Fertilization Strategies in Huanglongbing-Infected Citrus latifolia and Their Physiological and Hormonal Effects
Abstract
1. Introduction
2. Results
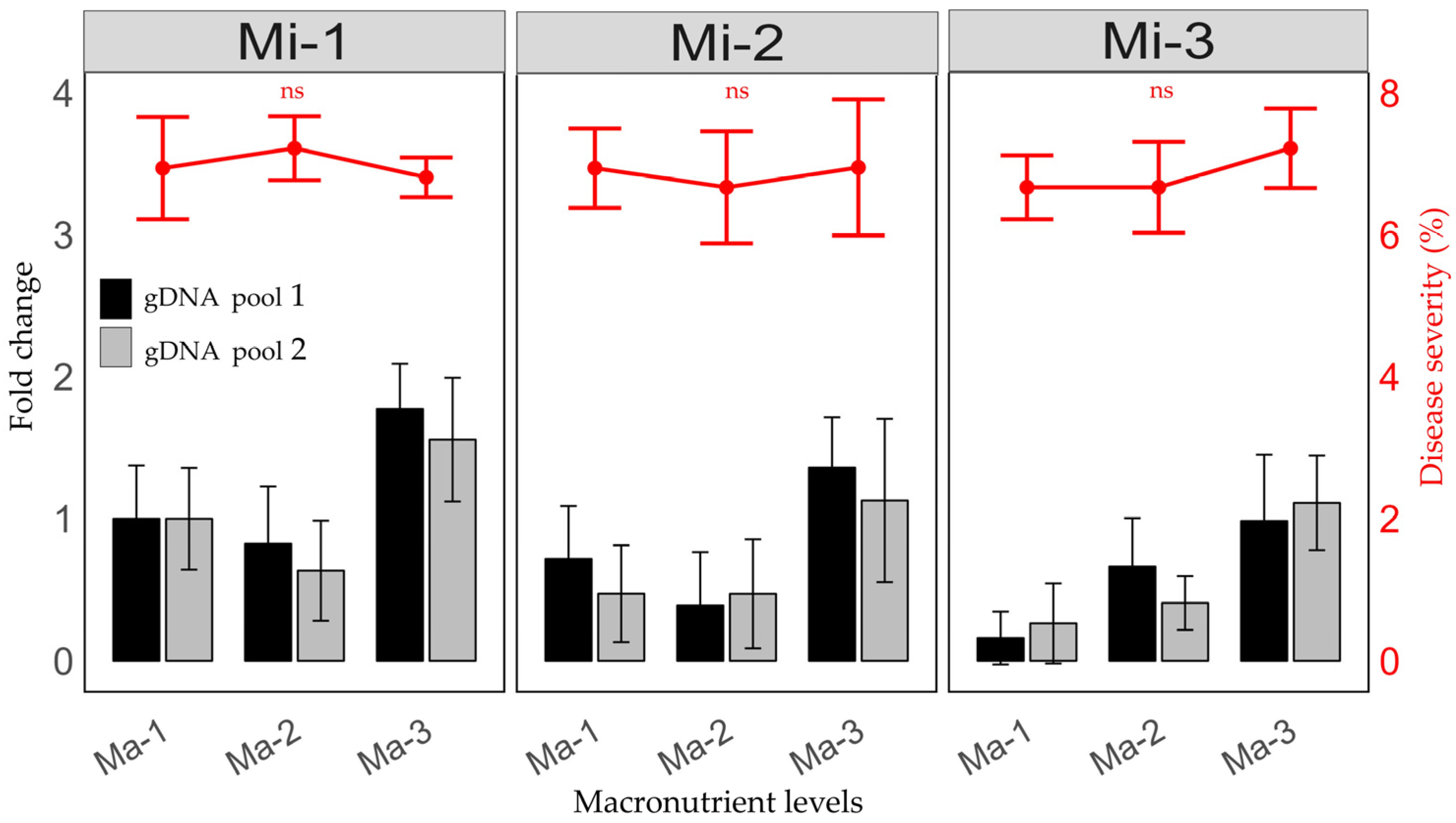
2.1. Relative Quantification of CLas 16S rDNA Gene After Fertilization Treatments
2.2. Fertilization and the Percentage of Severity of HLB Symptoms in Leaves
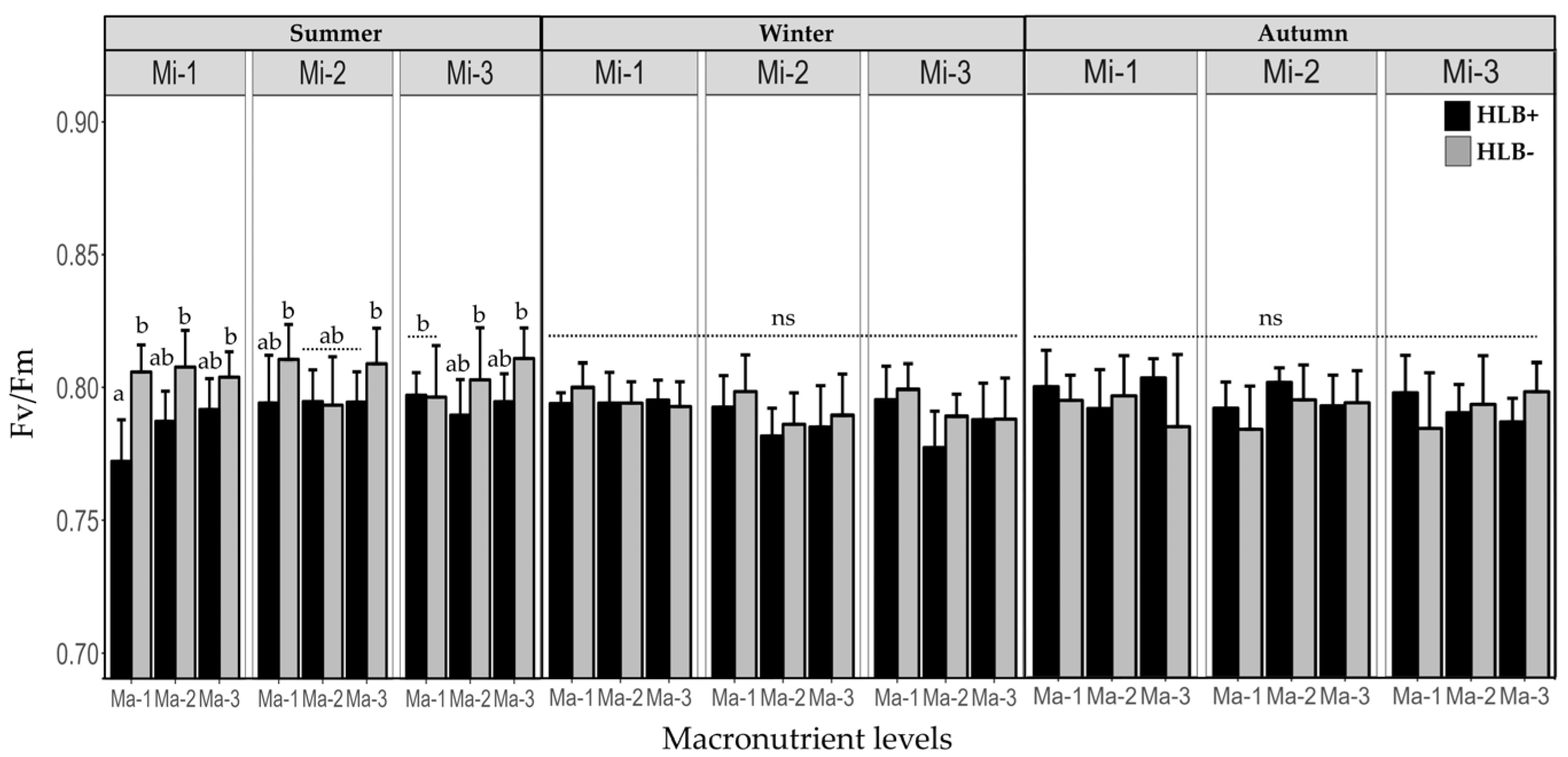
2.3. Fertilization on the Efficiency of Photosystem II (Fv/Fm) in Healthy and Diseased Plants
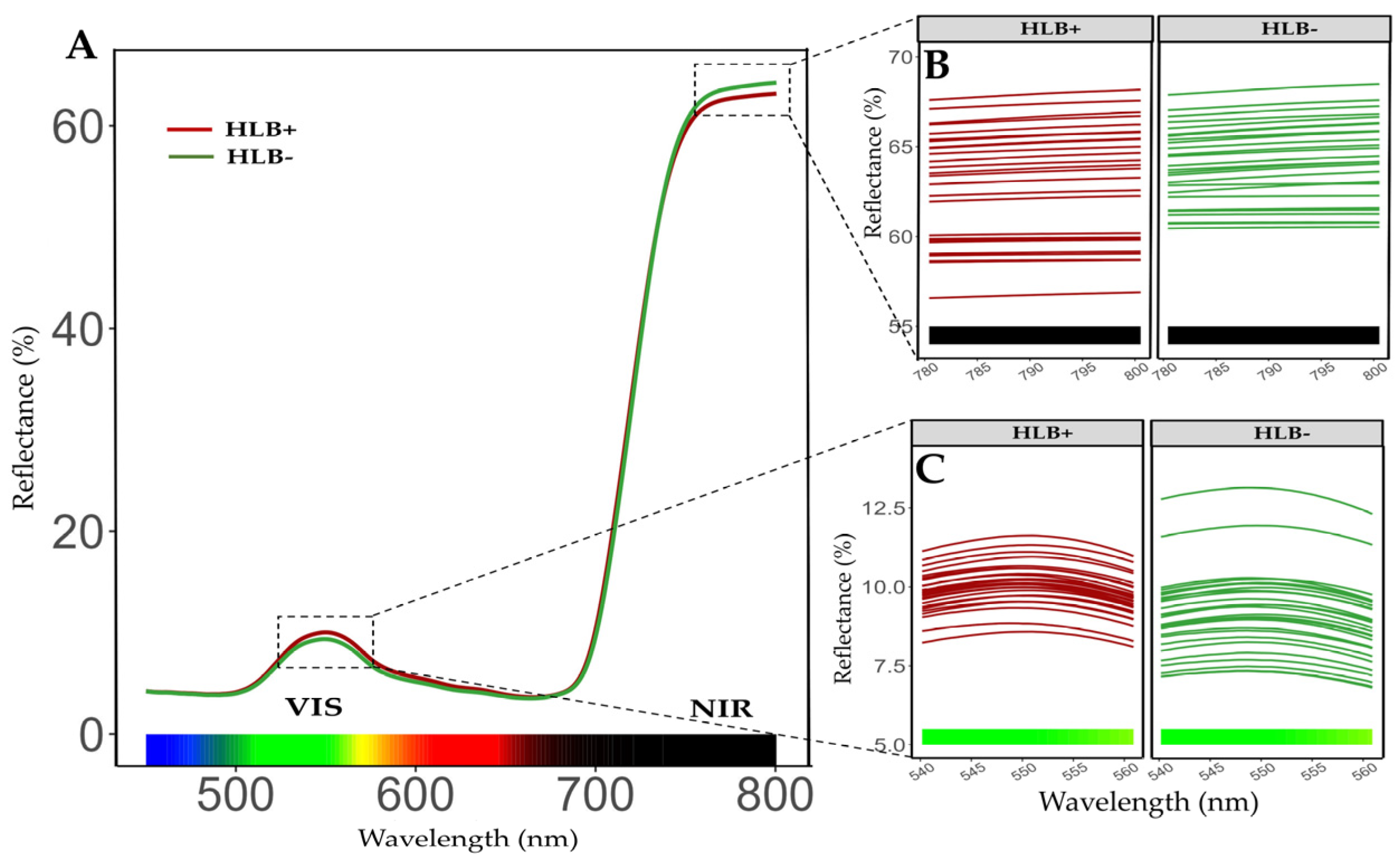
2.4. Reflectance Spectrum on Leaves of Healthy and HLB-Diseased Plants After Fertilization Treatments
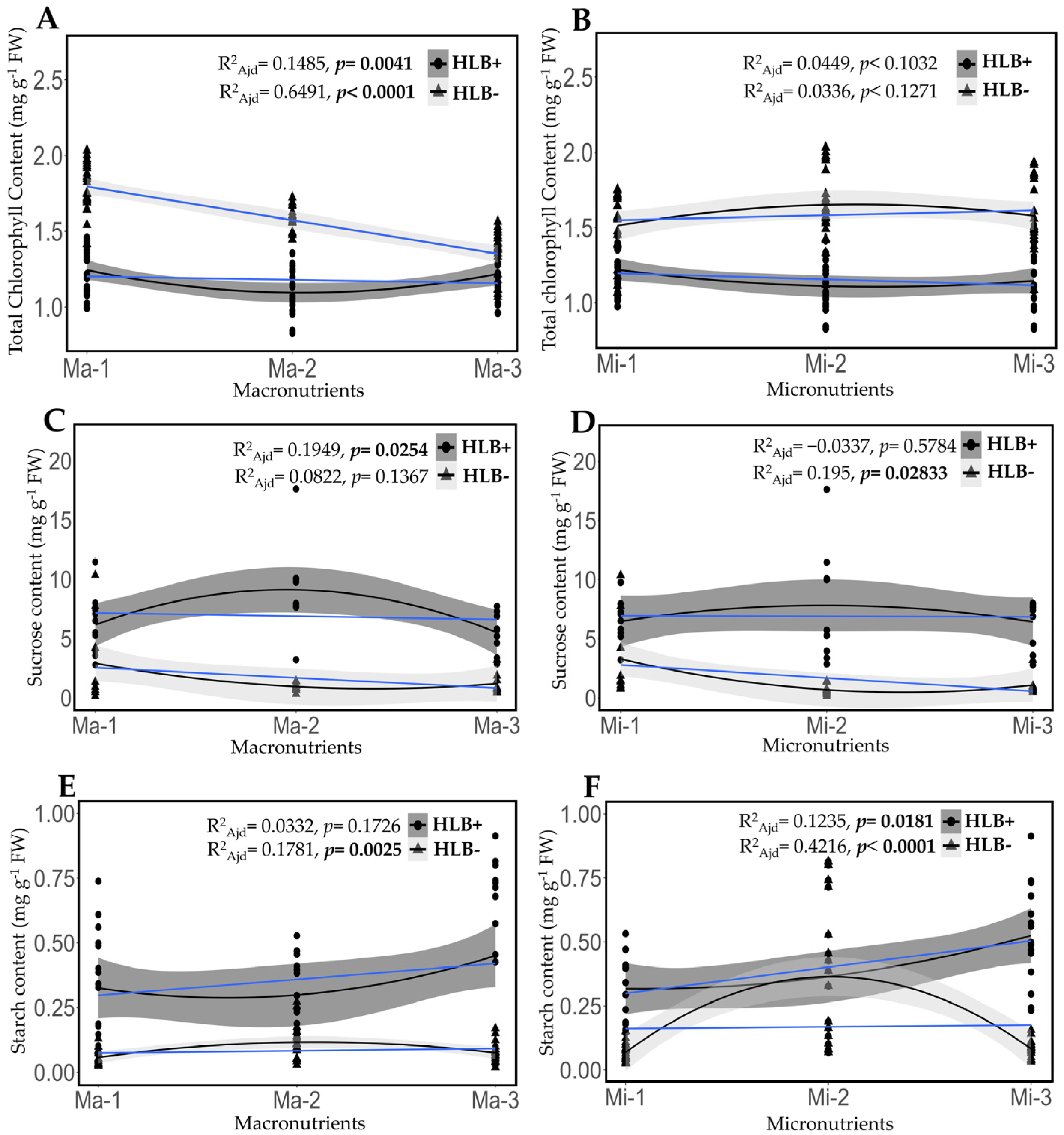
2.5. Fertilization Impact on the Content of Chlorophyll, Sucrose, and Starch in Healthy and HLB-Diseased Leaves
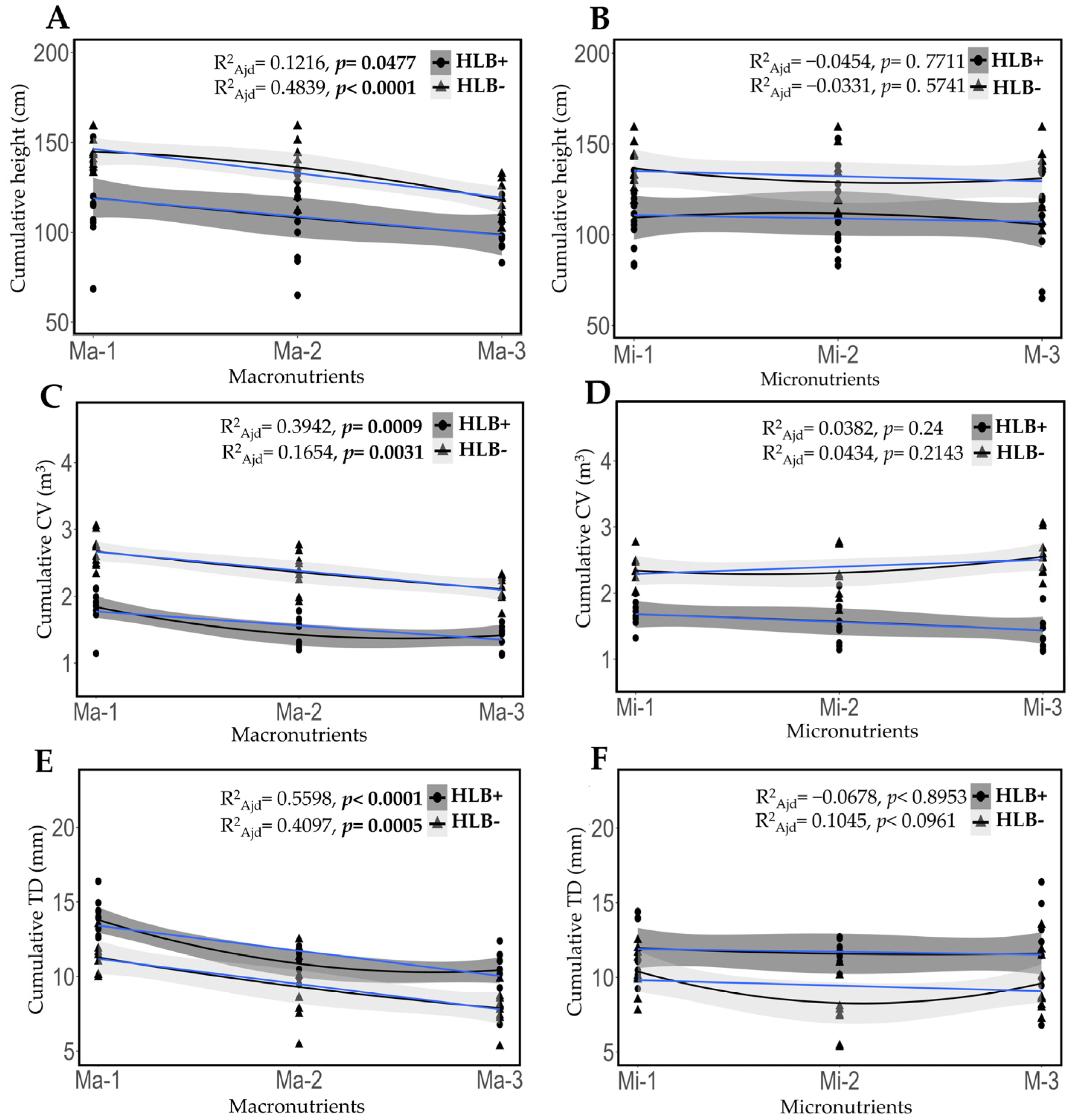
2.6. Fertilization Impact on Cumulative Growth in Height, Canopy Volume, and Trunk Diameter in Healthy and HLB-Diseased Plants
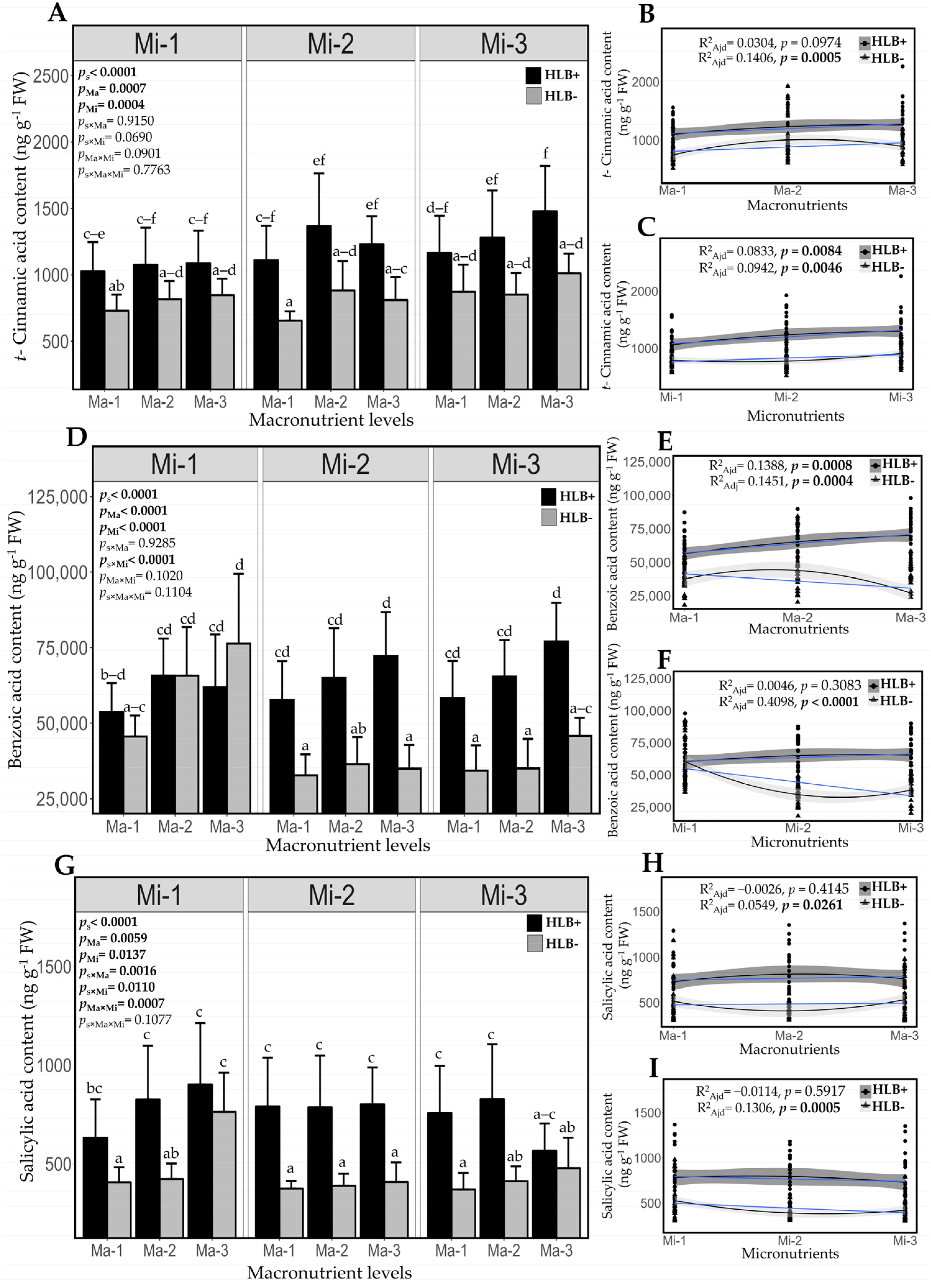
2.7. Fertilization Impact on Endogenous Content of Salicylic Acid and Its Biosynthetic Precursors in Healthy and HLB-Diseased Plants
2.8. Variables Associated with Healthy and HLB-Diseased Plant Groups Obtained by Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Study Site Description and Plant Material Used
4.2. Fertilization Treatments
4.3. Severity of Foliar Symptoms
4.4. DNA Extraction and CLas Detection
4.5. Growth Variables
4.6. Photosystem II (Fv/Fm) Efficiency
4.7. Chlorophyll Quantification in Leaves
4.8. Quantification of Sucrose and Starch in Leaves
4.9. Reflectance Measurement Using Spectrometry
4.10. Quantification of Benzoic Acid, Trans-Cinnamic Acid, and Salicylic Acid in Leaves Using GC-MS
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus: Citrus Huanglongbing. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N. Made for each other: Vector–pathogen interfaces in the Huanglongbing pathosystem. Phytopathology 2022, 112, 26–43. [Google Scholar] [CrossRef]
- Mora-Aguilera, G.; Robles-García, P.; López-Arroyo, J.I.; Flores-Sánchez, J.; Acevedo-Sánchez, G.; Domínguez-Monge, S.; Gutierrez-Espinosa, A.; Loeza-Kuk, E.; González-Gómez, R. Situación Actual y perspectivas del manejo del HLB de los cítricos. Rev. Mex. Fitopatol. 2014, 32, 108–119. [Google Scholar]
- Farnsworth, D.; Grogan, K.A.; van Bruggen, A.H.C.; Moss, C. The potential economic cost and response to greening in Florida citrus. Choices Mag. Food Farm Resour. Issues 2014, 29, 188996. [Google Scholar]
