Chemical Profile Analysis of Prosopis laevigata Extracts and Their Topical Anti-Inflammatory and Antibacterial Activities
Abstract
1. Introduction
2. Results
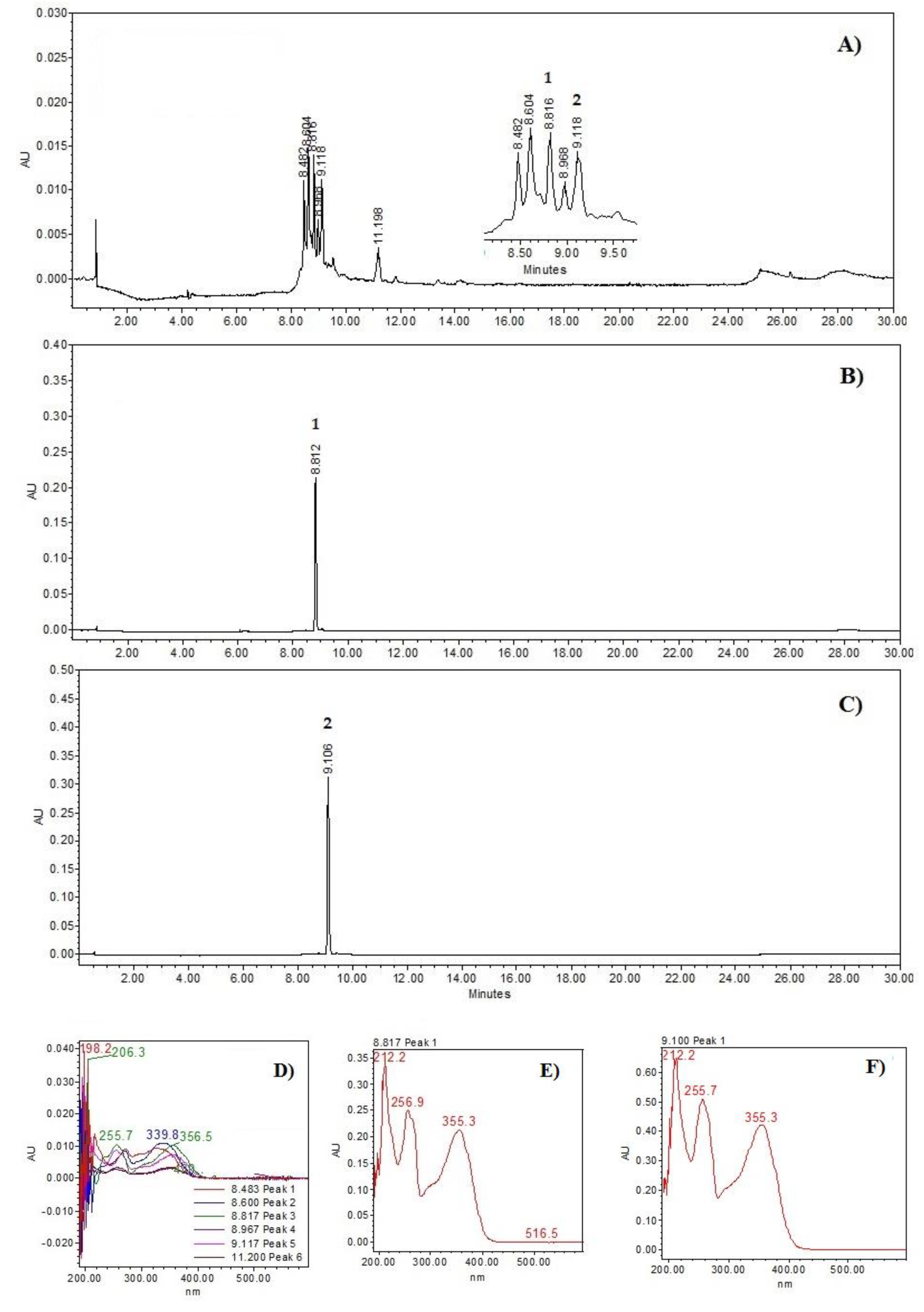
2.1. High-Performance Liquid Chromatography (HPLC) Analysis of the PM Extract
2.2. The Gas Chromatography–Mass Spectrometry (GS-MS) Profiles of the PH and PD Extracts
2.3. Anti-Inflammatory Effect of the P. laevigata Extracts
Structural Elucidation of Compound (1)
| Position |
δ1H (δ in ppm, J in Hz) 1 |
δ13C (HSQC) 1 | HMBC (J2–3) |
|---|---|---|---|
| 1 | 166.2 | 7.57, 7.45, 6.77, 4.26 | |
| 2 | 122.9 | ||
| 3 | 7.45 (1H, d, 2.0) | 110.1 | 123.3, 148.5, 152.8, 166.5 |
| 4 | 148.5 | ||
| 5 | 152.8 | ||
| 6 | 6.77 (1H, d, 8.9) | 111.8 | 122.9, 148.5, 152.8 |
| 7 | 7.57 (1H, dd, 2.0, 8.9) | 123.3 | 111.8, 148.5, 152.8, 166.2 |
| 1′ | 4.25 (2H, q, 7.5) | 60.6 | 14.2, 166.2 |
| 2′ | 1.29 (3H, t, 6.8) | 14.2 | 60.6 |
| OCH3 | 3.83, s | 55.8 | 152.8 |
| OCH3 | 3.82, s | 55.8 | 148.5 |

2.4. Anti-Inflammatory Effect of PD Extract Fractions and Compound (1)
Quantification of Pro-Inflammatory the Cytokines IL-10 and TNF-α
2.5. Antibacterial Activity
2.6. Antibacterial Activity of Fractions and Compound 1
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. HPLC–Photo Diode Array (PDA) Analysis of the PM Extract
4.4. Identification by GS-MS
4.5. Chromatographic Fractionation of the PD Extract
4.6. Chromatographic Separation of the PDR7 Fraction to Obtain VE (1)
4.7. Chromatographic Fractionation of the PM Extract
4.8. Determination of the Anti-Inflammatory Activity
4.8.1. Experimental Animals
4.8.2. Mouse TPA-Induced Ear Oedema Model
4.8.3. Quantification of the Pro-Inflammatory the Cytokines IL-10 and TNF-α
4.9. Antimicrobial Activity
4.9.1. Microorganisms
4.9.2. MIC
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drugs development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public. Health 2024, 2, 100081. [Google Scholar]
- Pham, M.N.; Nishimura, F.; Wei-Lan, J.C.; Shiong-Khoo, K. Recent advancement of eliminating antibiotic bacteria and antibiotic resistance genes in livestock waste: A review. Environ. Technol. Innov. 2024, 36, 103751. [Google Scholar]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [PubMed]
- Macias, J.; Kahly, O.; Pattik-Edward, R.; Khan, S.; Qureshi, A.; Shaik, A.; Shala, A.; Shah, D. Sepsis: A systematic review of antibiotic resistance and antimicrobial therapies. Mod. Res. Inflamm. 2022, 11, 9–23. [Google Scholar]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. In Review on Antimicrobial Resistance; Wellcome Trust & HM Government: London, UK, 2016; pp. 10–16. [Google Scholar]
