013.1 | CpWOX1 | 159 | 18669.12 | 8.62 | 56.15 | 71.7 | −0.798 | Nucleus |
| Cpra02G01741.1 | CpWOX2 | 185 | 21441.46 | 9.05 | 54.1 | 55.35 | −0.839 | Nucleus |
| Cpra03G00351.1 | CpWOX3 | 207 | 23340.11 | 6.66 | 49.71 | 65.99 | −0.821 | Nucleus |
| Cpra03G01103.1 | CpWOX4 | 313 | 35020.28 | 8.93 | 63.2 | 61.47 | −0.583 | Nucleus |
| Cpra04G00358.1 | CpWOX5 | 214 | 24178.15 | 9.13 | 54.81 | 60.19 | −0.943 | Nucleus |
| Cpra05G00919.1 | CpWOX6 | 192 | 21821.53 | 5.79 | 62.76 | 66.51 | −0.827 | Nucleus |
| Cpra06G01843.1 | CpWOX7 | 346 | 37937.55 | 6.51 | 61.19 | 66.24 | −0.539 | Nucleus |
| Cpra08G00857.1 | CpWOX8 | 311 | 34981.89 | 8.48 | 67.5 | 60.29 | −0.727 | Nucleus |
| Cpra08G00936.1 | CpWOX9 | 236 | 26214.51 | 5.25 | 76.21 | 76.78 | −0.424 | Nucleus |
| Cpra09G00234.1 | CpWOX10 | 235 | 26738.95 | 6.97 | 64.77 | 58.81 | −0.762 | Nucleus |
| Cpra09G00715.1 | CpWOX11 | 181 | 20892.75 | 8.52 | 54.47 | 56.08 | −0.851 | Nucleus |
| Cpra11G00217.1 | CpWOX12 | 279 | 31555.08 | 5.68 | 65.45 | 60.47 | −0.92 | Nucleus |
| Cpra11G00833.1 | CpWUS | 270 | 30006.96 | 5.86 | 54.08 | 49.11 | −0.857 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, B.; Cao, Y.; Ma, G.; Zheng, X.; Zhu, H.; Sui, S. Genome-Wide Identification of WOX Gene Family in Chimonanthus praecox and a Functional Analysis of CpWUS. Plants 2025, 14, 1144. https://doi.org/10.3390/plants14071144
Wu H, Liu B, Cao Y, Ma G, Zheng X, Zhu H, Sui S. Genome-Wide Identification of WOX Gene Family in Chimonanthus praecox and a Functional Analysis of CpWUS. Plants. 2025; 14(7):1144. https://doi.org/10.3390/plants14071144
Chicago/Turabian StyleWu, Huafeng, Bin Liu, Yinzhu Cao, Guanpeng Ma, Xiaowen Zheng, Haoxiang Zhu, and Shunzhao Sui. 2025. "Genome-Wide Identification of WOX Gene Family in Chimonanthus praecox and a Functional Analysis of CpWUS" Plants 14, no. 7: 1144. https://doi.org/10.3390/plants14071144
APA StyleWu, H., Liu, B., Cao, Y., Ma, G., Zheng, X., Zhu, H., & Sui, S. (2025). Genome-Wide Identification of WOX Gene Family in Chimonanthus praecox and a Functional Analysis of CpWUS. Plants, 14(7), 1144. https://doi.org/10.3390/plants14071144