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Domínguez-Monge, S.; Acevedo-Sánchez, G.; Robles-García, P.; Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; et al. Pérdidas en producción inducidas por Candidatus Liberibacter asiaticus en limón Persa, en Yucatán México. Rev. Mex. Fitopatol. 2015, 33, 195–210. [Google Scholar]
- Villar-Luna, H.; Santos-Cervantes, M.E.; Rodríguez-Negrete, E.A.; Méndez-Lozano, J.; Leyva-López, N.E. Economic and social impact of huanglongbing on the mexico citrus industry: A Review and future perspectives. Horticulturae 2024, 10, 481. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. Revisiting the complex pathosystem of huanglongbing: Deciphering the role of citrus metabolites in symptom development. Metabolites 2020, 10, 409. [Google Scholar] [CrossRef]
- Ribeiro, C.; Stitt, M.; Hotta, C.T. How Stress affects your budget—Stress impacts on starch metabolism. Front. Plant Sci. 2022, 13, 774060. [Google Scholar] [CrossRef]
- Kumar, N.; Kiran, F.; Etxeberria, E. Huanglongbing-Induced anatomical changes in citrus fibrous root orders. HortScience 2018, 53, 829–837. [Google Scholar] [CrossRef]
- Deng, H.; Achor, D.; Exteberria, E.; Yu, Q.; Du, D.; Stanton, D.; Liang, G.; Gmitter, F.G., Jr. Phloem Regeneration is a mechanism for huanglongbing-tolerance of “Bearss” Lemon and “LB8-9” Sugar Belle® mandarin. Front. Plant Sci. 2019, 10, 277. [Google Scholar] [CrossRef]
- Suh, J.H.; Tang, X.; Zhang, Y.; Gmitter, F.G.; Wang, Y. Metabolomic analysis provides new insight into tolerance of Huanglongbing in citrus. Front. Plant Sci. 2021, 12, 710598. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the responses of different genotypes of citrus to Huanglongbing (citrus greening) under Different conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Brat, P.; Gros, O.; Ollitrault, P.; Morillon, R. Specific physiological and anatomical traits associated with polyploidy and better detoxification processes contribute to improved Huanglongbing tolerance of the Persian lime compared with the Mexican lime. Front. Plant Sci. 2021, 12, 685679. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, S.; Domínguez-Monge, S.; Allende-Molar, R.; Mendoza-García, J.D.; Pérez-hernández, O.; Rodríguez-Quibrera, C.G.; Curti-Díaz, S.A.; Flores-Sánchez, J.L.; Sarmiento-Tejeda, R. Influencia de Huanglongbing en pérdidas de producción y calidad de frutos de limón Persa en Veracruz. Supl. Rev. Mex. Fitopatol. 2022, 40, S40. [Google Scholar]