- Rojas-Jiménez, S.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Pérez-Ramos, J.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Campos-Xolalpa, N. Anti-inflammatory and cytotoxic compounds isolated from plants of Euphorbia genus. Molecules 2024, 29, 1083. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar]
- Matotoka, M.M.; Mashabela, G.T.; Masoko, P. Phytochemical content, antibacterial activity, and antioxidant, anti-Inflammatory, and cytotoxic effects of traditional medicinal plants against respiratory tract bacterial pathogens. Evid. Based Complement. Alternat Med. 2023, 2023, 1243438. [Google Scholar]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- El-Saber-Batiha, G.; Magdy-Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd-El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad-Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green tea (Camellia sinensis): A review of its phytochemistry, pharmacology, and toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Sha, Z.; Chen, X.; Zhang, W.; Shi, L.Y. Rosmarinic acid bolsters antibacterial immunity activity of macrophages by up-regulating PINK1/Parkin-mediated mitophagy. Zhongguo Zhong Yao Za Zhi 2022, 47, 6450–6456. [Google Scholar] [PubMed]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A critical review of Ginger’s (Zingiber officinale) antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar]
- Henciya, S.; Seturaman, P.; James, R.A.; Tsai, Y.; Nikam, R.; Wu, Y.; Dahms, H.; Chang, F.R. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J. Food Drug Anal. 2017, 25, 187–196. [Google Scholar]
- Hernández-Ruiz, J.; Aguilar-Marcelino, L.; Marín, J.A.H.; Avalos, A.M.C.; Huerta, I.A.; Arriaga, A.I.M. Use of Prosopis on feed nutrition: Challenges and opportunities. In Complementary and Alternative Medicine: Feed Additives; Abbas, R.Z., Akhtar, T., Asrar, R., Khan, A.M.A., Saeed, Z., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 163–170. [Google Scholar]
- Pasiecznik, N.M. Prosopis: Pest or providence, weed or wonder tree? Eur. Trop. For. Res. Netw. Newslett 1999, 28, 12e4. [Google Scholar]
- Argueta Villamar, A.; Cano Asseleih, L.M.; Rodarte, M.L. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Mexico City, Mexico, 1994. [Google Scholar]
- Nava-Solis, U.; Rodriguez-Canales, M.; Hernandez-Hernandez, A.B.; Velasco-Melgoza, D.A.; Moreno-Guzman, B.P.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Antimicrobial activity of the methanolic leaf extract of Prosopis laevigata. Sci. Rep. 2022, 12, 20807. [Google Scholar]