- Alquézar, B.; Carmona, L.; Bennici, S.; Miranda, M.P.; Bassanezi, R.B.; Peña, L. Cultural management of Huanglongbing: Current status and ongoing research. Phytopathology® 2022, 112, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant Mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Ahmad, K.; Ismail, S.I. Enhanced nutritional programme: An innovative approach to controlling plant diseases in the tropics. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 225–251. ISBN 978-3-319-27455-3. [Google Scholar]
- Ramirez, D.; Chaparro, J.; Wright, A.; Boman, B.; Gruber, B.; Ritenour, M.; Marino, S. Mitigation of Huanglongbing Effects on grapefruit trees using enhanced nutritional programs. Proc. Fla. State Hortic. Soc. 2016, 129, 51–55. [Google Scholar]
- Nwugo, C.C.; Duan, Y.; Lin, H. Study on citrus response to Huanglongbing highlights a down-regulation of defense-related proteins in lemon plants upon ‘Ca. Liberibacter Asiaticus’ infection. PLoS ONE 2013, 8, e67442. [Google Scholar] [CrossRef]
- Dong, Z.; Srivastava, A.K.; Liu, X.; Riaz, M.; Gao, Y.; Liang, X.; Tan, Q.; Sun, X.; Wu, S.; Hu, C. Interactions between nutrient and Huanglongbing pathogen in citrus: An overview and implications. Sci. Hortic. 2021, 290, 110511. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Manganese Adsorption, availability, and uptake in citrus under microsprinkler irrigation. Agrosystems Geosci. Environ. 2020, 3, e20061. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Mahmoud, K.A. The effect of foliar and ground-applied essential nutrients on Huanglongbing-affected mature citrus trees. Plants 2021, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.R.; de Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta Filho, H.D.; Mattos, D. Following the Effects of micronutrient supply in HLB-infected trees: Plant responses and ‘Candidatus Liberibacter Asiaticus’ acquisition by the Asian Citrus Psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M.; Mahmoud, K.A. Water and soil nutrient dynamics of Huanglongbing-affected citrus trees as impacted by ground-applied nutrients. Agronomy 2020, 10, 1485. [Google Scholar] [CrossRef]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground Application of overdoses of manganese have a therapeutic effect on sweet orange trees infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar applications of essential nutrients on growth and yield of ‘Valencia’ sweet orange infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Vashisth, T.; Grosser, J. Comparison of controlled release fertilizer (CRF) for newly planted sweet orange trees under Huanglongbing prevalent conditions. J. Hortic. 2018, 5, 244. [Google Scholar] [CrossRef]
- Ghimire, L.; Grosser, J.; Vashisth, T. Differences in nutrient uptake can influence the performance of citrus rootstocks under Huanglongbing conditions. HortScience 2023, 58, 40–46. [Google Scholar] [CrossRef]
- Hallman, L.M.; Kadyampakeni, D.M.; Ferrarezi, R.S.; Wright, A.L.; Ritenour, M.A.; Rossi, L. Uptake of micronutrients in severely HLB-affected grapefruit trees grown on Florida Indian River flatwood soils. J. Plant Nutr. 2023, 46, 4110–4124. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A.; Koo, R.; Alferez, F.M. Irrigation, Nutrition, and Citrus Fruit Quality; University of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Kadyampakeni, D.M.; Chinyukwi, T. Are macronutrients and micronutrients therapeutic for restoring performance of trees affected by citrus greening? A discussion of current practices and future research opportunities. J. Plant Nutr. 2021, 44, 2949–2969. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Kamal, M.A. Effect of soil and/or foliar applied nutrients on leaf nutrient accumulation and water uptake on Huanglongbing affected ‘Valencia’ citrus trees. Proc. Fla. State Hortic. Soc. 2018, 131, 58–64. [Google Scholar]
- Morgan, E.K.T.; Kadyampakeni, D.M. Nutrition of Florida Citrus Trees, 3rd ed.; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2020; p. 113. [Google Scholar]
- Kwakye, S.; Kadyampakeni, D.M.; Morgan, K.; Vashisth, T.; Wright, A. Effects of iron rates on growth and development of young Huanglongbing-affected citrus trees in Florida. HortScience 2022, 57, 1092–1098. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M. Interaction of soil boron application with leaf B concentration, root length density, and canopy size of citrus affected by Huanglongbing. J. Plant Nutr. 2020, 43, 186–193. [Google Scholar] [CrossRef]