- Salinas-Sánchez, D.O.; Arteaga-Najera, G.L.; León-Rivera, I.; Dorado-Ramírez, O.; Valladares-Cisneros, M.G.; Navarro-García, V.M. Antimicrobial activity of medicinal plants from the Huautla Sierra Biosphere Reserve in Morelos (Mexico). Polibotánica 2009, 28, 1405–2768. [Google Scholar]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A nematocidal flavonoid from Prosopis laevigata leaves against Haemonchus contortus eggs and larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef]
- Ramirez-Arteaga, M.; Valladares, M.G.; González-Rodríguez, J.G. Use of Prosopis laevigata as a corrosion inhibitor for al in H2SO4. Int. J. Electrochem. Sci. 2013, 8, 6864–6877. [Google Scholar]
- Matta, D.; Nanda, H.; Mahalingam, G. Phytopharmaceutical potentials of Prosopis laevigata, Symplocos cochinchinensis and Nymphacea alba: A review. Asian J. Pharm. Clin. Res. 2017, 10, 63–68. [Google Scholar]
- Palacios, R.A. Los mezquites mexicanos: Biodiversidad y distribución geográfica; Sociedad Argentina de Botánica. Bol. Soc. Argent. Bótanica 2006, 41, 99–121. [Google Scholar]
- Galindo-Almanza, S.; García-Moya, E. Usos del mezquite (Prosopis L.) en el Altiplano Potosino. Agrociencia 1986, 63, 7–16. [Google Scholar]
- Hernández, T.; Canales, M.; Avila, J.G.; Duran, A.; Caballero, J.; De Vivar, A.R.; Lira, R. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México). J. Ethnopharmacol. 2003, 88, 181–188. [Google Scholar] [PubMed]
- García-Andrade, M.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Rosales-Castro, M.; Medina-Torres, L. Mesquite leaves (Prosopis laevigata), a natural resource with antioxidant capacity and cardioprotection potential. Ind. Crops Prod. 2017, 44, 336–342. [Google Scholar]
- You, B.R.; Yoo, J.M.; Baek, S.Y.; Kim, M.R. Anti-inflammatory effect of aged black garlic on 12-O-tetradecanoylphorbol-13-acetate-induced dermatitis in mice. Nutr. Res. Pract. 2019, 13, 189–195. [Google Scholar]
- Nakamura, A.; Uratsuji, H.; Yamada, Y.; Hashimoto, K.; Nozawa, N.; Matsumoto, T. Anti-inflammatory effect of lanoconazole on 12-O-tetradecanoylphorbol-13-acetate-and 2, 4, 6-trinitrophenyl chloride–induced skin inflammation in mice. Mycoses 2020, 63, 189–196. [Google Scholar]
- Murakawa, M.; Yamaoka, K.; Tanaka, Y.; Fukuda, Y. Involvement of tumor necrosis factor (TNF)-α in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem. Pharmacol. 2006, 71, 1331–1336. [Google Scholar]
- Darwish, S.R.; Fares, M.; Hammada, H.; Yassin, A.; Ghareeb, D.; Harraz, F.; Shawky, E. Comparative metabolomics applied for valorization of Mesquite (Prosopis juliflora Sw. DC.) by-products as potential source of anti-inflammatory functional constituents. Ind. Crops Prod. 2022, 176, 114344. [Google Scholar]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.B.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.A.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar]