- Kwakye, S.; Kadyampakeni, D.M.; Morgan, K.; Wright, A. Foliar Micronutrient applications enhance growth and yield of Huanglongbing (HLB)-affected sweet orange. Soil Sci. Soc. Am. J. 2022, 87, 365–377. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Transcriptional response of susceptible and tolerant citrus to infection with Candidatus Liberibacter Asiaticus. Plant Sci. 2012, 185–186, 118–130. [Google Scholar] [CrossRef]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Hufnagel, B.; Cebrian-Torrejon, G.; Doménech-Carbó, A.; Gros, O.; Ollitrault, P.; Morillon, R. Better tolerance to Huanglongbing Is conferred by tetraploid swingle citrumelo rootstock and is influenced by the ploidy of the scion. Front. Plant Sci. 2022, 13, 1030862. [Google Scholar] [CrossRef]
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Gutiérrez-Espinosa, M.A.; Velázquez-Monreal, J.J.; Domínguez-Monge, S.; Bassanezi, R.B.; Acevedo-Sánchez, G.; Robles-García, P. Diffusion model for describing the regional spread of Huanglongbing from first-reported outbreaks and basing an area wide disease management strategy. Plant Dis. 2017, 101, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Guo, Q.; Zhu, Z.; Zhang, L. Effects of different water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biol. Res. 2016, 49, 12. [Google Scholar] [CrossRef]
- Shahzad, F.; Chun, C.; Schumann, A.; Vashisth, T. Nutrient uptake in Huanglongbing-affected sweet orange: Transcriptomic and physiological analysis. J. Am. Soc. Hortic. Sci. 2020, 145, 349–362. [Google Scholar] [CrossRef]
- Flores-de la Rosa, F.R.; Santillán-Mendoza, R.; Rodríguez-Quibrera, C.G.; Martínez-Ruiz, A.; Adame-García, J.; Luna-Rodriguez, M. Antioxidant gene expression, chlorophyll, and starch content in Persian lime (Citrus latifolia Tanaka Ex Q. Jiménez) trees with HLB by application of elicitors of plant resistance. Mex. J. Biotechnol. 2021, 6, 86–102. [Google Scholar] [CrossRef]
- Smeekens, S. Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 49–81. [Google Scholar] [CrossRef]
- Curtolo, M.; de Souza Pacheco, I.; Boava, L.P.; Takita, M.A.; Granato, L.M.; Galdeano, D.M.; de Souza, A.A.; Cristofani-Yaly, M.; Machado, M.A. Wide-Ranging transcriptomic analysis of Poncirus trifoliata, Citrus sunki, Citrus sinensis and Contrasting hybrids reveals HLB tolerance mechanisms. Sci. Rep. 2020, 10, 20865. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.; Burns, J.K. Phytohormone changes and carbohydrate status in sweet orange fruit from Huanglongbing-Infected trees. J. Plant Growth Regul. 2011, 30, 312–321. [Google Scholar] [CrossRef]