- Pankaj, H.N.; Narendra, D.P.; Dhananjay, V.M. Ethanopharmacological anti-inflammatory study of phytol in ethanol extract of Woodfordia floribunda Salisb. Ann. Phytomedicine 2022, 11, 426–437. [Google Scholar]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Quintans-Júnior, L.J. Phytol, a chlorophyll component, produces antihyperalgesic, anti-inflammatory, and antiarthritic effects: Possible NFκB pathway involvement and reduced levels of the proinflammatory cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [PubMed]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginet, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Wei, W.C.; Lin, S.Y.; Chen, Y.J.; Wen, C.C.; Huang, C.Y.; Palanisamy, A.; Yang, N.S.; Sheu, J.H. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J. Biomed. Sci. 2011, 18, 94. [Google Scholar]
- Ekinci, B.; Süleyman, B.; Mammadov, R.; Bulut, S.; Özçiçek, A.; Ergul, C.; Süleyman, H. Beneficial interaction of pycnogenol with indomethacin in rats. Gen. Physiol. Biophys. 2022, 41, 473–481. [Google Scholar] [PubMed]
- Cattaneo, F.; Sayago, J.E.; Alberto, M.R.; Zampini, I.C.; Ordoñez, R.M.; Chamorro, V.; Isla, M.I. Anti-inflammatory and antioxidant activities, functional properties and mutagenicity studies of protein and protein hydrolysate obtained from Prosopis alba seed flour. Food Chem. 2014, 161, 391–399. [Google Scholar]
- Saánchez, E. Antibacterial and antibiofilm activity of methanolic plant extracts against nosocomial microorganisms. Evid. Based Complement. Altern. Med. 2016, 2016, 1572697. [Google Scholar]
- Khan, R.; Zakir, M.; Afaq, S.H.; Latif, A.; Khan, A.U. Activity of solvent extracts of Prosopis spicigera, Zingiber officinale and Trachyspermum ammi against multidrug resistant bacterial and fungal strains. J. Infect. Dev. Ctries. 2010, 4, 292–300. [Google Scholar]
- Raghavendra, M.; Satish, S.; Raveesha, K. Alkaloid extracts of Prosopis juliflora (Sw) DC. (Mimosaceae) against Alternaria alternata. Biopestic. Int. 2009, 2, 56–59. [Google Scholar]
- Lee, W.; Woo, E.R.; Lee, D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar]
- Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Pérez, S.; Jiménez-Ferrer, E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299. [Google Scholar] [CrossRef]
- Payá, M.; Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Blasco, R.; Ríos, J.L.; Alcaráz, M.J. Study of the antioedema activity of some seaweed and sponge extracts from the Mediterranean coast in mice. Phytother. Res. 2010, 7, 159–162. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [PubMed]