- Weng, H.; Lv, J.; Cen, H.; He, M.; Zeng, Y.; Hua, S.; Li, H.; Meng, Y.; Fang, H.; He, Y. Hyperspectral reflectance imaging combined with carbohydrate metabolism analysis for diagnosis of citrus Huanglongbing in different seasons and cultivars. Sens. Actuators B Chem. 2018, 275, 50–60. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and Near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Martínez-Martínez, V.; Gomez-Gil, J.; Machado, M.L.; Pinto, F.A.C. Leaf and canopy reflectance spectrometry applied to the estimation of angular leaf spot disease severity of common bean crops. PLoS ONE 2018, 13, e0196072. [Google Scholar] [CrossRef]
- Deng, X.; Huang, Z.; Zheng, Z.; Lan, Y.; Dai, F. Field detection and classification of citrus Huanglongbing based on hyperspectral reflectance. Comput. Electron. Agric. 2019, 167, 105006. [Google Scholar] [CrossRef]
- He, C.; Li, X.; Liu, Y.; Yang, B.; Wu, Z.; Tan, S.; Ye, D.; Weng, H. Combining multicolor fluorescence imaging with multispectral reflectance imaging for rapid citrus Huanglongbing detection based on lightweight convolutional neural network using a handheld device. Comput. Electron. Agric. 2022, 194, 106808. [Google Scholar] [CrossRef]
- Miles, G.P.; Stover, E.; Ramadugu, C.; Keremane, M.L.; Lee, R.F. Apparent tolerance to huanglongbing in citrus and citrus-related germplasm. HortScience 2017, 52, 31–39. [Google Scholar] [CrossRef]
- Koh, E.-J.; Zhou, L.; Williams, D.S.; Park, J.; Ding, N.; Duan, Y.-P.; Kang, B.-H. Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with “Candidatus Liberibacter Asiaticus”. Protoplasma 2012, 249, 687–697. [Google Scholar] [CrossRef]
- Esquivel-Chávez, F.; Valdovinos-Ponce, G.; Mora-Aguilera, G.; Gómez-Jaimes, R.; Velázquez-Monreal, J.J.; Manzanilla-Ramírez, M.Á.; Flores-Sánchez, J.L.; López-Arroyo, J.I. Análisis histológico foliar de cítricos agrios y naranja dulce con síntomas ocasionados por Candidatus Liberibacter Asiaticus. Agrociencia 2012, 46, 769–782. [Google Scholar]
- Gilroy, E.; Breen, S. Interplay between phytohormone signalling pathways in plant defence—Other than salicylic acid and jasmonic acid. Essays Biochem. 2022, 66, 657–671. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Phytohormone profiling of the sweet orange (Citrus Sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J. Plant Physiol. 2016, 199, 12–17. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Citrus Phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol. Mol. Plant Pathol. 2018, 102, 24–35. [Google Scholar] [CrossRef]
- Zou, X.; Bai, X.; Wen, Q.; Xie, Z.; Wu, L.; Peng, A.; He, Y.; Xu, L.; Chen, S. Comparative analysis of tolerant and susceptible citrus reveals the role of methyl salicylate signaling in the response to Huanglongbing. J. Plant Growth Regul. 2019, 38, 1516–1528. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Zheng, Z.; Dai, Z.; Tao, Y.; Deng, X. Transcriptional analyses of mandarins seriously infected by ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2015, 10, e0133652. [Google Scholar] [CrossRef]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef]
- Coquoz, J.-L.; Buchala, A.; Métraux, J.-P. The Biosynthesis of salicylic acid in potato plants. Plant Physiol. 1998, 117, 1095–1101. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. Gamma-aminobutyric acid supplementation boosts the phytohormonal profile in ‘Candidatus Liberibacter asiaticus’-infected citrus. Plants 2023, 12, 3647. [Google Scholar] [CrossRef]
- Torres, L.M.F.; Olivas, A.F.; Fuentes, Y.M.O.; Arroyo, J.I.L.; Portugal, V.O.; Mendoza, A.B.; Morales, S.G.; Villa, V.M.Z. Comparison of Enzymes and phenolic compounds in three citrus species infected with Candidatus Liberibacter asiaticus. Mex. J. Phytopathol. 2017, 35, 314–325. [Google Scholar] [CrossRef]
- Esteves, E.; Maltais-Landry, G.; Zambon, F.; Ferrarezi, R.S.; Kadyampakeni, D.M. Nitrogen, Calcium, and magnesium inconsistently affect tree growth, fruit yield, and juice quality of Huanglongbing-affected orange trees. HortScience 2021, 56, 1269–1277. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Albrecht, U.; Padmanabhan, C.; Wang, A.; Coffey, M.D.; Girke, T.; Wang, Z.; Close, T.J.; Roose, M.; et al. Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus Huanglongbing disease. Mol. Plant 2013, 6, 301–310. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, C.; Yang, J.; Wang, Q. Pathogen Infection drives patterns of nutrient resorption in citrus plants. Sci. Rep. 2015, 5, 14675. [Google Scholar] [CrossRef]
- Gottwald, T.R.; Graham, J.H.; Irey, M.S.; McCollum, T.G.; Wood, B.W. Inconsequential effect of nutritional treatments on Huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Prot. 2012, 36, 73–82. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef]
- Ozores-Hampton, M.; Roka, F.; Rouse, R.; Roberts, P. Dual treatment tested for HLB trees. Citrus Ind. News, May 2017; 28–31. Available online: https://crec.ifas.ufl.edu/media/crecifasufledu/extension/extension-publications/2017/2017_May_dual.pdf (accessed on 16 December 2024).