| Peak No | Name | Retention Time (min) | Relative Area (%) | Mw (g/mol) | Type of Compound |
|---|---|---|---|---|---|
| 1 | 1,54-Dibromotetrapentacontane | 21.37 | 4.27 | 917.2 | Alkane |
| 2 | Hexanedioic acid | 22.22 | 5.64 | 370.6 | Fatty acid |
| 3 | Tetracontane | 23.13 | 3.47 | 563.1 | Alkane |
| 4 | 6,6′-di-tert-butyl-4,4′-diethyl-2,2′-methylenediphenol | 23.36 | 9.64 | 368.55 | Alkylated phenol |
| 5 | Heptacosane | 24.24 | 2.72 | 380.7 | Alkane |
| 6 | Tetratetracontane | 25.64 | 3.68 | 619.2 | Alkane |
| 7 | Squalene | 27.89 | 1.48 | 410.73 | Triterpene |
| Peak No. | Name | Retention Time (min) | Relative Area (%) | Mw (g/mol) | Type of Compound |
|---|---|---|---|---|---|
| 1 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | 13.77 | 12.87 | 196.24 | Benzofuran |
| 2 | Phytol | 19.77 | 70.11 | 296.53 | Diterpene alcohol |
| 3 | Bis[(2S)-2-ethylhexyl] hexanedioate | 22.22 | 17.0 | 370.6 | Fatty acid |
| Treatment | Oedema (mg) | Oedema Inhibition (%) |
|---|---|---|
| VEH | 11.43 ± 0.87 | – |
| INDO | 4.00 ± 1.40 * | 65.01 |
| PH | 4.48 ± 1.83 * | 60.81 |
| PD | 2.74 ± 0.68* | 75.96 |
| PM | 4.54 ± 1.41 * | 60.29 |
| Treatment | Oedema (mg) | Oedema Inhibition (%) |
|---|---|---|
| VEH | 12.78 ± 2.64 | – |
| INDO | 4.00 ± 1.40 * | 65.01 |
| PDR3 | 5.55 ± 2.03 * | 56.58 |
| PDR6 | 4.41 ± 2.55 * | 65.45 |
| PDR7 | 6.23 ± 1.70 * | 51.23 |
| VE (1) | 1.90 ± 0.63 * | 85.13 |
| Bacterial Clinical Isolate | Minimum Inhibitory Concentration (μg/mL) | ||
|---|---|---|---|
| PH | PD | PM | |
| Staphylococcus aureus ATCC 29213 | 200 | 200 | 6.25 |
| Methicillin-resistant Staphylococcus aureus ATCC 43300 | 200 | 12.5 | 6.25 |
| Staphylococcus epidermidis ATCC 35984 | 200 | 12.5 | 6.25 |
| Staphylococcus epidermidis ATCC 12228 | 50 | 6.25 | 6.25 |
| Staphylococcus epidermidis ATCC 49134 | - | 200 | 6.25 |
| Staphylococcus haemolyticus derived from ATCC 29970 | - | 200 | 6.25 |
| Enterococcus faecalis ATCC 29212 | 6.25 | 6.25 | 6.25 |
| Klebsiella pneumoniae ATCC 700603 | 6.25 | 12.5 | 6.25 |
| Pseudomonas aeruginosa ATCC 27853 | 6.25 | 12.5 | 6.25 |
| Escherichia coli ATCC 8739 | - | - | 12.5 |
| Escherichia coli ATCC 25922 | 6.25 | 25 | 12.5 |
| Salmonella dublin ATCC 9676 | 6.25 | 6.25 | 6.25 |
| Enterobacter cloacae ATCC 700323 | 12.5 | 12.5 | 12.5 |
| Candida albicans ATCC 10231 | 6.25 | 6.25 | 6.25 |
| Bacterial Clinical Isolate | Minimum Inhibitory Concentration (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Fractions | |||||||
| PMR2 | PMR5 | PMR6 | PMR7 | PMR10 | PDR7 | 1 | |
| Staphylococcus aureus ATCC 29213 | - | - | 6.25 | 25 | 6.25 | - | - |