- Bassanezi, R.B.; Primiano, I.V.; Vescove, H.V. Effect of enhanced nutritional programs and exogenous auxin spraying on huanglongbing severity, fruit drop, yield and economic profitability of orange orchards. Crop Prot. 2021, 145, 105609. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P.; Kwakye, S.; Barlas, N.T. Boron, Manganese, and zinc sorption and leaf uptake on citrus cultivated on a sandy soil. Plants 2022, 11, 638. [Google Scholar] [CrossRef]
- Curti-Díaz, S.A.; Loredo-Salazar, R.X.; Díaz-Zorrilla, U.; Sandoval-Rincón, J.A.; Hernández, H.J. Tecnología para Producir Limón Persa.; INIFAP-CIRGOC. Campo Experimental Ixtacuaco; Libro Técnico Núm. 8.: Veracruz, Mexico, 2000; 145p, ISBN 978-968-800-473-9. [Google Scholar]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of essential nutrients on roots growth and lifespan of Huanglongbing affected citrus trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.B. Citrus Huanglongbing: The Pathogen and Its Impact. Online. Plant Health Progress. Available online: https://apsjournals.apsnet.org/doi/10.1094/PHP-2007-0906-01-RV (accessed on 11 August 2024).
- Shokrollah, H.; Lee Abdullah, T.; Sijam, K.; Akmar Abdullah, S.N. Potential use of selected citrus rootstocks and interstocks against HLB disease in Malaysia. Crop Prot. 2011, 30, 521–525. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Bao, M.; Zheng, Z.; Sun, X.; Chen, J.; Deng, X. Enhancing PCR capacity to detect Candidatus Liberibacter asiaticus utilizing whole genome sequence information. Plant Dis. 2020, 104, 527–532. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P. Effect of irrigation pattern and timing on root density of young citrus trees infected with Huanglongbing disease. HortTechnology 2014, 24, 209–221. [Google Scholar] [CrossRef]
- Das, D.; Seal, P.; Biswas, A.K. Influence of selenium on growth, antioxidants production and physiological parameters of rice (Oryza Sativa L.) seedlings and its possible reversal by coapplication of sulphate. Am. J. Plant Sci. 2019, 10, 2236–2278. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G., Jr.; Li, Z.-G. Changes in carbohydrate metabolism in Citrus Sinensis infected with Candidatus Liberibacter Asiaticus. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Zheng, Y.; Kumar, N.; Gonzalez, P.; Etxeberria, E. Strigolactones restore vegetative and reproductive developments in Huanglongbing (HLB) affected, greenhouse-grown citrus trees by modulating carbohydrate distribution. Sci. Hortic. 2018, 237, 89–95. [Google Scholar] [CrossRef]
- Hijaz, F.; Killiny, N. Collection and chemical composition of phloem sap from Citrus Sinensis L. Osbeck (Sweet Orange). PLoS ONE 2014, 9, e101830. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, C.; Kamphuis, L.G.; Gummer, J.P.A.; Singh, K.B.; Trengove, R.D. A rapid method for profiling of volatile and semi-volatile phytohormones using methyl chloroformate derivatisation and GC–MS. Metabolomics 2015, 11, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Enviroment for R (Version 4.3.1) R. RStudio PBC 2023. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 20 February 2024).
- Brochu, A.-S.; Durrivage, J.; Torres, D.; Pérez-López, E. Diet and injection, important recommendations to characterize Clavibacter Michiganensis –tomato interactions. Plant Health Prog. 2023, 24, 475–481. [Google Scholar] [CrossRef]
- Kozak, M.; Piepho, H.-P. What’s normal anyway? residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop Sci. 2018, 204, 86–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Zarate, L.A.; Martínez-Hernández, A.; Osorio-Acosta, F.; García-Pérez, E.; Morales-Trejo, F.; Villanueva-Jiménez, J.A. Fertilization Strategies in Huanglongbing-Infected Citrus latifolia and Their Physiological and Hormonal Effects. Plants 2025, 14, 1086. https://doi.org/10.3390/plants14071086
Pérez-Zarate LA, Martínez-Hernández A, Osorio-Acosta F, García-Pérez E, Morales-Trejo F, Villanueva-Jiménez JA. Fertilization Strategies in Huanglongbing-Infected Citrus latifolia and Their Physiological and Hormonal Effects. Plants. 2025; 14(7):1086. https://doi.org/10.3390/plants14071086
Chicago/Turabian StylePérez-Zarate, Luis A., Aída Martínez-Hernández, Francisco Osorio-Acosta, Eliseo García-Pérez, Fredy Morales-Trejo, and Juan A. Villanueva-Jiménez. 2025. "Fertilization Strategies in Huanglongbing-Infected Citrus latifolia and Their Physiological and Hormonal Effects" Plants 14, no. 7: 1086. https://doi.org/10.3390/plants14071086
APA StylePérez-Zarate, L. A., Martínez-Hernández, A., Osorio-Acosta, F., García-Pérez, E., Morales-Trejo, F., & Villanueva-Jiménez, J. A. (2025). Fertilization Strategies in Huanglongbing-Infected Citrus latifolia and Their Physiological and Hormonal Effects. Plants, 14(7), 1086. https://doi.org/10.3390/plants14071086