| Methicillin-resistant Staphylococcus aureus ATCC 43300 | - | - | 6.25 | 25 | 12.5 | 100 | - |
| Staphylococcus epidermidis ATCC 35984 | 25 | - | 6.25 | 6.25 | 6.25 | 25 | - |
| Staphylococcus epidermidis ATCC 12228 | 200 | - | 6.25 | 25 | 25 | - | - |
| Staphylococcus epidermidis ATCC 49134 | - | - | 6.25 | 12.5 | 12.5 | - | - |
| Staphylococcus haemolyticus derived from ATCC 29970 | - | - | 6.25 | 12.5 | 12.5 | - | - |
| Enterococcus faecalis ATCC 29212 | 25 | 25 | 6.25 | 6.25 | 50 | 100 | - |
| Klebsiella pneumoniae ATCC 700603 | 25 | - | 6.25 | 6.25 | 6.25 | - | 2 |
| Pseudomonas aeruginosa ATCC 27853 | 25 | 25 | 6.25 | 25 | 6.25 | 100 | 8 |
| Escherichia coli ATCC 8739 | - | - | 25 | 200 | 50 | 200 | - |
| Escherichia coli ATCC 25922 | 25 | 25 | 12.5 | 25 | 25 | - | 8 |
| Salmonella dublin ATCC 9676 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 25 | 2 |
| Enterobacter cloacae ATCC 700323 | 12.5 | 12.5 | 12.5 | 12.5 | 12.25 | 25 | - |
| Candida albicans ATCC 10231 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cortazar, M.; Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Hernández-Hernández, P.; Zamilpa, A.; Jiménez-Ferrer, E.; Utrera-Hernández, B.E.; Pérez-García, M.D.; Gutiérrez-Roman, A.S.; Ble-González, E.A. Chemical Profile Analysis of Prosopis laevigata Extracts and Their Topical Anti-Inflammatory and Antibacterial Activities. Plants 2025, 14, 1118. https://doi.org/10.3390/plants14071118
González-Cortazar M, Salinas-Sánchez DO, Herrera-Ruiz M, Hernández-Hernández P, Zamilpa A, Jiménez-Ferrer E, Utrera-Hernández BE, Pérez-García MD, Gutiérrez-Roman AS, Ble-González EA. Chemical Profile Analysis of Prosopis laevigata Extracts and Their Topical Anti-Inflammatory and Antibacterial Activities. Plants. 2025; 14(7):1118. https://doi.org/10.3390/plants14071118
Chicago/Turabian StyleGonzález-Cortazar, Manasés, David Osvaldo Salinas-Sánchez, Maribel Herrera-Ruiz, Paulina Hernández-Hernández, Alejandro Zamilpa, Enrique Jiménez-Ferrer, Beatriz E. Utrera-Hernández, Ma. Dolores Pérez-García, Ana S. Gutiérrez-Roman, and Ever A. Ble-González. 2025. "Chemical Profile Analysis of Prosopis laevigata Extracts and Their Topical Anti-Inflammatory and Antibacterial Activities" Plants 14, no. 7: 1118. https://doi.org/10.3390/plants14071118
APA StyleGonzález-Cortazar, M., Salinas-Sánchez, D. O., Herrera-Ruiz, M., Hernández-Hernández, P., Zamilpa, A., Jiménez-Ferrer, E., Utrera-Hernández, B. E., Pérez-García, M. D., Gutiérrez-Roman, A. S., & Ble-González, E. A. (2025). Chemical Profile Analysis of Prosopis laevigata Extracts and Their Topical Anti-Inflammatory and Antibacterial Activities. Plants, 14(7), 1118. https://doi.org/10.3390/plants14071118











